Abstract
Objectives:
This study explores the mechanism of tanshinone IIA (TSN)-mediated inhibition of myocardial fibrosis by investigating the effect of TSN on transforming growth factor β1 (TGFβ1) signal transduction in rat cardiac fibroblasts (CFs).
Materials and Methods:
CFs were isolated from neonatal Sprague–Dawley rats by trypsin digestion and differential adhesion and stimulated with 5 ng/mL TGFβ1 and TSN (10−6, 10−5, or 10−4 mol/L). The expression of fibronectin (FN) mRNA in the CFs was determined using reverse transcriptase-polymerase chain reaction and the protein expression of FN and Smads in CFs was detected using Western blot. The intracellular expression and localization of Smads in the CFs were analyzed using immunocytochemistry.
Results:
TGFβ1 induced the expression of FN and Smads in a time-dependent manner. At the end of the culture treatment, the mRNA expression of FN and the expression of phosphorylated Smad2/3 (p-Smad2/3) increased significantly (P < 0.01). TSN pretreatment (10−5 and 10−4 mol/L) reduced the expression of FN and p-Smad2/3 (P < 0.01) following TGFβ1 stimulation and led to a significant decrease in the nuclear staining intensity and a positive rate of p-Smad2/3 (P < 0.05 and P < 0.01, respectively).
Conclusion:
The inhibitory effect of TSN on myocardial fibrosis may be associated with its inhibition of TGFβ1-induced Smad2/3 phosphorylation and p-Smad2/3 nuclear translocation, which blocks the TGFβ1/Smad signaling pathway in CFs.
KEY WORDS: Cardiac fibroblasts, smads, tanshinone II A, transforming growth factor β1
Introduction
Cardiac fibroblasts (CFs) that are over-stimulated by a variety of growth factors are the main pathological reason for myocardial fibrosis. These hyperactive cells proliferate and transform into myofibroblasts, causing large amounts of extracellular matrix (ECM) secretion and deposition in the interstitial sites of the heart.[1,2] Although there are many growth factors implicated in fibrosis, many investigators currently postulate that transforming growth factor β (TGFβ) is a key regulatory factor in the proliferation and differentiation of fibroblasts. The role of TGFβ in promoting fibrosis is primarily mediated by the Smad signaling pathway.[3,4] In recent years, research on drugs targeting the TGFβ receptor, its ligand, and Smad proteins for the treatment of myocardial fibrosis has achieved positive results,[5] but the clinical efficacy of these drugs has not yet been confirmed.
Tanshinone II A (TSN) is a liposoluble active ingredient isolated from the traditional Chinese medical plant Salvia miltiorrhiza. TSN has been found to possess antioxidant properties, inhibit platelet aggregation and smooth muscle cell proliferation, and inhibit expansion of the coronary artery, in addition to its anti-inflammatory properties and other pharmacological effects.[6,7,8] Recent studies have shown that TSN effectively inhibits the proliferation and phenotypic transformation of rat CFs, as well as reduces ECM production and delays the progression of myocardial fibrosis.[9,10,11] As a result, this study was designed to explore the mechanism underlying TSN-mediated inhibition of myocardial fibrosis by observing the effects of TSN on the TGFβ1 signal transduction pathway in CFs. Our objective was to provide a mechanistic basis for the clinical application of TSN in the treatment of myocardial fibrosis.
Materials and Methods
Animals
Twenty pathogen-free grade neonatal Sprague–Dawley (SD) rats (4–7 days old), weighing 10–15 g and irrespective of gender, were purchased from the Experimental Animal Center (Animal certification No. 19–050). This study was carried out in strict accordance with the recommendations in the guide for the care and use of Laboratory Animals of the National Institutes of Health. The animal use protocol had been reviewed and approved by the Institutional Animal Care and Use Committee of Tongji Hospital.
