Abstract
Hyponatremia is a known adverse effect of duloxetine, and it can lead to potentially life-threatening complications. Administration of thiazide diuretics also has been the cause of hyponatremia. We report a case of duloxetine-induced hyponatremia in an elderly patient treated with thiazide diuretics. An 86-year-old woman treated with the trichlormethiazide was admitted for vertebral compression fracture with disorientation and nausea on the 6th day of treatment with duloxetine. Laboratory findings revealed hyponatremia, hypo-osmolality, concentrated urine, and increased urine sodium. Syndrome of inappropriate antidiuretic hormone was considered, therefore, duloxetine, and trichlormethiazide was discontinued and treated with fluid restriction, furosemide and sodium chloride administered orally. Disorientation and nausea were improved after correction of hyponatremia. Health care practitioners should be aware of the possibility of duloxetine-induced hyponatremia, particularly in patients treated with thiazide diuretics.
KEY WORDS: Drug interaction, duloxetine, syndrome of inappropriate secretion of antidiuretic hormone syndrome, trichlormethiazide
Introduction
Duloxetine is a selective serotonin and noradrenaline reuptake inhibitor (SNRI), indicated for the treatment of major depressive disorder and diabetic peripheral neuropathy. In rare cases, hyponatremia associated with duloxetine has been reported in female patients.[1] Thiazide diuretics are among the most commonly prescribed antihypertensive medications, which have been implicated in multiple electrolyte abnormalities including hyponatremia.[2] We report a case of severe hyponatremia that developed after the duloxetine was added to thiazide diuretics therapy.
Case Report
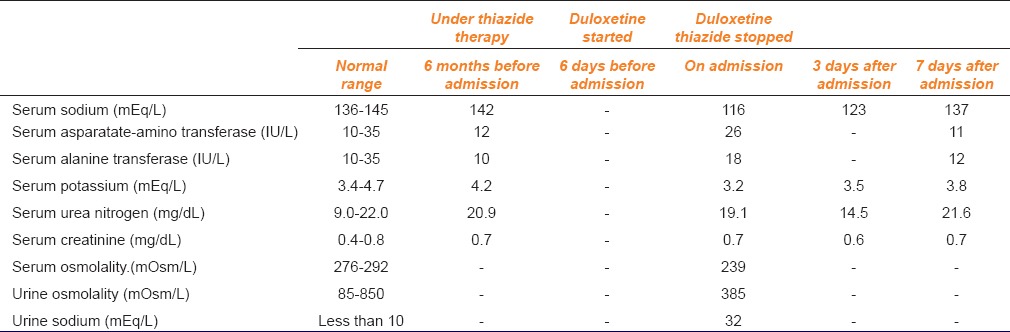
An 86-year-old woman with a history of hypertension, vertigo and insomnia was admitted to the orthopedic department complaining of low back pain, nausea and disorientation. Her height was 151 cm, and she weighed 48 kg. According to the information obtained from the patient and her family members, she complained of nausea and disorientation a few days before admission and fell down at that time. On admission, her magnetic resonance imaging of the thoracic-lumbar vertebra showed fresh compression fracture of the twelfth thoracic vertebra. She was prescribed trichlormethiazide 2 mg/day, Doxazosin 1 mg/day, tocopherol nicotinate 600 mg/day, oxybutynin 4 mg/day, diphenidol 75 mg/day, bethahistine 18 mg/day, domperidone 30 mg/day, triazolam 0.25 mg/day, lorazepam 0.5 mg/day and duloxetine 20 mg/day before admission. According to her medication history, duloxetine was started for depression 6 days before admission and trichlormethiazide was started more than 1-year before admission. At the time of admission, blood investigation showed serum sodium level of 116 mEq/L (normal range: 136–145 mEq/L). Other clinical laboratory findings were: Serum aspartate-amino transferase level - 26 IU/L (normal range: 10–35 IU/L), serum alanine transaminase level - 18 IU/L (10–35 IU/L), serum potassium level - 3.2 mEq/L (3.4–4.7 mEq/L), serum urea nitrogen level - 19.1 mg/dL (9.0–22.0 mg/dL) and serum creatinine level-0.7 mg/dL (0.4–0.8 mg/dL). Additional clinical laboratory investigations revealed serum osmolality of 239 mOsm/L (normal range: 276–292 mOsm/L), simultaneous urine osmolality of 385 mOsm/L (85–850 mOsm/L) and urine sodium of 32 mEq/L (<10 mEq/L). According to medical records from her primary care physician, serum sodium level was 142 mEq/L before 3 months. Other clinical laboratory findings were also within normal range. Her medications were not changed, except duloxetine. Hence based on laboratory reports, the diagnosis of the syndrome of inappropriate secretion of antidiuretic hormone (SIADH) was made. It was suspected that SIADH was associated with concurrent use of duloxetine and trichlormethiazide, which were, therefore, discontinued. The severe hyponatremia was treated with oral fluid restriction (600 ml/day), oral furosemide (20 mg/day) and oral sodium chloride (6 g/day). On the third day after admission, serum sodium concentration increased to 123 mEq/L. On the next day, disorientation and nausea improved remarkably. On the 7th day after admission, serum sodium concentration returned to the normal range (137 mEq/L). Liver and renal functions also remained normal [Table 1]. Oral fluid restriction and oral sodium chloride were stopped a week later. After that serum sodium concentration remained in normal range.
