Abstract
Zea mays is an economically important crop, but its molecular mechanism of flowering remains largely uncharacterized. The gene, SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1), integrates multiple flowering signals to regulate floral transition in Arabidopsis. In this study, ZmSOC1 was isolated from Zea mays. Sequence alignment and phylogenetic analysis demonstrated that the ZmSOC1 protein contained a highly conserved MADS domain and a typical SOC1 motif. ZmSOC1 protein was localized in the nucleus in protoplasts and showed no transcriptional activation activity in yeast cells. ZmSOC1 was highly expressed in maize reproductive organs, including filaments, ear and endosperm, but expression was very low in embryos; on the other hand, the abiotic stresses could repress ZmSOC1 expression. Overexpression of ZmSOC1 resulted in early flowering in Arabidopsis through increasing the expression of AtLFY and AtAP1. Overall, these results suggest that ZmSOC1 is a flowering promoter in Arabidopsis.
Keywords: Zea mays, ZmSOC1, flowering, Arabidopsis, transcription factor
1. Introduction
Flowering is regulated by both environmental and endogenous factors [1]. In Arabidopsis thaliana, six genetically defined pathways that control flowering have been identified: vernalization, photoperiod, ambient temperature, gibberellin, autonomous and age pathways [2]. Various signals in these pathways regulate flowering, but eventually converge to several integration factors, including FLOWERING LOCUS C (FLC), FLOWERING LOCUS T (FT), SUPPRESSOR OF OVEREXPRESSION OF CO1 (SOC1) and LEAFY (LFY) [3,4,5,6,7].
The MADS-box family of transcription factors in plants is known for its role in developmental processes [8]. Most of the members of this family are involved in flowering regulation, vegetative development, meristem identity regulation, floral organ development and the formation of fruit and seed [9,10,11,12,13]. The MADS-box family contains a DNA binding domain of about 58 amino acids that binds DNA sequences known as the CArG box (CC(A/T)6GG) [14,15].
Models for the integration of genetic networks for flowering have been proposed in model plants, and SOC1, FT and LFY act as flowering pathway integrators [16,17,18]. SOC1 encodes a MADS-box protein and contains a highly conserved DNA-binding domain. Studies have shown that SOC1 integrates the GA pathway [19]. Furthermore, CO acts as a major positive factor underlying long day conditions, whereas the GA pathway acts as a positive factor underlying short day conditions [20,21,22]. SOC1 interacts with multiple MADS box proteins, including AGAMOUS-LIKE24 (AGL24), FRUITFUL (FUL) and AP1, and regulates the expression of several flowering genes, such as SVP, AGL15, AGL18 and SEP3, by binding directly to regulatory sequences [20,23,24,25,26]. SOC1 had been isolated from Arabidopsis [7,19,25], Oryza sativa [27,28,29], Petunia hybrida [9], Citrus sinensis [30], Trillium camtschatcense [31] and Triticum aestivum L. [32] and promoted flowering in transgenic Arabidopsis.
Maize is an economically important crop, and a comprehensive understanding of flowering behaviors is required to increase production and improve seed quality. However, the molecular mechanisms of flowering control in maize remain poorly understood. The ZmMADS1/ZMM5, which is a SOC1/TM3-like gene in Zea mays, is expressed in vegetative organs at a low level, increased in reproductive tissues and parallels the expression pattern in the development of OsSOC1 and AtSOC1 [7,28,33,34,35,36]. ZmMADS1/ZMM5 not only contains the typical Mikc domain, but also has a highly conserved SOC1 motif at the C-terminal, which uniquely exists in the SOC1/TM3-like gene [36,37]. Based on the similarity between ZmMADS1/ZMM5 and SOC1, we renamed ZmMADS1/ZMM5 as ZmSOC1 [38].
In the present study, we isolated the ZmSOC1 gene from Zea mays (B73). The protein sequence information, subcellular localization, transcriptional activation activity and expression pattern of ZmSOC1 in maize were investigated. Our results show that ZmSOC1 promotes flowering in Arabidopsis and suggest that the gene may play a similar role in maize.
2. Results and Discussion
2.1. Isolation and Characterization of ZmSOC1
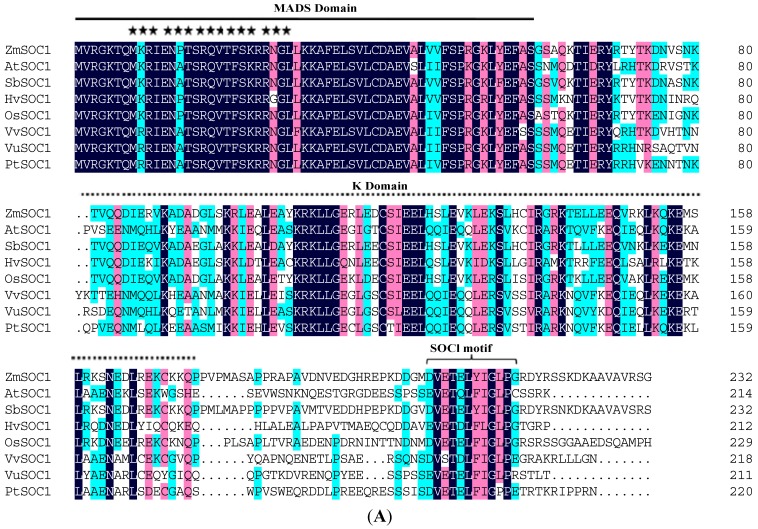
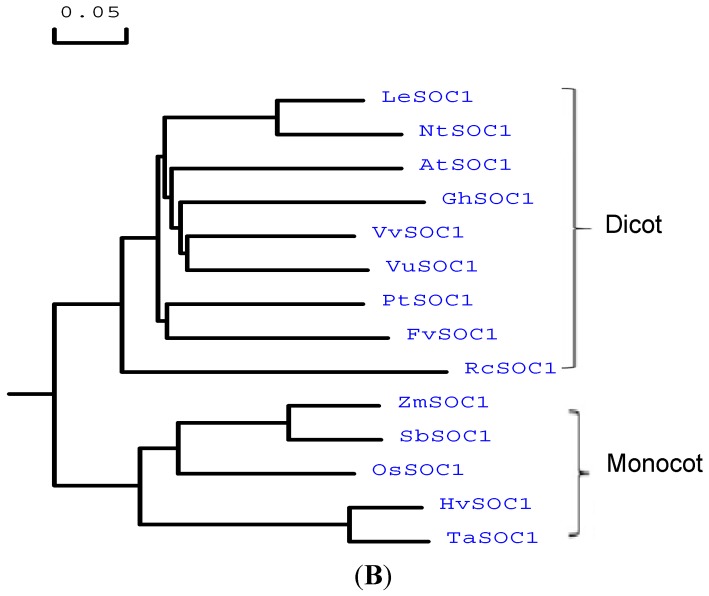
The ORF of the ZmSOC1 gene was 696 base pairs (bp) in length and identical to ZmMADS1 (GenBank ID: NM_001111682.1), encoding a protein of 232 amino acids with an estimated molecular mass of 26.4 kDa. A BLAST search of GenBank revealed that the ZmSOC1 protein was similar to SbSOC1 (87.50% identity), OsSOC1 (75.11% identity), AtSOC1 (53.99% identity), HvSOC1 (65.09% identity), VvSOC1 (54.17% identity), PtSOC1 (52.05% identity) and VuSOC1 (50.48% identity), respectively (Figure 1A). Multiple sequence alignment indicated that ZmSOC1 contained a well-conserved MADS domain and a less-conserved K domain (Figure 1A). However, there are significant variations appearing in their C-terminals. In addition, a nuclear localization signal was predicted (Figure 1A). To investigate the relationship between ZmSOC1 and other SOC1-like genes, a phylogenetic tree was constructed based on the amino acid sequences in their MADS-box domain (Figure 1B). The phylogenetic tree showed that all members could be divided into dicot and monocot clades, and the ZmSOC1 protein belonged to the monocot clade together with other SOC1 homologs from the Gramineae (Figure 1B).