Isolation, Culture, and Identification of Cardiac Fibroblasts
As previously reported,[11] neonatal SD rats underwent thoracotomy under sterile conditions to remove the heart. Once the large vessels were cut and the cardiac pericardium removed, the heart was rinsed with phosphate-buffered saline (PBS), minced into pieces of about 1 mm3, and digested with 0.25% trypsin (Beyotime Institute of Biotechnology, Shanghai, China) repeatedly at 37°C. After two centrifugation steps at 1000 rpm for 5 min, the cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM, Hangzhou Sijiqing Biological Engineering Co., Ltd., Zhejiang, China) containing 20% fetal calf serum at 37°C and 5% CO2 for 60 min. The differential adhesion method was used to remove cardiomyocytes. T-enriched cardiomyocytes were identified with streptavidin-biotin complex (SABC) immuno-enzymatic staining with vimentin and desmin antibodies. The cells that were vimentin-positive and desmin-negative were considered to be CFs and cultured continually. As the cells grew to 80% confluence, they were passaged at a ratio of 1:2, and the second through fourth passages of cells were used for the following experiments.
Cell Culture
The second through fourth passages of CFs were cultured in a serum-free DMEM/F12 medium (Gibco, Grand Island, USA) and incubated at 37°C and 5% CO2 for 24 h. When the cells were synchronized, they were separated into three groups: TGFβ1-stimulated, TSN pretreated, and control groups. TGFβ1 (5 ng/mL; PeproTech, New York, USA) was added to CFs in the TGFβ1 group, and cells were cultured for 0, 6, 12, or 24 h. CFs in the TSN pretreatment group were pretreated with 10−6, 10−5, or 10−4 mol/L TSN (Shanghai No. 1 Biochemical Pharmaceutical Co., Ltd., State Drug Approval Document Number: H31022558) and incubated for 2 h, and then, the cells were treated as in the TGFβ1 group. CFs in the control group were treated with the same volume of PBS instead of drug. Each experiment was performed in triplicate.
Reverse Transcription Polymerase Chain Reaction
Total RNA was extracted from CFs using Trizol reagent (Invitrogen, Carlsbad, USA). cDNA was made from the RNA product using the PrimeScript™ RT reagents kit (TaKaRa, Tokyo, Japan) following the manufacturer's directions. The cDNA was amplified by polymerase chain reaction (PCR) using the following gene-specific primer pairs: Fibronectin (FN): (forward) 5ʹ-TTA TGA CGA TGG GAA GAC CTA-3ʹ and (reverse) 5ʹGTG GGG CTG GAA AGA TTA CTC-3ʹ; β-actin: (forward) 5ʹ-TGG AGA AGA GCT ATG AGC TGC CTG-3ʹ and (reverse) 5ʹ-GTG CCA CCA GAC AGC ACT GTG TTG-3ʹ. The PCR product (308 bp) was visualized using 1.5% agarose gel electrophoresis and scanned using the Gel Image Analysis System (manufacturer: Media Cybernetics, New York, USA). The scanned absorbance of each PCR product was analyzed and normalized to the β-actin absorbance value (A value) using the Gel Image Analysis software (manufacturer: Media Cybernetics, New York, USA).
Western Blot
Total protein was extracted using radioimmunoprecipitation buffer, separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to polyvinylidene difluoride membranes (Nanjing Kangruipu Biological Technology Co., Ltd., Nanjing, China) for immunoblotting. The membranes were blocked with 5% defatted milk powder (diluted in TBST) for 1 h and then incubated with rabbit anti-rat FN polyclonal antibody (1:500, Wuhan Boster Biological Engineering Co., Ltd., Wuhan, China) or goat anti-rat Smad2, Smad3, or phosphorylated Smad2/3 (p-Smad2/3) polyclonal antibody (1:500, Abcam, Cambridge, UK) at 4°C overnight. After three washes with PBST, membranes were incubated with peroxidase-conjugated goat anti-rabbit IgG (1:5000, Protein Tech Group Inc., Chicago, USA) or rabbit anti-goat IgG (1:9000, Protein Tech Group Inc., Chicago, USA) for 1 h at room temperature. Specific protein bands on the membranes were visualized using enhanced chemiluminescence (Amersham, Piscataway, NJ, USA) according to the manufacturer's instructions, and their absorbance was analyzed using LabWorks 4.5 software (manufacturer: UVP, Upland, USA) and normalized to the β-actin absorbance value.
