Abstract
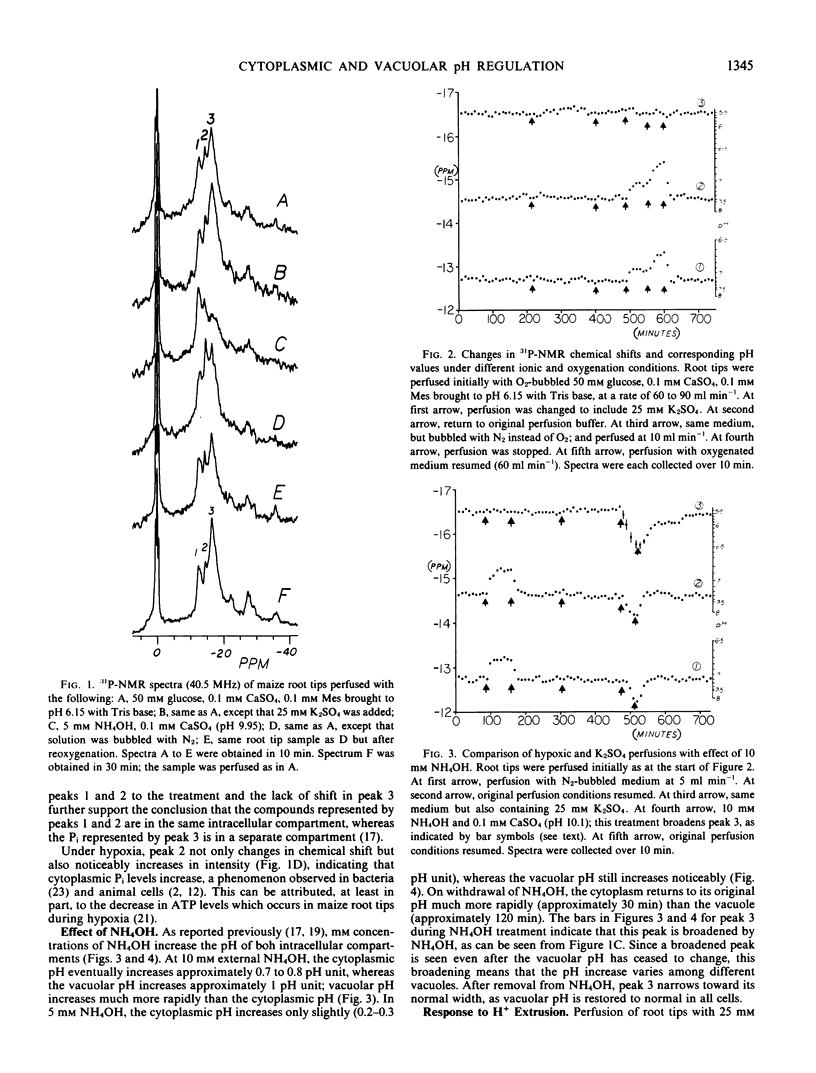
31P-Nuclear magnetic resonance spectra of perfused maize (Zea mays L., hybrid WW x Br 38) root tips, obtained at 10-minute intervals over 12 hours or longer, indicate that no cytoplasmic or vacuolar pH changes occur in these cells in the presence of 25 millimolar K2SO4, which induces extrusion of 4 to 5 microequivalents H+ per gram per hour. In contrast, hypoxia causes cytoplasmic acidification (0.3-0.6 pH unit) without a detectable change in vacuolar pH. The cytoplasm quickly returns to its original pH on reoxygenation. Dilute NH4OH increases the vacuolar pH more than it does the cytoplasmic pH; after NH4OH is removed, the vacuole recovers its original pH more slowly than does the cytoplasm. The results indicate that regulation of cytoplasmic pH and that of vacuolar pH in plant cells are separate processes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burt C. T., Cohen S. M., Bárány M. Analysis with intact tissue with 31P NMR. Annu Rev Biophys Bioeng. 1979;8:1–25. doi: 10.1146/annurev.bb.08.060179.000245. [DOI] [PubMed] [Google Scholar]
- Chance B., Eleff S., Leigh J. S., Jr Noninvasive, nondestructive approaches to cell bioenergetics. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7430–7434. doi: 10.1073/pnas.77.12.7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Contraction and recovery of living muscles studies by 31P nuclear magnetic resonance. J Physiol. 1977 Jun;267(3):703–735. doi: 10.1113/jphysiol.1977.sp011835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKSON P. C., ADAMS H. R. Cation-anion balance during potassium and sodium absorption by barley roots. J Gen Physiol. 1963 Jan;46:369–386. doi: 10.1085/jgp.46.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkdjian A., Guern J. Vacuolar pH Measurement in Higher Plant Cells : I. EVALUATION OF THE METHYLAMINE METHOD. Plant Physiol. 1981 May;67(5):953–957. doi: 10.1104/pp.67.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkdjian A., Leguay J. J., Guern J. Measurement of intracellular pH and aspects of its control in higher plant cells cultivated in liquid medium. Respir Physiol. 1978 Apr;33(1):75–89. doi: 10.1016/0034-5687(78)90086-5. [DOI] [PubMed] [Google Scholar]
- McLaughlin A. C., Takeda H., Chance B. Rapid ATP assays in perfused mouse liver by 31P NMR. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5445–5449. doi: 10.1073/pnas.76.11.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon R. B., Richards J. H. Determination of intracellular pH by 31P magnetic resonance. J Biol Chem. 1973 Oct 25;248(20):7276–7278. [PubMed] [Google Scholar]
- Rabkin S. W., Mathewson F. A., Tate R. B. Chronobiology of cardiac sudden death in men. JAMA. 1980 Sep 19;244(12):1357–1358. [PubMed] [Google Scholar]
- Roberts J. K., Jardetzky O. Monitoring of cellular metabolism by NMR. Biochim Biophys Acta. 1981 Nov 9;639(1):53–76. doi: 10.1016/0304-4173(81)90005-7. [DOI] [PubMed] [Google Scholar]
- Roberts J. K., Wade-Jardetzky N., Jardetzky O. Intracellular pH measurements by 31P nuclear magnetic resonance. Influence of factors other than pH on 31P chemical shifts. Biochemistry. 1981 Sep 15;20(19):5389–5394. doi: 10.1021/bi00522a006. [DOI] [PubMed] [Google Scholar]
- Saglio P. H., Raymond P., Pradet A. Metabolic Activity and Energy Charge of Excised Maize Root Tips under Anoxia: CONTROL BY SOLUBLE SUGARS. Plant Physiol. 1980 Dec;66(6):1053–1057. doi: 10.1104/pp.66.6.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]


