Abstract
An extensive number of pathologies are associated with mitochondrial dysfunction (MDF) and oxidative stress (OS). Thus, mitochondrial cofactors termed “mitochondrial nutrients” (MN), such as α-lipoic acid (ALA), Coenzyme Q10 (CoQ10), and l-carnitine (CARN) (or its derivatives) have been tested in a number of clinical trials, and this review is focused on the use of MN-based clinical trials. The papers reporting on MN-based clinical trials were retrieved in MedLine up to July 2014, and evaluated for the following endpoints: (a) treated diseases; (b) dosages, number of enrolled patients and duration of treatment; (c) trial success for each MN or MN combinations as reported by authors. The reports satisfying the above endpoints included total numbers of trials and frequencies of randomized, controlled studies, i.e., 81 trials testing ALA, 107 reports testing CoQ10, and 74 reports testing CARN, while only 7 reports were retrieved testing double MN associations, while no report was found testing a triple MN combination. A total of 28 reports tested MN associations with “classical” antioxidants, such as antioxidant nutrients or drugs. Combinations of MN showed better outcomes than individual MN, suggesting forthcoming clinical studies. The criteria in study design and monitoring MN-based clinical trials are discussed.
Keywords: mitochondrial nutrients, mitochondrial dysfunction, oxidative stress, oxidative phosphorylation, Krebs cycle
1. Introduction
The functions of mitochondria, unconfined to bioenergetic pathways, were linked to OS by studies in early 1990’s reporting on reactive oxygen species (ROS) formation as by-products of oxygen metabolism [1,2]. The implications for OS in Parkinson’s disease were discovered by Di Monte et al. [3] and associated with MDF by a correlation of complex I–III deficiency with lower-than-normal levels of CoQ10, a cofactor in the oxidative phosphorylation (OXPHOS) pathway [4,5].
The association of OS and MDF has been reported in in mitochondrial diseases [6,7], and our recent reviews evaluated the literature showing that OS/MDF is involved in broad-ranging pathologies, including some genetic diseases [8], aging and age-associated disorders, neurologic and psychiatric diseases, malignancies and autoimmune diseases [9]. This state-of-art has prompted a growing body of literature from clinical studies, aimed at compensating OS/MDF by means of MN administration [10,11,12,13,14]. These endogenous cofactors, such as ALA, CoQ10 and CARN (or CARN derivatives) are essential in mitochondrial functions.
ALA exists in two redox states, and the reduced thiol form is a potent mitochondrial antioxidant, a metal chelator and a glutathione (GSH) repletor. It is involved as an essential cofactor in Krebs cycle for mitochondrial α-ketoacid dehydrogenases. CoQ10 is a benzoquinone derivative playing a central role in the mitochondrial respiratory chain through shuttling between three redox forms, the quinone, the semiquinone and the hydroquinone. It acts as a carrier accepting electrons from mitochondrial complex I and complex II and transferring them to complex III. CARN (bioactive l-form) is biosynthesized from lysine and methionine and is concentrated in tissues that use fatty acids as their primary source of energy. It is involved in the transport of long-chain fatty acids from the intermembraneous space to the matrix in the mitochondria for generation of metabolic energy.
A number of studies have demonstrated MN deficiencies in several diseases [9], either related to deficiencies in OXPHOS activities [15,16,17,18] or caused by their degradation due to OS-related by-products as, e.g., by ALA oxidative modification induced by 4-hydroxynonenal in Alzheimer disease brain [19]. The present review is to provide an overall survey of the currently published clinical trials having tested each of the above MN, or their combinations, and/or their associations with “typical” antioxidants, including antioxidant nutrients and/or herbal preparations and/or disease-specific drugs. For each disease, or disease group, the total numbers of clinical trials were recorded, along with the relative frequencies of controlled, randomized trials. No further attempt was made to evaluate the clinical and/or laboratory outcomes of the evaluated reports. The major objective of the present survey was providing an overall state-of-art, and suggesting some prospects in study design and in monitoring the outcomes of forthcoming clinical trials. These should be aimed at verifying any specific MN deficiency(ies) associated to a given disorder, and at prompting adequate follow-up in monitoring the effects, if any, of mitochondria-targeted interventions.