Immunocytochemical Staining
The third passage of CFs was seeded in a 24-well plate and incubated at 37°C and 5% CO2. After the cells had been grown to 60% confluence, the medium was removed, and the cells were washed twice with PBS. Then, the cells were treated with 5 ng/mL TGFβ1, followed by culture for 15, 60, or 120 min. In addition, a set of CFs was treated with 10−6, 10−5, or 10−4 mol/L TSN, followed by stimulation with 5 ng/mL of TGFβ1 and further cultured for 120 min. One group with no treatment was used as a control. The experiments in the control group and each treatment group were repeated 3 times. After treatments, cells were stained with 1:100 p-Smad2/3 polyclonal antibody (Abcam, Cambridge, UK) using the SABC immunocytochemical method according to the manufacturer's instructions.[12]
Statistical Analysis
All data are shown as mean ± standard deviation and were analyzed using SPSS v11.0 (manufacturer: IBMSPSS software, New York, USA). Comparisons between groups were carried out using analysis of variance. P <0.05 was considered significant.
Results
Effect of Tanshinone on Transforming Growth Factor B1-induced Fibronectin Expression
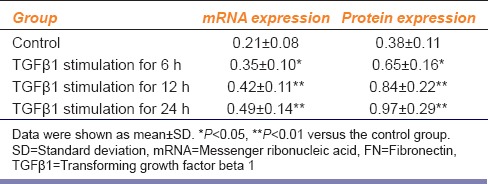
At certain concentrations, TGFβ1 can modify FN expression in CFs in a time-dependent manner [Table 1]. The mRNA and protein expression of FN began to increase 6 h after TGFβ1 stimulation. At 12 h and 24 h after stimulation, the mRNA expression of FN increased 1.2-fold and 1.4-fold, respectively (P < 0.01), and the protein expression of FN increased 1.3-fold and 1.5-fold, respectively (P < 0.01).
Table 1.
mRNA and protein expression of FN after TGFβ1 stimulation
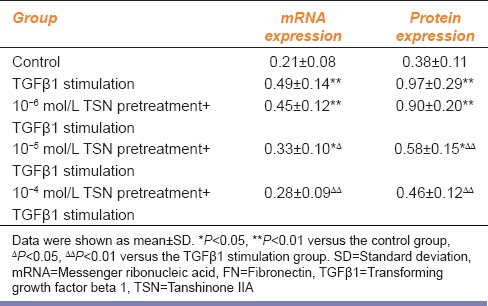
Different concentrations of TSN had different effects on TGFβ1-induced FN expression in CFs [Figures 1 and 2]. Pretreatment with 10−6 mol/L TSN had no effect on the expression of FN (P > 0.05), but pretreatment with 10−5 and 10−4 mol/L TSN reduced FN mRNA expression by 32.7% and 42.9%, respectively (P < 0.05 and P < 0.01, respectively) and reduced FN protein expression by 45.8% and 57.1%, respectively [P < 0.01 and P < 0.01, respectively; Table 2].
Figure 1.
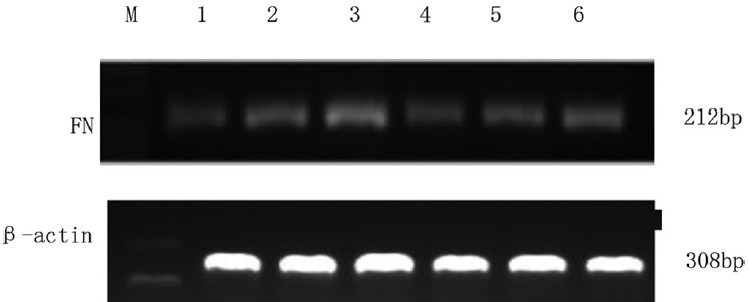
The fibronectin mRNA expression of cardiac fibroblasts in different groups determined using real time-polymerase chain reaction. M, DNA ladder; 1, control; 2, 5 ng/mL transforming growth factor beta 1 (TGFβ1) stimulation for 6 h; 3, 5 ng/mL TGFβ1 stimulation for 24 h; 4, 10−6 mol/L tanshinone (TSN) pretreatment + 5 ng/mL TGFβ1 stimulation; 5, 10−5 mol/L TSN pretreatment + 5 ng/mL TGFβ1 stimulation; 6, 10−4 mol/L TSN pretreatment + 5 ng/mL TGFβ1 stimulation
Figure 2.