Figure 1.
Bioinformatical analysis of ZmSOC1. (A) Amino acid sequence alignment of ZmSOC1 and its homologs from other plant species. The proteins were initially aligned using DNAMAN software. Identical and similar amino acids are shown in black and red shading, respectively. The MADS domain, K domain and SOC1 motif are marked with a solid line, dotted line and brackets, respectively. The locations of potential nuclear localization signals are marked with asterisks within the ZmSOC1 amino acid sequence; (B) Phylogenetic analysis of ZmSOC1 protein and its homologs from various plant species. The DNAMAN software was used for phylogenetic analysis. ZmSOC1 protein could be divided into dicot and monocot clades. The accession numbers of the SOC1-like genes are as follows: Arabidopsis thaliana SOC1: AtSOC1 (NP_182090.1); Hordeum vulgare SOC1: HvSOC1 (BAJ99551.1); Lycopersicon esculentum SOC1: LeSOC1 (NP_001276829.1); Nicotiana tabacum SOC1: NtSOC1 (CAA53782.1); Oryza sativa SOC1: OsSOC1 (NP_001048801.1); Populus tremuloides SOC1: PtSOC1 (AAP46287.1); Ricinus communis SOC1: RcSOC1 (XP_002510866.1); Sorghum bicolor SOC1: SbSOC1 (XP_002465961.1); Vitis vinifera SOC1: VvSOC1 (NP_001267909.1); Fragaria vesca SOC1: FvSOC1 (NP_001266966.1); Vigna unguiculata SOC1: VuSOC1 (BAJ22387.1); Gossypium hirsutum SOC1: GhSOC1 (AEA29618.1); and Triticum aestivum SOC1: TaSOC1 (ABF57922.1).
2.2. Expression Profiles of ZmSOC1 in Maize
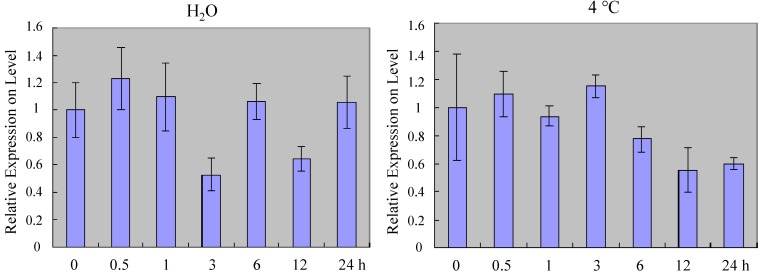
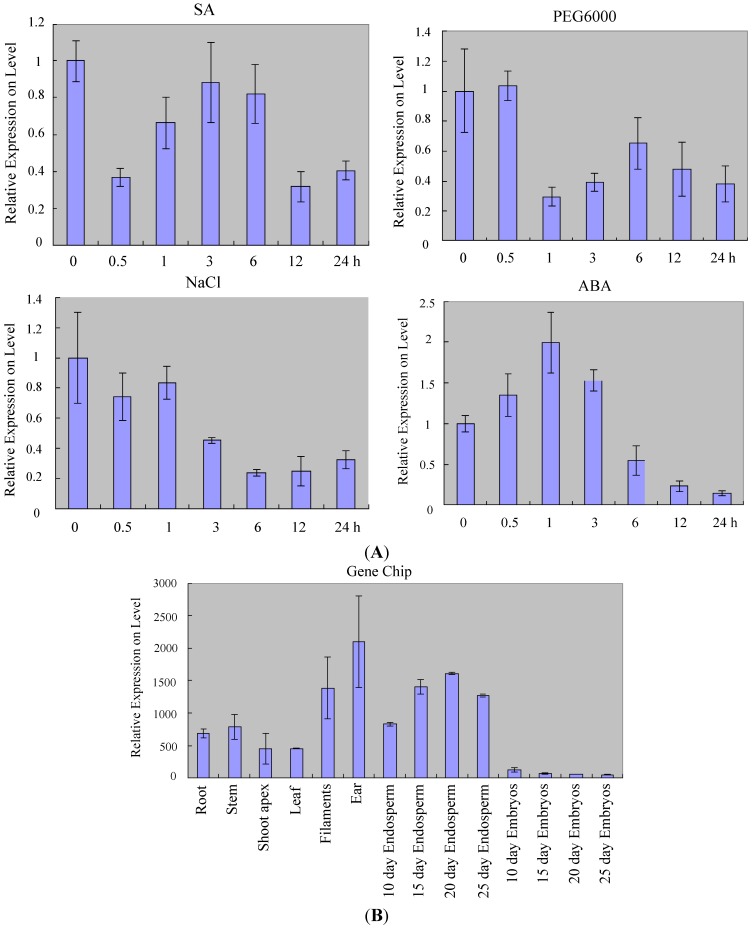
To explore whether ZmSOC1 expression was regulated by abiotic stresses, maize seedlings were treated with NaCl, PEG6000, Abscisic Acid (ABA), Salicylic acid (SA), low temperature (4 °C) and water without nutrients. At 4 °C, ZmSOC1 was slightly downregulated after 6 h; for NaCl treatment, ZmSOC1 was significantly repressed after 3 h; for PEG6000 treatment, ZmSOC1 was repressed quickly; for ABA treatment, ZmSOC1 increased within 3 h and subsequently decreased after 6 h; for SA treatment, ZmSOC1 expression decreased (but not significantly before 6 h), with a 40% reduction after 12 h, but the expression level of ZmSOC1 was lightly changed in the water without nutrients (Figure 2A). Taken together, these results indicate that ZmSOC1 expression was downregulated by the abiotic stresses.