Table 1.
Serial laboratory findings in the patient
Discussion
Hyponatremia is defined as a low serum sodium level <136 mEq/L.[3] Patients with mild hyponatremia whose serum sodium levels are >125 mEq/L are usually asymptomatic.[3] Symptoms of hyponatremia include lethargy, anorexia, headache, fatigue, nausea, and muscle cramps. If sodium levels continue to decrease, symptoms may progress to confusion, seizure, coma and irreversible brain damage, which are noted with serum sodium levels <120 mEq/L.[3]
In our case, symptoms of hyponatremia included nausea and disorientation, which seemed to be relatively modest. However, her serum sodium level was 116 mEq/L, which could lead to severe symptoms. If appropriate treatments were not conducted at an early stage, patient might have developed permanent neurological complications.
The exact mechanism of hyponatremia associated with thiazide is unclear. Some proposed mechanisms involve impairment of the urinary diluting ability, stimulation of the antidiuretic hormone (ADH) secretion, urinary sodium loss and intracellular potassium depletion by inhibiting sodium chloride reabsorption in the distal convoluted tubule. Furthermore, increased water ingestion due to dipsogenic effect of thiazides can develop more severe hyponatremia.[2]
The mechanism of SNRI-related hyponatremia is also unclear. Animal experiments suggest that both norepinephrine and serotonin can stimulate ADH secretion.[4] Duloxetine inhibits the reuptake of both serotonin and norepinephrine, which may explain why our patient developed hyponatremia. Hyponatremia associated with selective serotonin reuptake inhibitors (SSRIs) developed a few weeks after initiation of SSRIs, while duloxetine-induced hyponatremia developed a few days following the start of duloxetine,[1] which also occurred in our patient.
According to Horn's drug interaction probability scale,[5] hyponatremia was probably related to concomitant use of duloxetine and trichlormethiazide (score 6). Furthermore, according to the Naranjo adverse drug reaction probability scale,[6] hyponatremia was also probably related to the use of these drugs (score 7). Serum sodium level before and after the initiation of duloxetine were 142 mEq/L and 116 mEq/L, respectively. The risk factors for hyponatremia include advanced age, female, lower body weight, lower baseline serum sodium.[4] Our patient had these risk factors except lower baseline serum sodium. All previous cases of duloxetine-related hyponatremia had lower baseline sodium levels.[2] Our case suggests that the rapid decrease in serum sodium level developed due to coadministration of SNRI with thiazides through complementary and synergistic manners. Our report has two limitations. First, serum sodium level immediately before the initiation of duloxetine was unclear. Second, the role of thiazide in the rapid decrease of serum sodium level is unclear because thiazide was started >1-year before the initiation of duloxetine.
This case highlights the development of severe hyponatremia in elderly female patient treated with duloxetine and thiazide diuretics concurrently. Although the exact mechanism is unclear, SIADH is suspected as a contributing cause based on the clinical findings (normal renal function, low serum osmolality, concentrated urine and high level of urine sodium). The combination of duloxetine and thiazide may result in a rapid decrease of serum sodium level. When SNRI and thiazide diuretics are prescribed concurrently, health care practitioners should be aware of the possibility of hyponatremia. Careful monitoring of serum electrolytes within a week of the initiation of these medications is recommended.
Footnotes
Source of Support: Nil.
Conflict of Interest: No.
References
- 1.Safdieh JE, Rudominer R. A case of hyponatremia induced by duloxetine. J Clin Psychopharmacol. 2006;26:675–6. doi: 10.1097/01.jcp.0000246207.73034.96. [DOI] [PubMed] [Google Scholar]
- 2.Friedman E, Shadel M, Halkin H, Farfel Z. Thiazide-induced hyponatremia. Reproducibility by single dose rechallenge and an analysis of pathogenesis. Ann Intern Med. 1989;110:24–30. doi: 10.7326/0003-4819-110-1-24. [DOI] [PubMed] [Google Scholar]
- 3.Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342:1581–9. doi: 10.1056/NEJM200005253422107. [DOI] [PubMed] [Google Scholar]
- 4.Kirby D, Ames D. Hyponatraemia and selective serotonin re-uptake inhibitors in elderly patients. Int J Geriatr Psychiatry. 2001;16:484–93. doi: 10.1002/gps.367. [DOI] [PubMed] [Google Scholar]
- 5.Horn JR, Hansten PD, Chan LN. Proposal for a new tool to evaluate drug interaction cases. Ann Pharmacother. 2007;41:674–80. doi: 10.1345/aph.1H423. [DOI] [PubMed] [Google Scholar]
- 6.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]