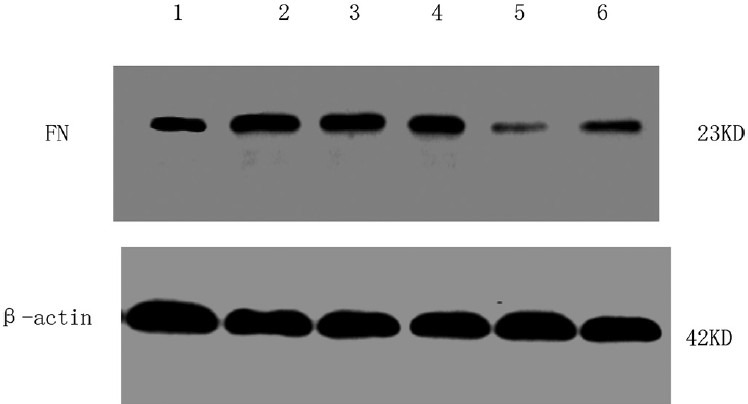
The fibronectin protein expression of cardiac fibroblasts in different groups determined using Western blot. 1, Control; 2, 5 ng/mL transforming growth factor beta 1 (TGFβ1) stimulation for 6 h; 3, 5 ng/mL TGFβ1 stimulation for 24 h; 4, 10−6 mol/L tanshinone (TSN) pretreatment + 5 ng/mL TGFβ1 stimulation; 5, 10−5 mol/L TSN pretreatment + 5 ng/mL TGFβ1 stimulation; 6, 10−4 mol/L TSN pretreatment + 5 ng/mL TGFβ1 stimulation
Table 2.
mRNA and protein expression of FN after TGFβ1 stimulation with TSN pretreatment
Effect of Tanshinone on the Transforming Growth Factor B1-induced Smads Expression and its Nuclear Translocation
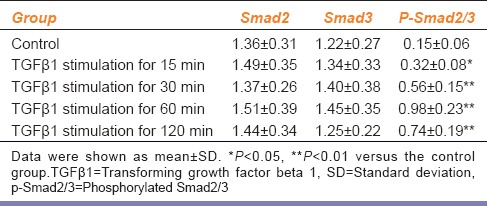
Within the range of concentrations, TGFβ1 induced the expression of p-Smad2/3 in CFs in a time-dependent manner [Table 3]. 15 min after TGFβ1 stimulation, the expression of p-Smad2/3 began to increase and reached a peak at 60 min. At 120 min after TGFβ1 stimulation, the expression of pSmad2/3 decreased but was still 3.9-fold higher than the expression of the control group. There was no significant difference in total Smad2 or Smad3 expression between the TGFβ1 simulation and control groups (P > 0.05).
Table 3.
Expression of Smads after TGFβ1 stimulation
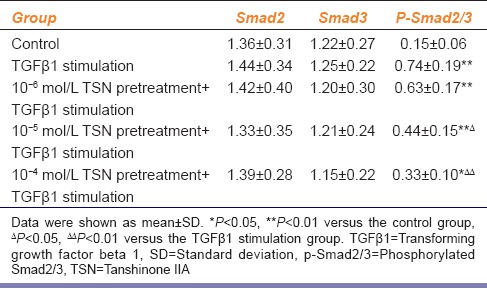
Different concentrations of TSN differently affected the expression of TGFβ1-induced p-Smad2/3 by CFs [Figure 3]. Low-dose TSN pretreatment (10−6 mol/L) played no significant role in the expression of p-Smad2/3 in CFs (P > 0.05). However, pretreatment with 10−5 mol/L and 10−4 mol/L TSN reduced the expression of p-Smad2/3 by 40.5% and 55.4%, respectively [P < 0.05 and P < 0.01, respectively; Table 4]. No concentration of TSN influenced the expression of total Smad2 or total Smad3 (P > 0.05).