Figure 2.
Expression profiles of ZmSOC1. (A) ZmSOC1 expression under stress conditions. ZmSOC1 expression was analyzed using qRT-PCR in Z31 leaves under different stress treatments (i.e., NaCl (250 mM), PEG6000 (20%), Abscisic Acid (ABA, 100 µM), Salicylic acid (SA, 100 µM), 4 °C, and H2O without nutrients) for different period of time (0, 0.5, 1, 3, 6, 12 and 24 h); (B) ZmSOC1 expression levels in maize tissues. The standardized data from the GeneChip of maize were used for the expression analysis; the data of eight tissues were extracted from root, stem, leaf, shoot apex, filament, ear, embryo and the endosperm compartment.
To profile expression of ZmSOC1 in different plant tissues and different developmental stages, we analyzed its expression using a GeneChip. ZmSOC1 maintained expression at a moderate level with relatively stable expression in roots, stems, leaves and the shoot apex (Figure 2B). The expression level increased two- to three-fold in the filaments and ears, demonstrating that ZmSOC1 plays an important role in maize floral organ development. Interestingly, its expression remained high for 10–25 days in endosperms, but in the embryos, the expression levels decreased to almost undetectable levels, indicating that ZmSOC1 may be an essential factor for endosperm development (Figure 2B).
MADS-box gene-specific effects are closely related to internal and external environmental factors. Zhang and Forde [39] found NO3− as a signaling molecule stimulating lateral root elongation in Arabidopsis and is dependent of the expression of the MADS-box gene, ANR1. Wang [40] found that the RM1 (MADS-box) gene in rice callus tissue regulates plant cell dedifferentiation and re-differentiation processes and acts on target genes. After the target genes are activated, the cells transform into a distinct morphology. Overall, ZmSOC1 as a MADS-box gene may play an important role in plant growth and developmental processes.
2.3. Subcellular Localization and Yeast Transcriptional Activation of ZmSOC1
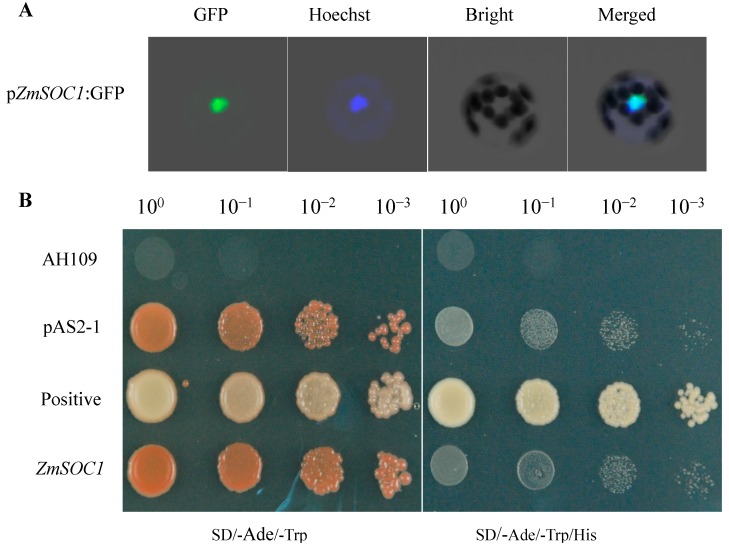
ZmSOC1 amino acid sequence analysis revealed a nuclear localization signal in the MADS-box domain (Figure 1A). To validate the subcellular localization of ZmSOC1 protein, ZmSOC1:GFP was transformed into Arabidopsis mesophyll protoplasts. The fluorescence of ZmSOC1:GFP was localized in the nucleus as a blue spot according to Hoechst staining (Figure 3A). These data demonstrate that ZmSOC1 entered the nucleus as a transcription factor and was involved in transcriptional regulation.
Figure 3.
Subcellular localization and yeast transcriptional activation analysis of ZmSOC1. (A) Subcellular localization of the ZmSOC1:GFP fusion protein. The Arabidopsis protoplasts were transformed with the plasmid ZmSOC1:GFP. After the transfected protoplasts were incubated for 12–16 h at 22 °C in darkness, they were stained with 5 mg/mL Hoechst 33342 (CalBiochem) for 0.5–2.0 h to visualize the locations of the nucleus; (B) Analysis of ZmSOC1 transcriptional activation activity in yeast. The empty plasmids, pAS2-1, pAS2-1:ZmSOC1 and pGBK-GAL4-SV40-T53 (positive), were transferred into yeast strain AH109. Yeast were diluted in four gradient solutions.
The transcriptional activation activity of ZmSOC1 protein was analyzed using a yeast two-hybrid system. The recombinant plasmid pAS2-1:ZmSOC1, together with the positive control pGBK-GAL4-SV40-T53 and the negative control pAS2-1 plasmids, respectively, were transformed into the yeast strain AH109 containing the reporter gene HIS. All transformants grew well on the SD (Synthetic Dropout)/-Ade/-Trp media (Figure 3B), whereas on SD/-Ade/-Trp/-His media, only the positive control pGBK-GAL4-SV40-T53 transformants survived. These results demonstrate that ZmSOC1 could not activate the expression of HIS genes in yeast cells, suggesting that ZmSOC1 protein may have no transcriptional activation activity in yeast.
Transcription factors containing sequence-specific DNA-binding motifs are key molecular switches for nuclear localization, DNA binding and dimerization [24,25,41]. In the present study, a predicted nuclear localization signal was present in the MADS domain of ZmSOC1 (Figure 1). As expected, the ZmSOC1 protein mainly localized to the Arabidopsis nucleus (Figure 3). However, MADS-box transcriptional activation has not been widely investigated. The C-terminus in some MADS-box proteins functions as a core transcriptional activation domain [37,42]. In the present study, we demonstrated that the ZmSOC1 protein had no transcriptional activation activity in yeast cells (Figure 3), similar to other MADS-box proteins, such as AGAMOUS (AG), APETALA3 (AP3) and PISTILLATA (PI) [43]. Because the C-terminal domain is the most divergent region among the MADS-box proteins, it is well established that some MADS-box proteins have transcriptional activity, while others do not [43,44].