2. Methods
A MedLine retrieval up to July 2014 was carried out for each MN. The papers reporting on clinical trials for each MN were evaluated according to: (a) treated diseases (or disease groups); (b) dosages, number of enrolled patients and duration of treatment; (c) numbers of trials and frequencies of randomized, controlled studies (opposed to open-label and pilot studies); and (d) an empyrical “success ratio” (SR) of trials testing each of the individual MN or their combinations, as reported by authors; SR was calculated by dividing the number of successful results by the total of trials for each agent. No clinical trials involving healthy volunteers were included for evaluation. The reports failing to provide clear-cut data for dosages, the numbers of patients and/or treatment duration were not included in survey, nor were included self-repeating reports of previous or contemporary studies.
3. α-Lipoic Acid
As shown in Table 1, ALA testing was reported in 81 evaluated clinical trials on a total of 2980 patients, since the pioneering report by Marshall et al. on alcohol-related liver disease [10], and since early studies in 1990’s in counteracting diabetes-associated neuropathies [20,21,22]. Both Type 1 and Type 2 diabetes mellitus (DM) have been the major focus for ALA administration, encompassing 42 trials (of which 30 controlled trials) on a total of 2980 patients and with a SR = 0.93 [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61]. A major outcome of ALA treatment in diabetic patients consisted of the amelioration of neurologic damage [22,23,24,26,27,28,29,30,31,33,34,35,37,39,42,46,49,51,52,60,61]. Moreover, ALA-treated diabetic patients displayed decreased serum concentrations of thiobarbituric acid reactive substances (TBARS) [20,43], increased insulin sensitivity [21,25,36,58], an improvement in endothelium function [38,43,55], decreased erectile dysfunction [59] and decreased urinary PGF2α-isoprostanes, a marker of oxidative damage [50].
Table 1.
Clinical studies utilizing α-lipoic acid aimed at compensating oxidative stress (OS)/mitochondrial dysfunction (MDF)-related pathogenetic mechanisms.
| Diseases/Conditions | No. Studies (Controlled Studies) | No. Treated Patients | Success Ratio | References |
|---|---|---|---|---|
| Type 1 and Type 2 diabetes | 42 (30) | 2980 | 0.93 | [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61] |
| Neurological diseases | 9 (5) | 509 | 0.89 | [62,63,64,65,66,67,68,69,70] |
| Liver and metabolic diseases | 8 (5) | 417 | 0.86 | [10,71,72,73,74,75,76,77] |
| Heart and vessel diseases | 4 (4) | 137 | 1.00 | [78,79,80,81] |
| Kidney diseases | 4 (3) | 288 | 0.25 | [82,83,84,85] |
| Genetic and mitochondrial diseases | 3 (2) | 129 | 0.33 | [86,87,88] |
| Burning mouth syndrome | 5 (5) | 293 | 0.60 | [89,90,91,92,93] |
| Other diseases § | 6 (4) | 525 | 1.00 | [94,95,96,97,98,99] |
| Total | 81 | 5278 |
Beyond the literature of ALA-centered clinical trials in DM, a broad clinical use of ALA is recognized as a prescription generic drug for DM in Germany (German Drug Index), thus the use of ALA in diabetic patients may be seen as an established practice in the specialist community.
Other clinical trials successfully tested ALA in neurological diseases [62,63,64,65,66,67,68,69,70], in liver and metabolic diseases [10,71,72,73,74,75,76,77], and in heart and vessel diseases [78,79,80,81] (Table 1).
Lesser, if any, positive effects were reported for three ALA trials in kidney diseases [82,83,84,85]. Other clinical trials tested the effects of ALA administration in genetic and mitochondrial diseases [86,87,88], burning mouth syndrome [89,90,91,92,93], cancer cachexia [94,95], mitochondrial function in HIV-1-related lipoatrophy [96,97], in vitiligo [98], and in osteoporosis [99].
Among these studies, some reports investigated the comparative effects of different MN formulations, and/or provided evidence for combined effects in clinical and laboratry endpoints.
Li et al. [79] showed that ALA administration to patients with acute coronary syndrome caused a decrease in an OS marker, 8-iso-prostaglandin F2α and an increase in aldehyde dehydrogenase-2, responsible for acetaldehyde oxidation in ethanol metabolism that also provides protection against OS. Protective effects were reported by Martins et al. [87] in sickle cell trait subjects and sickle cell patients following ALA administration, with a significant increase in blood catalase (CAT) and a significant decrease in malondialdehyde (MDA) and in carbonyl levels.