Figure 3.
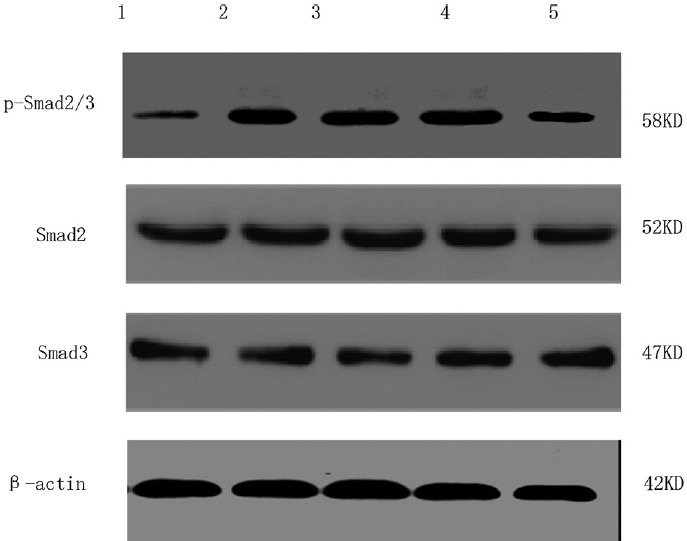
The expression of p-Smad2/3 in cardiac fibroblasts with different treatments determined using Western blot 1, Control; 2, 5 ng/mL transforming growth factor beta 1 (TGFβ1) stimulation for 120 min; 3, 10−6 mol/L tanshinone (TSN) pretreatment + 5 ng/mL TGFβ1 stimulation; 4, 10−5 mol/L TSN pretreatment + 5 ng/mL TGFβ1 stimulation; 5, 10−4 mol/L TSN pretreatment + 5 ng/mL TGFβ1 stimulation
Table 4.
Expression of Smads after TGFβ1 stimulation with TSN pretreatment
Based on the above results, we, immunocytochemistry, examined the expression and localization of p-Smad2/3 in CFs. The nuclear expression of p-Smad2/3 in CFs in the control group was weak, but this expression was induced by TGFβ1 stimulation in a time-dependent manner. During TGFβ1 stimulation, the nuclear expression of p-Smad2/3 increased gradually over time, reaching a peak after 60 min but decreasing slightly after 120 min. If the cells were pretreated with 10−5 or 10−4 mol/L but not 10−6 mol/L TSN, the intensity and positive rate of p-Smad2/3 expression in the nucleus reduced remarkably [Figure 4].
Figure 4.