2.4. Overexpression of ZmSOC1 Promotes Flowering in Arabidopsis
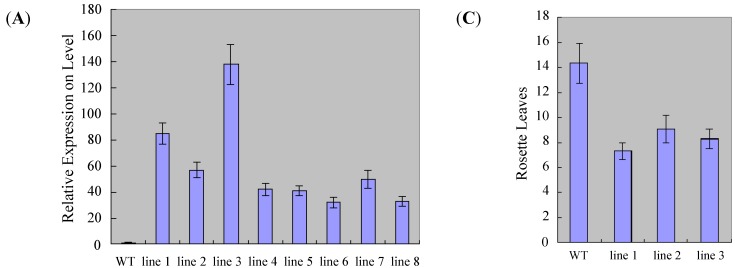
To examine the role of ZmSOC1 at flowering time, transgenic ZmSOC1 Arabidopsis plants driven by the Ubi promoter were generated. The transgenic plants were analyzed by qRT-PCR to confirm the expression level of ZmSOC1, and the transgenic lines all showed high ZmSOC1 expression levels compared to wild-type (Figure 4A). Three independent T3 lines were selected for flowering time analysis under LD (Long-day) conditions. Compared to the wild-type, overexpression of ZmSCOC1 significantly promoted flowering in Arabidopsis. The number of rosette leaves showing bolting ranged from 7.2 to 9.3 in ZmSOC1-overexpressing plants and was 14.5 for wild-type plants. In addition, the transgenic lines showed no morphological changes (excluding the leaves becoming slightly smaller). However, overexpression of another nine maize MADS-box genes was not observed to promote flowering in transgenic Arabidopsis. These results indicate that ZmSOC1 functions as a flowering activator in Arabidopsis.
Figure 4.
Overexpression of ZmSOC1 promotes flowering in Arabidopsis. (A) ZmSOC1 expression in transgenic plants. The expression level of AtActin2 was used as an internal references. Values represent the means, and the error bars represent standard errors for three independent experiments; (B) The phenotype of ZmSOC1 transgenic lines under the LD (Long-day) condition (14/10 h light/dark, 22 °C) after 30 days (above) and 40 days (below); (C) The number of rosette leaves during flowering in transgenic plants and wild-type plants.
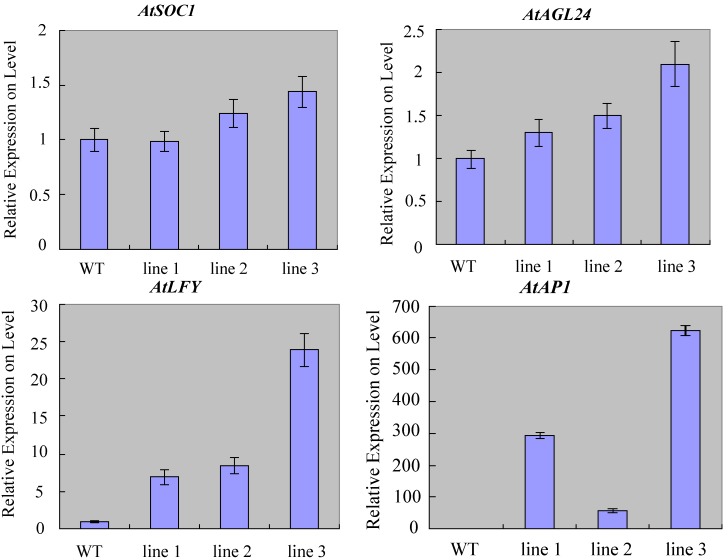
To examine the molecular mechanisms by which ZmSOC1 promotes flowering, the expression patterns of AtAGL24, AtSOC1, AtLFY and AtAP1 were analyzed using qRT-PCR in wild-type and transgenic seedlings under LD conditions. AtSOC1 expression levels in transgenic plants were similar to the wild-type. AtAGL24 expression levels increased slightly, demonstrating that ZmSOC1 overexpression did not influence the SOC1 upstream gene in the flowering pathway. The downstream gene, AtLFY, was obviously upregulated in transgenic plants (Figure 5), suggesting that upregulation of AtLFY was the result of ZmSOC1. The expression of AP1 (downstream of AtLFY) was significantly upregulated in transgenic compared to wild-type plants (Figure 5). These results demonstrate that the high expression of AtLFY and AtAP1, which are upregulated by the overexpression of ZmSOC1, should be involved in the promotion of flowering time in transgenic plants.
Figure 5.
Expression patterns of AtAGL24, AtSOC1, AtLFY and AtAP1 using qRT-PCR in wild-type and transgenic seedlings. The expression level of AtActin2 was used as an internal reference. Values represent the means, and the error bars represent standard errors for three independent experiments.
The overexpression of ZmSOC1 in Arabidopsis suggests that ZmSOC1 plays an evolutionarily conserved role in the promotion of flowering. First, it caused early flowering (Figure 4), in agreement with previous studies [45,46,47]. Second, it upregulated the expression of AtLFY and AtAP1 (Figure 5), which were directly or indirectly upregulated by SOC1 during the floral transition [7,25,34]. Taken together, these results demonstrate that ZmSOC1 may be a flowering promoter in maize. Because heterodimerization of AtSOC1 and AtAGL24 is a key mechanism activating AtLFY expression, we predicted that the interaction between ZmSOC1 and AtAGL24 would result in early flowering in transgenic Arabidopsis by activating AtLFY expression [7,25,34,48,49].
3. Experimental Section
3.1. Plant Material and Growth Conditions
Arabidopsis thaliana Columbia plants were grown in a greenhouse with a 14/10 h light/dark cycle at 22 °C. Seeds were sterilized for 10 min in 75% ethanol with 0.1% Triton X-100, for 5 min in 95% ethanol and finally washed with absolute ethanol on filter paper for drying. Seeds were germinated on half-strength Murashige and Skoog selective plates with 50 mg/L kanamycin. After 10 days of incubation in a growth chamber (14/10 h light/dark, 22 °C), resistant plants were transferred to soil.
3.2. Gene Cloning and Vector Construction
To clone the ZmSOC1 gene, the AtSOC1 protein (GenBank ID: NP_182090.1) was used for a BLAST search in the Maize Genetics and Genomics Database [50], and a protein (GRMZM2G171365) with high similarity to AtSOC1 was identified. Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) from the ear of maize (B73). Total RNA was treated with DNase I (Promega, Madison, WI, USA), and first-strand cDNA synthesis was performed using a First Strand cDNA Synthesis Kit following the manufacturer’s instructions (Promega, Madison, WI, USA).