A clinical trial reported by Galasko et al. [64] tested the effects of combined ALA with Vit E and Vit C (E/C/ALA), or CoQ10, or placebo in three groups of patients with Alzheimer's disease, finding a decrease in CSF F2-isoprostane levels in the E/C/ALA group that suggested a reduction of OS in the brain. However, this treatment raised the caution of faster cognitive decline.
Altogether, the current body of evidence may suggest further interventions with ALA in several disorders, beyond DM and the other disorders where ALA treatment led both to clinical improvements and to compensation of OS-related endpoints.
4. Coenzyme Q10
A recent review has focused on the multiple implications of CoQ10 deficiency and of CoQ10 administration encompassing an extensive number of disorders [100]. Out of 101 reports on clinical trials testing CoQ10, 39 studies were focused on support to patients with heart and vessel disorders or undergoing heart surgery [11,12,81,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136], including 32 controlled studies, as shown in Table 2. The pioneering studies by the groups of Tanaka [11] and of Langsjoen [12] since 1982 provided the avenue for a number of clinical trials assessing the successful outcomes of CoQ10 administration to patients with coronary artery disease, cardiomyopathy, heart failure, or heart surgery, with an overall SR = 0.89. Among these studies, Dai et al. [105] evaluated mitochondrial function in patients with ischemic left ventricular systolic dysfunction receiving CoQ10 vs. placebo, in terms of plasma lactate/pyruvate (LP) ratio. After an 8-week treatment, CoQ10-treated patients had significant increases in plasma CoQ10 concentration, brachial flow-mediated dilation, and decreased LP ratio, showing positive effects both on heart function and in balancing mitochondrial activities [105]. Lee et al. [106] tested CoQ10 administration in patients with coronary artery disease and found a decrease in the inflammatory markers C-reactive protein (hs-CRP) and interleukin 6 (IL-6), and decreased MDA and superoxide dismutase (SOD) activities, along with increased CoQ10 levels. Direct evidence for a CoQ10-induced compensation of mitochondrial function was reported by Rosenfeldt et al. [133] in patients undergoing heart surgery, which resulted in increased CoQ10 levels in serum, atrial trabeculae, and isolated mitochondria compared with patients receiving placebo, with an improvement in mitochondrial respiration, as adenosine diphosphate/oxygen ratio, and a decrease in mitochondrial MDA content.
Table 2.
Clinical studies utilizing coenzyme Q10 aimed at compensating OS/MDF-related pathogenetic mechanisms.
| Diseases/Conditions | No. Studies (Controlled Studies) | No. Treated Patients | Success Ratio | References |
|---|---|---|---|---|
| Heart and vessel diseases | 39 (32) | 3386 | 0.89 | [11,12,81,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136] |
| Genetic and mitochondrial diseases | 18 (8) | 680 | 0.75 | [13,14,88,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151] |
| Neurological diseases | 16 (10) | 1185 | 0.87 | [65,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166] |
| Type 1 and Type 2 Diabetes | 9 (7) | 370 | 0.89 | [41,166,167,168,169,170,171,172,173,174] |
| Malignancies | 6 (2) | 301 | 0.67 | [175,176,177,178,179,180] |
| Kidney diseases | 4 (2) | 171 | 0.50 | [181,182,183,184] |
| Other diseases § | 15 (13) | 555 | 0.59 | [76,185,186,187,188,189,190,191,192,193,194,195,196,197,198] |
| Total | 107 | 6648 |
Eighteen studies (of which 8 controlled studies) have focused on clinical trials testing CoQ10 in a number of genetic and mitochondrial diseases [13,14,88,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151] on 680 patients (Table 2) with different success across the different diseases investigated (SR = 0.75).
Among these studies, Friedreich ataxia (FRDA) is relevant for a deficiency in the gene encoding frataxin, a mitochondrial protein implicated in iron metabolism and glutathione balance [139], and lower-than-normal CoQ10 levels [139,140]. FRDA was investigated by Cooper’s group [140,141] for CoQ10 and Vit E administration finding a significant improvement in cardiac and skeletal muscle bioenergetics and in International Co-operative Ataxia Ratings Scale. Rodriguez et al. [88], as mentioned above, tested the effect of a combination therapy with CoQ10 with ALA and creatine monohydrate on several outcome variables in patients with mitochondrial diseases, both achieving amelioration of clinical and of OS/MDF endpoints [88]. Limited evidence for a CoQ10-induced improvement in brain and muscle bioenergetics in patients with mitochondrial diseases was reported by Barbiroli et al. [142] and by Glover et al. [145].