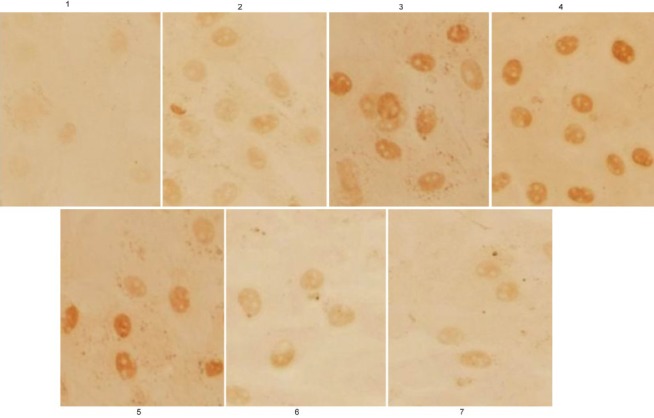
The translocation of p-Smad2/3 in cardiac fibroblasts with different treatments determined using immunocytochemistry. 1, Control; 2, 5 ng/mL (transforming growth factor beta 1 (TGFβ1) stimulation for 15 min; 3, 5 ng/mL TGFβ1 stimulation for 60 min; 4, 5 ng/mL TGFβ1 stimulation for 120 min; 5, 10−6 mol/L tanshinone (TSN) pretreatment + 5 ng/mL TGFβ1 stimulation; 6, 10−5 mol/L TSN pretreatment + 5 ng/mL TGFβ1 stimulation; 7, 10−4 mol/L TSN pretreatment + 5 ng/mL TGFβ1 stimulation
Discussion
Hypertension, myocardial infarction, diabetes, and other diseases can cause CF proliferation and transformation into myofibroblasts, which may lead to increased ECM synthesis, secretion, and deposition in the interstitial sites of the heart. Consequently, this process can result in myocardial fibrosis and severely impair cardiac function.[1,2] Thus, regulation of fibroblast proliferation and CF phenotypic transformation may prevent myocardial fibrosis and improve the prognosis of patients with heart disease.[13,14]
Currently, investigators have hypothesized that the proliferation and transformation of fibroblasts are primarily related to over-activation of the TGFβ signaling pathway.[3,4,15] TGFβ1 is the strongest pro-fibrosis factor that has been discovered thus far. TGFβ1 stimulation can up-regulate the expression of actin in CFs, providing fibroblasts with contractile properties and contributing to the irreversible transformation of fibroblast to myofibroblasts.[3] Smads are key proteins in the TGFβ signaling pathway.[4] TGFβ1 is activated at its extracellular region and then binds to its specific membrane receptor-TGFβ I/II receptor (TβR I/II) to activate Smad-mediated intracellular signal transduction. Smads are a class of highly conserved proteins with a molecular weight of 42–60 kDa. According to their structural and functional characteristics, Smads can be divided into three categories: Receptor modulators, common channel types, and receptor inhibitors. Receptor modulator Smads include Smad2 and Smad3, which are related to the TGFβ1 signal transduction pathway. Common channel-type Smads are characterized by Smad4. Inhibitory-type Smads include Smad6 and Smad7. Smads 2 and 3 are substrates for TβR I intracellular kinase, which can bind to Smad4 as it is phosphorylated into p-Smad2/3 via the receptor kinase. Then, it can regulate the gene expression of procollagen αI and connective tissue growth factor after nuclear translocation and consequently promote the proliferation and phenotypic transformation of fibroblasts. Smad4 can also promote the synthesis and secretion of large amounts of ECM, playing a key role in intracellular TGFβ1 signal transduction.[16,17] FN is the major component of ECM, with a potent ability to bind fibrin, fibrinogen, and collagen. Its synthesis and expression levels show a significant positive correlation with the extent of tissue fibrosis.[18,19] This study finds that TGFβ1 can stimulate CFs to express p-Smad2/3 in a time-dependent manner, which is consistent with the promoting effect on FN expression. This suggests that an active TGFβ1/Smad signaling pathway in CFs. Excessive activation of this pathway could play an important role in the occurrence and development of myocardial fibrosis.
Tanshinone is the liposoluble active ingredient of the traditional Chinese medical plant S. miltiorrhiza. TSN can effectively inhibit the proliferation of hepatic stellate cells and renal interstitial fibroblasts, thus reversing or delaying hepatic fibrosis or kidney fibrosis.[20,21] Recent studies have also found that TSN can alleviate myocardial fibrosis caused by increased pressure overload in rats,[22] probably by inhibiting the proliferation of CFs and their phenotypic transformation, and by reducing the production of ECM.[9,10,11] Based on these previously published data, this study further explored the possible mechanisms underlying TSN inhibition of fibroblast proliferation and transformation.