The ZmSOC1 CDS was cloned into the pGWCVP64B vector using the Gateway recombination method, which contains an Ubi promoter and Nos terminator at the C-terminus. Based on the ZmMADS1 sequence, the Building Gateway Enter Cloning System vector primers used were 5'-GGGGACAAGTTTGTACAAAAAAGCAGGCTTCATGGTGCGGGGCAAGACGCA-3' (forward) and 5'-GGGGACCACTTTGTACAAGAAAGCTGGGTTGCCTGACCTGACCGCCACTG-3' (reverse). The BP reaction mixture included attB-PCR product (100 ng), pDONR vector (100 ng) and BP Clonase™ Enzyme Mix (1 µL) (Invitrogen, Carlsbad, CA, USA). The LR reaction mixture included the Enter Cloning System Vector (100 ng), pGWVP64 vector (100 ng) and LR Clonase™ Enzyme Mix (1 µL) (Invitrogen, Carlsbad, CA, USA).
3.3. RNA Preparation and Gene Expression Assays
Total RNA was isolated from 3-week-old Arabidopsis and treated with DNase I. Quantitative real-time PCR (qRT-PCR) analysis was performed with an Applied Biosystems [51] Prism 7500 instrument and GoTaq® qPCR Master Mix (Promega, Madison, WI, USA). For each genotype, three independent replicates consisting of five individual plants were used for analysis. The relative expression levels of target genes were calculated with the formula 2−ΔΔCt [52]. In Arabidopsis, we used qRT-PCR to investigate the genes expression levels, and the AtActin2 gene was used as an internal control. The gene-specific primers used were 5'-CCCTCGTCGTCTTCTCCC-3' (forward) and 5'-CCATCCGCATCAGCTTTT-3' (reverse) for ZmSOC1; 5'-CCAACAGAGAGAAGATGACT-3' (forward) and 5'-ATGTCTCTTACAATTTCCCG-3' (reverse) for AtActin2; 5'-TCGAGTCAGCACCAAACCG-3' (forward) and 5'-TTGAGCATGTTCCTATGCCTTC-3' (reverse) for AtSOC1; 5'-ACTAGAGACGTTGGAAAGGG-3' (forward) and 5'-TCATTCCCAAGATGGAAGCCC-3' (reverse) for AtAGL24; 5'-AAGCCTAAAATGCGACACTACG-3' (forward) and 5'-GAGGATGAGCGTTAAAGACGG-3' (reverse) for AtLFY; and 5'-GGGATCAGCATAACCAAGGC-3' (forward) and 5'-ACGGGTTCAAGAGTCAGTTCG-3' (reverse) for AtAP1.
3.4. Bioinformatics Analysis
Genes homologous to ZmSOC1 were identified using BLAST searches against GenBank [53], and potential sequence motifs were identified using SMART [54]. Protein sequence similarity ratio analysis was performed using DNAMAN software. Based on the e-value and identity, the orthologous of ZmSOC1 were selected for the construction of the phylogenetic tree using DNAMAN software.
3.5. Subcellular Localization of ZmSOC1
The open reading frame (ORF) of ZmSOC1 was amplified with the primer pair 5'-ATCTAGAATGGTGCGGGGCAAGACGCA-3' (containing a XbaI site) and 5'-ACCATGGTGCCTGACCTGACCGCCACTG-3' (containing a NcoI site). The PCR product was cloned into the pCAMBIA1302 vector N-terminus to generate pZmSOC1:GFP. pZmSOC1:GFP was transiently transformed into Arabidopsis mesophyll cell protoplasts using 10 μg plasmid DNA. For protoplast transient expression assays, mesophyll protoplasts were isolated from 6 to 8 4-week-old Arabidopsis rosette leaves, and the leaves were cut into 0.5–1 mm thin strips, then dropped into enzymatic solution (1.5% Cellulase R10, 0.4% macerozyme R10, 20 mM MES, 0.4 M mannitol, 10 mM CaCl2, 20 mM KCl, 0.1% bovine serum albumin, pH 5.7) and digested for 3 h. Plasmid DNA was prepared using the EndoFree Plasmid Midi Kit (CWBIO, Beijing, China). Protoplast transformation steps were performed as described by Yoo and Luan [55,56]. We washed protoplasts with W5 solution (2 mM MES, 154 mM NaCl, 125 mM CaCl2, 5 mM KCl, pH 5.7) and centrifuged them for collection. After cold treatment, protoplasts were resuspended with MMg solution (15 mM MgCl2, 4 mM MES, 0.4 M mannitol, pH 5.6), then an equal volume of PEG solution was added (40% PEG3350, 0.2 M mannitol, 0.1 M CaCl2), as well as 10 μg of the plasmid. After incubating for 10–15 min, transformed protoplasts were washed with W5, incubated with WI solution (0.5 M mannitol, 20 mM KCl, 4 mM MES, pH 5.7) at room temperature for 12–16 h in the dark. Hoechst 33342 dye (CalBiochem, San Diego, CA, USA) was added for 30 min, and the nuclei of living cells were stained blue [56]. GFP and Hoechst 33342 fluorescence in the nucleus was detected using LSM700 (ZEISS, Oberkochen, Germany).
3.6. Yeast Transcriptional Activation Assay
The full-length coding sequence of ZmSOC1 was amplified with the primer pair 5'-ACCATGGTGCGGGGCAAGACGCAG-3' (NdeI site) and 5'-AGGATCCGCCTGACCTGACCGCCACT-3' (BamHI site). The PCR product was cloned into the pAS2-1 vector. For interaction studies, the plasmid was transformed into yeast strain AH109. Yeast cells were made competent for transformation by incubation in lithium acetate solution. Then transformation was performed by incubating the cells with transforming DNA, carrier DNA and PEG3350. The Yeast Transformation Kit (SIGMA, St. Louis, MO, USA) used the lithium acetate method. The transformed strains were cultured on SD/-Ade/-Trp and SD/-Ade/-His/-Trp synthetic complete drop-out media (SC drop-out) with 3-amino-1,2,4-triazole at 30 °C.
3.7. Stress Treatments in Maize
The maize inbred line Z31 was used in this study. Z31 seeds were washed with distilled water and set in a sprout machine at 28 °C for 4 days in the dark. Seeds with 2–3 cm germs were transferred to vermiculite to grow at 28 °C/22 °C in the 14/10 h light/dark cycle with nutrient solution irrigation. The nutrients in solution were (mmol·L−1): 0.75 K2SO4, 0.1 KCl, 0.25 KH2PO4, 0.65 MgSO4·7H2O and 0.2 EDTA-Fe; and (in µmol·L−1) 1.0 MnSO4·H2O, 1.0 ZnSO4·7H2O, 0.1 CuSO4·5H2O and 0.005 (NH4)6Mo7O24·4H2O [57]. After 10 days, the maize contained two leaves and a core stop providing nutrient solution. Subsequently, maize seedlings were treated with NaCl (250 mM), PEG6000 (20%), ABA (100 μM), and SA (100 μM) at 4 °C, with H2O as a control, and sampled at 0, 0.5, 1, 3, 6, 12 and 24 h. The samples were immediately frozen in liquid nitrogen, with three seedlings for each replicate.
3.8. Material Collecting for Microarray
Maize plants of inbred line B73 have been sequenced and have a mature GeneChip, so this was used to harvest these tissues. We harvested vegetative tissues at the big trumpet stage, including the roots, stems, leaves and the shoot apex, as well as reproductive tissues, including endosperms and embryos at 10, 15, 20 and 25 days after pollination; filaments and ear were harvested before pollination [58]. The above material was divided in three to get total RNA, and we used the Affymetrix GeneChip of maize to analysis in triplicate.
3.9. Statistics of Transgenic Arabidopsis in Phenotypes and Expression
The T3 transgenic and wild-type Arabidopsis were directly planted in nutritive soil, and total RNA was extracted from leaves after four weeks. qRT-PCR was used to analyze ZmSOC1 expression levels in transgenic Arabidopsis. The number of rosette leaves was counted when these Arabidopsis began bolting, and 30 transgenic plants and wild-type Arabidopsis were counted, respectively.
4. Conclusions
ZmSOC1 was isolated from maize and characterized. It showed high identity to other SOC1 homologs and contained the well-conserved MADS domain and SOC1 motif. The protein was localized to the Arabidopsis nucleus and showed no transcriptional activation activity in yeast cells. Overexpression of ZmSOC1 promoted flowering through increasing the expression of AtLFY and AtAP1 in transgenic Arabidopsis. ZmSOC1 has a high expression level in floral organs, suggesting that ZmSOC1 should be a flowering promoter in maize. These findings increase our understanding of the mechanism of flowering control in maize.
Acknowledgments
This work was supported by the National High-Tech R&D Program (Grant No. 2012AA10A306), the National Key Basic Research Program (Grant No. 2014CB138200) and the National Special Program for Transgenic Research (2010ZX08010).
Author Contributions
Suzhou Zhao and Yanzhong Luo cloned the gene and constructed vectors and transferred ZmSOC1 into Arabidopsis. Zhanlu Zhang and Yangmin Zhao designed the primers and performed the real-time PCR. Miaoyun Xu and Weibu Wang treated maize seedlings with the stressors. Lan Zhang planted the Arabidopsis. Lei Wang and Yunliu Fan supervised the project and edited the manuscript. All authors discussed the results and implications and commented on the manuscript at all stages. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hwan Lee J., Joon Kim J., Ahn J.H. Role of SEPALLATA3 (SEP3) as a downstream gene of miR156-SPL3-FT circuitry in ambient temperature-responsive flowering. Plant Signal. Behav. 2012;7:1151–1154. doi: 10.4161/psb.21366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu M.Y., Zhang L., Li W.W., Hu X.L., Wang M.B., Fan Y.L., Zhang C.Y., Wang L. Stress-induced early flowering is mediated by miR169 in Arabidopsis thaliana. J. Exp. Bot. 2014;65:89–101. doi: 10.1093/jxb/ert353. [DOI] [PubMed] [Google Scholar]
- 3.Benlloch R., Kim M.C., Sayou C., Thevenon E., Parcy F., Nilsson O. Integrating long-day flowering signals: A LEAFY binding site is essential for proper photoperiodic activation of APETALA1. Plant J. 2011;67:1094–1102. doi: 10.1111/j.1365-313X.2011.04660.x. [DOI] [PubMed] [Google Scholar]
- 4.Deng W., Ying H., Helliwell C.A., Taylor J.M., Peacock W.J., Dennis E.S. FLOWERING LOCUS C (FLC) regulates development pathways throughout the life cycle of Arabidopsis. Proc. Natl. Acad. Sci. USA. 2011;108:6680–6685. doi: 10.1073/pnas.1103175108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruokolainen S., Ng Y.P., Albert V.A., Elomaa P., Teeri T.H. Over-expression of the Gerbera hybrida At-SOC1-like1 gene Gh-SOC1 leads to floral organ identity deterioration. Ann. Bot. 2011;107:1491–1499. doi: 10.1093/aob/mcr112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turck F., Fornara F., Coupland G. Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annu. Rev. Plant Biol. 2008;59:573–594. doi: 10.1146/annurev.arplant.59.032607.092755. [DOI] [PubMed] [Google Scholar]
- 7.Lee J., Lee I. Regulation and function of SOC1, a flowering pathway integrator. J. Exp. Bot. 2010;61:2247–2254. doi: 10.1093/jxb/erq098. [DOI] [PubMed] [Google Scholar]
- 8.Lei H.J., Yuan H.Z., Liu Y., Guo X.W., Liao X., Liu L.L., Wang Q., Li T.H. Identification and characterization of FaSOC1, a homolog of SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 from strawberry. Gene. 2013;531:158–167. doi: 10.1016/j.gene.2013.09.036. [DOI] [PubMed] [Google Scholar]