In this study, we found that TGFβ1 induced the expression of p-Smad2/3 in CFs in a time-dependent manner, and this observation was consistent with the role of TGFβ1 in the promotion of FN expression, suggesting the presence of the TGFβ1/Smad signaling pathway in CFs. TSN pretreatment inhibited TGFβ1-induced p-Smad2/3 and FN expression in a dose-dependent manner. Low-dose TSN pretreatment (10−6 mol/L) had almost no impact on the expression of p-Smad2/3, but 10−5 and 10−4 mol/L TSN pretreatment significantly reduced the expression of p-Smad2/3. Pretreatment with 10−5 and 10−4 mol/L TSN reduced the expression of p-Smad2/3 by 40.5% and 55.4% (P < 0.05 and P < 0.01, respectively) and reduced the mRNA expression of FN by 32.7% and 42.9% (P < 0.05 and P < 0.01, respectively). Moreover, at two concentrations (10−5 and 10−4 mol/L), TSN pretreatment significantly reduced the expression of nuclear p-Smad2/3 (P < 0.05 and P < 0.01, respectively), indicating that TSN can interfere with the intracellular TGFβ1/Smad signaling pathway in CFs. These data also indicate that TSN may inhibit the synthesis and secretion of ECM by preventing the phosphorylation of Smad2/3 induced by TGFβ1, as well as the nuclear translocation of p-Smad2/3. This mechanism is similar to that demonstrated in our previous study, in which TSN prevented renal interstitial fibrosis.[23]
These experiments were completed in vitro using a TSN solution. To determine whether the TSN effects on the TGFβ1/Smads signal pathway was dose dependent, we tested three concentrations of TSN (10−6, 10−5, and 10−4 mol/L) based on existing reports,[6,10,15] but whether these concentrations are optimal remains unclear. In addition, TGFβ1 signal transduction is very complex. Results from this study revealed that TSN can inhibit TGFβ1-induced Smad2/3 phosphorylation, but the precise cellular target of TSN remains unclear. TSN may directly bind Smad2/3 or may modify Smad2/3 function via upstream or downstream pathways, and these mechanisms require further investigation.
Based on this study and studies published by other groups, we hypothesize that TSN can partially block intracellular TGFβ1 signal transduction and can inhibit the proliferation and phenotypic transformation of CFs. In this way, TSN reduces ECM synthesis induced by TGFβ1, suggesting that it may have some clinical application in the prevention and treatment of myocardial fibrosis.
Footnotes
Source of Support: Nil.
Conflict of Interest: No.
References
- 1.Cuspidi C, Ciulla M, Zanchetti A. Hypertensive myocardial fibrosis. Nephrol Dial Transplant. 2006;21:20–3. doi: 10.1093/ndt/gfi237. [DOI] [PubMed] [Google Scholar]
- 2.von Knobelsdorff-Brenkenhoff F, Schulz-Menger J. Cardiomyopathies and myocarditis. Radiologe. 2013;53:8–14. doi: 10.1007/s00117-012-2380-6. [DOI] [PubMed] [Google Scholar]
- 3.Leask A. TGFbeta, cardiac fibroblasts, and the fibrotic response. Cardiovasc Res. 2007;74:207–12. doi: 10.1016/j.cardiores.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Yoshimatsu Y, Watabe T. Roles of TGF-β signals in endothelial-mesenchymal transition during cardiac fibrosis. J Mol Cell Cardiol. 2012;52:273–82. doi: 10.4061/2011/724080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang B, Zhu B, Liang Y, Bi L, Hu Z, Chen B, et al. Asiaticoside suppresses collagen expreesion and TGF-β/Samd signaling through inducing Smad7 and inhibiting TGF-βR Iand TGF-βR II in keloid fibroblasts. Arch Dermatol Res. 2011;303:563–72. doi: 10.1007/s00403-010-1114-8. [DOI] [PubMed] [Google Scholar]