- 9.Ferrario S., Busscher J., Franken J., Gerats T., Vandenbussche M., Angenent G.C., Immink R.G. Ectopic expression of the petunia MADS box gene UNSHAVEN accelerates flowering and confers leaf-like characteristics to floral organs in a dominant-negative manner. Plant Cell. 2004;16:1490–1505. doi: 10.1105/tpc.019679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujisawa M., Nakano T., Shima Y., Ito Y. A large-scale identification of direct targets of the tomato MADS box transcription factor RIPENING INHIBITOR reveals the regulation of fruit ripening. Plant Cell. 2013;25:371–386. doi: 10.1105/tpc.112.108118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi K., Yasuno N., Sato Y., Yoda M., Yamazaki R., Kimizu M., Yoshida H., Nagamura Y., Kyozuka J. Inflorescence meristem identity in rice is specified by overlapping functions of three AP1/FUL-like MADS box genes and PAP2, a SEPALLATA MADS box gene. Plant Cell. 2012;24:1848–1859. doi: 10.1105/tpc.112.097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simonini S., Roig-Villanova I., Gregis V., Colombo B., Colombo L., Kater M.M. Basic pentacysteine proteins mediate MADS domain complex binding to the DNA for tissue-specific expression of target genes in Arabidopsis. Plant Cell. 2012;24:4163–4172. doi: 10.1105/tpc.112.103952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Theissen G., Becker A., di Rosa A., Kanno A., Kim J.T., Munster T., Winter K.U., Saedler H. A short history of MADS-box genes in plants. Plant Mol. Biol. 2000;42:115–149. doi: 10.1023/A:1006332105728. [DOI] [PubMed] [Google Scholar]
- 14.Parenicova L., de Folter S., Kieffer M., Horner D.S., Favalli C., Busscher J., Cook H.E., Ingram R.M., Kater M.M., Davies B., et al. Molecular and phylogenetic analyses of the complete MADS-box transcription factor family in Arabidopsis: New openings to the MADS world. Plant Cell. 2003;15:1538–1551. doi: 10.1105/tpc.011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riechmann J.L., Wang M., Meyerowitz E.M. DNA-binding properties of Arabidopsis MADS domain homeotic proteins APETALA1, APETALA3, PISTILLATA and AGAMOUS. Nucleic Acids Res. 1996;24:3134–3141. doi: 10.1093/nar/24.16.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Araki T. Transition from vegetative to reproductive phase. Curr. Opin. Plant Biol. 2001;4:63–68. doi: 10.1016/S1369-5266(00)00137-0. [DOI] [PubMed] [Google Scholar]
- 17.Fornara F., de Montaigu A., Coupland G. SnapShot: Control of flowering in Arabidopsis. Cell. 2010;141:550.e1–550.e2. doi: 10.1016/j.cell.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 18.Simpson G.G., Dean C. Arabidopsis, the Rosetta stone of flowering time? Science. 2002;296:285–289. doi: 10.1126/science.296.5566.285. [DOI] [PubMed] [Google Scholar]
- 19.Moon J., Suh S.S., Lee H., Choi K.R., Hong C.B., Paek N.C., Kim S.G., Lee I. The SOC1 MADS-box gene integrates vernalization and gibberellin signals for flowering in Arabidopsis. Plant J. 2003;35:613–623. doi: 10.1046/j.1365-313X.2003.01833.x. [DOI] [PubMed] [Google Scholar]
- 20.Hepworth S.R., Valverde F., Ravenscroft D., Mouradov A., Coupland G. Antagonistic regulation of flowering-time gene SOC1 by CONSTANS and FLC via separate promoter motifs. EMBO J. 2002;21:4327–4337. doi: 10.1093/emboj/cdf432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheldon C.C., Rouse D.T., Finnegan E.J., Peacock W.J., Dennis E.S. The molecular basis of vernalization: The central role of FLOWERING LOCUS C (FLC) Proc. Natl. Acad. Sci. USA. 2000;97:3753–3758. doi: 10.1073/pnas.97.7.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson R.N., Heckman J.W., Somerville C.R. Gibberellin is required for flowering in arabidopsis thaliana under short days. Plant Physiol. 1992;100:403–408. doi: 10.1104/pp.100.1.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui R., Han J., Zhao S., Su K., Wu F., Du X., Xu Q., Chong K., Theissen G., Meng Z. Functional conservation and diversification of class E floral homeotic genes in rice (Oryza sativa) Plant J. 2010;61:767–781. doi: 10.1111/j.1365-313X.2009.04101.x. [DOI] [PubMed] [Google Scholar]
- 24.Immink R.G., Gadella T.W., Jr., Ferrario S., Busscher M., Angenent G.C. Analysis of MADS box protein-protein interactions in living plant cells. Proc. Natl. Acad. Sci. USA. 2002;99:2416–2421. doi: 10.1073/pnas.042677699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J., Oh M., Park H., Lee I. SOC1 translocated to the nucleus by interaction with AGL24 directly regulates leafy. Plant J. 2008;55:832–843. doi: 10.1111/j.1365-313X.2008.03552.x. [DOI] [PubMed] [Google Scholar]
- 26.Seo E., Lee H., Jeon J., Park H., Kim J., Noh Y.S., Lee I. Crosstalk between cold response and flowering in Arabidopsis is mediated through the flowering-time gene SOC1 and its upstream negative regulator FLC. Plant Cell. 2009;21:3185–3197. doi: 10.1105/tpc.108.063883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S., Kim J., Han J.J., Han M.J., An G. Functional analyses of the flowering time gene OsMADS50, the putative SUPPRESSOR OF OVEREXPRESSION OF CO 1/AGAMOUS-LIKE 20 (SOC1/AGL20) ortholog in rice. Plant J. 2004;38:754–764. doi: 10.1111/j.1365-313X.2004.02082.x. [DOI] [PubMed] [Google Scholar]
- 28.Ryu C.H., Lee S., Cho L.H., Kim S.L., Lee Y.S., Choi S.C., Jeong H.J., Yi J., Park S.J., Han C.D., et al. OsMADS50 and OsMADS56 function antagonistically in regulating long day (LD)-dependent flowering in rice. Plant Cell Environ. 2009;32:1412–1427. doi: 10.1111/j.1365-3040.2009.02008.x. [DOI] [PubMed] [Google Scholar]
- 29.Tadege M., Sheldon C.C., Helliwell C.A., Upadhyaya N.M., Dennis E.S., Peacock W.J. Reciprocal control of flowering time by OsSOC1 in transgenic Arabidopsis and by FLC in transgenic rice. Plant Biotechnol. J. 2003;1:361–369. doi: 10.1046/j.1467-7652.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- 30.Tan F.C., Swain S.M. Functional characterization of AP3, SOC1 and WUS homologues from citrus (Citrus sinensis) Physiol. Plant. 2007;131:481–495. doi: 10.1111/j.1399-3054.2007.00971.x. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura T., Song I.J., Fukuda T., Yokoyama J., Maki M., Ochiai T., Kameya T., Kanno A. Characterization of TrcMADS1 gene of Trillium camtschatcense (Trilliaceae) reveals functional evolution of the SOC1/TM3-like gene family. J. Plant Res. 2005;118:229–234. doi: 10.1007/s10265-005-0215-5. [DOI] [PubMed] [Google Scholar]
- 32.Shitsukawa N., Ikari C., Mitsuya T., Sakiyama T., Ishikawa A., Takumi S., Murai K. Wheat SOC1 functions independently of WAP1/VRN1, an integrator of vernalization and photoperiod flowering promotion pathways. Physiol. Plant. 2007;130:627–636. doi: 10.1111/j.1399-3054.2007.00927.x. [DOI] [Google Scholar]