- 6.Yang Z, Qiu M. Cardiovascular effects of tanshinone II A and its mechanism research progress. Chin J Arterioscler. 2011;19:372–4. [Google Scholar]
- 7.Li YP, Gu B, Liu JT, Xiong XY, Zhou CL, Wu GJ. Research progress in tanshinone II A. Lishizhen Med Mater Med Res. 2010;21:1770–2. [Google Scholar]
- 8.Xu LJ, Huang GY. Overview of chemical constituents and pharmacological research of Salvia. Res Integr Tradit Chin West Med. 2009;1:45–8. [Google Scholar]
- 9.Zhang DM, Qin Y, Niu FL, Zhou LQ, Wang SR, Jiang LD. Effects of tanshinones II A on proliferation and type I collagen synthesis of rat cardiac fibroblasts. Liaoning J Tradit Chin Med. 2008;35:1934–6. [Google Scholar]
- 10.Cai H, Zhao ZM, Xiu CY, Guo JH, Zhao LJ. Tanshinone inhibits lysophosphatidic acid-induced cardiac fibroblast proliferation and TGF-β1 production in neonate rats. J Med Postgrad. 2008;21:126–8. [Google Scholar]
- 11.Zhou D, Li Z, Zhang L, Zhan C. Inhibitory effect of tanshinone II A on TGF II-β1-induced cardiac fibrosis. J Huazhong Univ Sci Technolog Med Sci. 2012;32:829–33. doi: 10.1007/s11596-012-1042-2. [DOI] [PubMed] [Google Scholar]
- 12.Huang Y, Gu B, Wu R, Zhang J, Li Y, Zhang M. Development of a rabbit monoclonal antibody group against Smads and immunocytochemical study of human and mouse embryonic stem cells. Hybridoma (Larchmt) 2007;26:387–91. doi: 10.1089/hyb.2007.0517. [DOI] [PubMed] [Google Scholar]
- 13.Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: Therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005;45:657–87. doi: 10.1146/annurev.pharmtox.45.120403.095802. [DOI] [PubMed] [Google Scholar]
- 14.Zeisberg M, Kalluri R. Cellular mechanisms of tissue fibrosis 1. Common and organ-specific mechanisms associated with tissue fibrosis. Am J Physiol Cell Physiol. 2013;304:C216–25. doi: 10.1152/ajpcell.00328.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74:184–95. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–27. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 17.Verrecchia F, Mauviel A. Transforming growth factor-beta signaling through the Smad pathway: Role in extracellular matrix gene expression and regulation. J Invest Dermatol. 2002;118:211–5. doi: 10.1046/j.1523-1747.2002.01641.x. [DOI] [PubMed] [Google Scholar]
- 18.Mao Y, Schwarzbauer JE. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol. 2005;24:389–99. doi: 10.1016/j.matbio.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Moriya K, Bae E, Honda K, Sakai K, Sakaguchi T, Tsujimoto I, et al. A fibronectin-independent mechanism of collagen fibrillogenesis in adult liver remodeling. Gastroenterology. 2011;140:1653–63. doi: 10.1053/j.gastro.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Che XH, Park EJ, Zhao YZ, Kim WH, Sohn DH. Tanshinone II A induces apoptosis and S phase cell cycle arrest in activated rat hepatic stellate cells. Basic Clin Pharmacol Toxicol. 2010;106:30–7. doi: 10.1111/j.1742-7843.2009.00465.x. [DOI] [PubMed] [Google Scholar]
- 21.Tang JH, Zhan CY. Inhibitive effect of tanshinone II A on renal interstitial fibrosis in rats. J Intern Intensive Med. 2008;14:16–8. [Google Scholar]
- 22.Chang WJ, Zhao ZM, Zhao ZJ, Dong XL, Cai H. Improvement effect of tanshinone II A on myocardial fibrosis caused by increased pressure overload in rats. Chin J Microcirc. 2013;23:34–9. [Google Scholar]
- 23.Tang J, Zhan C, Zhou J. Effects of tanshinone IIA on transforming growth factor beta1-Smads signal pathway in renal interstitial fibroblasts of rats. J Huazhong Univ Sci Technolog Med Sci. 2008;28:539–42. doi: 10.1007/s11596-008-0511-0. [DOI] [PubMed] [Google Scholar]