- 33.Heuer S., Hansen S., Bantin J., Brettschneider R., Kranz E., Lorz H., Dresselhaus T. The maize MADS box gene ZmMADS3 affects node number and spikelet development and is co-expressed with ZmMADS1 during flower development, in egg cells, and early embryogenesis. Plant Physiol. 2001;127:33–45. doi: 10.1104/pp.127.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee H., Suh S.S., Park E., Cho E., Ahn J.H., Kim S.G., Lee J.S., Kwon Y.M., Lee I. The AGAMOUS-LIKE 20 MADS domain protein integrates floral inductive pathways in Arabidopsis. Genes Dev. 2000;14:2366–2376. doi: 10.1101/gad.813600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song G.Q., Walworth A., Zhao D., Hildebrandt B., Leasia M. Constitutive expression of the K-domain of a Vaccinium corymbosum SOC1-like (VcSOC1-K) MADS-box gene is sufficient to promote flowering in tobacco. Plant Cell Rep. 2013;32:1819–1826. doi: 10.1007/s00299-013-1495-1. [DOI] [PubMed] [Google Scholar]
- 36.Theissen G., Kim J.T., Saedler H. Classification and phylogeny of the MADS-box multigene family suggest defined roles of MADS-box gene subfamilies in the morphological evolution of eukaryotes. J. Mol. Evol. 1996;43:484–516. doi: 10.1007/BF02337521. [DOI] [PubMed] [Google Scholar]
- 37.Vandenbussche M., Theissen G., van de Peer Y., Gerats T. Structural diversification and neo-functionalization during floral MADS-box gene evolution by C-terminal frameshift mutations. Nucleic Acids Res. 2003;31:4401–4409. doi: 10.1093/nar/gkg642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong Z., Danilevskaya O., Abadie T., Messina C., Coles N., Cooper M. A gene regulatory network model for floral transition of the shoot apex in maize and its dynamic modeling. PLoS One. 2012;7:e43450. doi: 10.1371/journal.pone.0043450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang H., Forde B.G. An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science. 1998;279:407–409. doi: 10.1126/science.279.5349.407. [DOI] [PubMed] [Google Scholar]
- 40.Wang G., Hu J., Zhao X., Cheng N., Qian X., Yang J. Differential expression of MADS-box genes in the morphogenesis of calli in rice. Acta Bot. Sin. 1997;39:1035–1041. [Google Scholar]
- 41.Huang H., Tudor M., Su T., Zhang Y., Hu Y., Ma H. DNA binding properties of two Arabidopsis MADS domain proteins: Binding consensus and dimer formation. Plant Cell. 1996;8:81–94. doi: 10.1105/tpc.8.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Y., Fanning L., Jack T. The K domain mediates heterodimerization of the Arabidopsis floral organ identity proteins, APETALA3 and PISTILLATA. Plant J. 2003;33:47–59. doi: 10.1046/j.0960-7412.2003.01473.x. [DOI] [PubMed] [Google Scholar]
- 43.Honma T., Goto K. Complexes of MADS-box proteins are sufficient to convert leaves into floral organs. Nature. 2001;409:525–529. doi: 10.1038/35054083. [DOI] [PubMed] [Google Scholar]
- 44.Chen M.K., Hsieh W.P., Yang C.H. Functional analysis reveals the possible role of the C-terminal sequences and PI motif in the function of lily (Lilium longiflorum) PISTILLATA (PI) orthologues. J. Exp. Bot. 2012;63:941–961. doi: 10.1093/jxb/err323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ding L., Wang Y., Yu H. Overexpression of DOSOC1, an ortholog of Arabidopsis SOC1, promotes flowering in the orchid Dendrobium Chao Parya Smile. Plant Cell Physiol. 2013;54:595–608. doi: 10.1093/pcp/pct026. [DOI] [PubMed] [Google Scholar]
- 46.Immink R.G., Pose D., Ferrario S., Ott F., Kaufmann K., Valentim F.L., de Folter S., van der Wal F., van Dijk A.D., Schmid M., et al. Characterization of SOC1’s central role in flowering by the identification of its upstream and downstream regulators. Plant Physiol. 2012;160:433–449. doi: 10.1104/pp.112.202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhong X., Dai X., Xv J., Wu H., Liu B., Li H. Cloning and expression analysis of GmGAL1, SOC1 homolog gene in soybean. Mol. Biol. Rep. 2012;39:6967–6974. doi: 10.1007/s11033-012-1524-0. [DOI] [PubMed] [Google Scholar]
- 48.Liu C., Chen H., Er H.L., Soo H.M., Kumar P.P., Han J.H., Liou Y.C., Yu H. Direct interaction of AGL24 and SOC1 integrates flowering signals in Arabidopsis. Development. 2008;135:1481–1491. doi: 10.1242/dev.020255. [DOI] [PubMed] [Google Scholar]
- 49.Torti S., Fornara F. AGL24 acts in concert with SOC1 and FUL during Arabidopsis floral transition. Plant Signal. Behav. 2012;7:1251–1254. doi: 10.4161/psb.21552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maize Genetics and Genomics Database. [(accessed on 30 October 2014)]. Available online: http://maizegdb.org/
- 51.Applied Biosystems by Life Technologies. [(accessed on 30 October 2014)]. Available online: http://www.AppliedBiosystems.com.
- 52.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 53.Basic Local Alignment Search Tool. [(accessed on 30 October 2014)]; Available online: http://www.ncbi.nlm.nih.gov/BLAST/
- 54.SMART. [(accessed on 30 October 2014)]. Available online: http://smart.embl-heidelberg.de/
- 55.Yoo S.D., Cho Y.H., Sheen J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007;2:1565–1572. doi: 10.1038/nprot.2007.199. [DOI] [PubMed] [Google Scholar]
- 56.Luan M., Xu M., Lu Y., Zhang Q., Zhang L., Zhang C., Fan Y., Lang Z., Wang L. Family-wide survey of miR169s and NF-YAs and their expression profiles response to abiotic stress in maize roots. PLoS One. 2014;9:e91369. doi: 10.1371/journal.pone.0091369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu Z., Zhong S., Li X., Li W., Rothstein S.J., Zhang S., Bi Y., Xie C. Genome-wide identification of microRNAs in response to low nitrate availability in maize leaves and roots. PLoS One. 2011;6:e28009. doi: 10.1371/journal.pone.0028009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu X., Tian J., Zhou X., Chen R., Wang L., Zhang C., Zhao J., Fan Y. Identification and characterization of promoters specifically and strongly expressed in maize embryos. Plant Biotechnol. J. 2014 doi: 10.1111/pbi.12227. [DOI] [PubMed] [Google Scholar]