A set of studies was focused on 16 clinical trials (of which 10 controlled trials) testing CoQ10 supplementation in 1185 patients with eight neurologic disorders [65,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166].
The above-mentioned report by Galasko et al. [65] tested CoQ10 as an alternative treatment to E/C/ALA in patients with Alzheimer’s disease, failing to report any effects of CoQ10, unlike E/C/ALA. A study by Shults et al. [152] in patients with Parkinson’s disease (PD) tested the effects of three CoQ10 dosages (300, 600, or 1200 mg/d) that were evaluated with the Unified Parkinson Disease Rating Scale at the baseline and up to 16-month visits. Less disability developed in subjects assigned to CoQ10 than in those assigned to placebo, with a significant benefit in patients receiving the highest dosage [152]. Moderate beneficial effects of CoQ10 in PD patients were also reported by Müller et al. [153], though at the low dosage (360 mg/d) reported by Shults et al. [152]. A study by Storch et al. [154] found that nanoparticular CoQ10 at a dosage of 300 mg/d was well tolerated, though failing to display symptomatic effects in midstage PD.
Sanoobar et al. [155,156] tested CoQ10 in multiple sclerosis (MS) patients, and found reduced OS and increased antioxidant enzyme activity [155], with a significant decrease of tumor necrosis factor alpha (TNF-α) and IL-6 levels [156] in the CoQ10 group compared to placebo group.
A series of studies focused on the role of CoQ10 in fibromyalgia (FM) in long-term treatment of fibromyalgic patients, resulting in significant pain reduction, fatigue, and morning tiredness [157,158,159,160]. CoQ10-treated patients underwent significant reduction in the pain visual scale and in tender points, recovery of inflammation, antioxidant enzymes, mitochondrial biogenesis, and AMPK gene expression levels [157,158]. A recent report by Miyamae et al. [159] measured plasma levels of ubiquinone-10, ubiquinol-10, free cholesterol, cholesterol esters, and free fatty acids in patients with juvenile FM vs. healthy control subjects. Plasma level of ubiquinol-10 was significantly decreased and the ratio of ubiquinone-10 to total CoQ10 was significantly increased in juvenile FM relative to healthy controls, with compensated plasma levels of lipid endpoints [159]. A previous uncontrolled trial by Lister [160] tested the effects of combined CoQ10 and and Ginkgo biloba extract reporting improved quality-of-life in FM patients. Altogether, independent studies support the usefulness of CoQ10 in FM pathogenesis and point to CoQ10 utilization in FM both in inducing clinical improvements and in compensating FM-associated OS/MDF.
No evidence of CoQ10-induced beneficial effects were found in a Phase II clinical trial in patients with amyotrophic lateral sclerosis [161].
Migraine has been investigated for deficiency of CoQ10, which showed lower-than-normal levels in patients with pediatric and adolescent migraine [162]. Clinical trials testing CoQ10 in migraine patients provided overall positive, though apparently transient outcomes [162,163,164].
Forester et al. [165] tested high-dose CoQ10 in patients with geriatric bipolar depression suggesting a reduction in depression symptom severity.
Stamelou et al. [166] tested CoQ10 in patients with progressive supranuclear palsy (PSP), and found that patients receiving CoQ10 displayed decreased concentration of low-energy phosphates, with increased ratio of high-energy phosphates to low-energy phosphates. Clinically, the PSP rating scale and the Frontal Assessment Battery improved upon CoQ10 treatment compared to placebo [166].
Nine clinical trials (seven controlled trials) were conducted by testing CoQ10 in 370 patients with Type 1 and Type 2 DM, particularly focusing on DM-associated vascular complications and on statin-induced CoQ10 depletion [167,168,169,170,171,172,173,174]. The outcomes of these studies showed beneficial effects of CoQ10, better if combined with fenofibrate [169]. By considering the established advantages of ALA treatment in DM patients [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61], one may suggest further clinical trials toward the clinical use of ALA and CoQ10 combinations in DM. This trial design was performed by Palacka et al. with successful outcomes both in improving heart left ventricular function and in decreasing lactate dehydrogenase activity in DM patients treated with ALA and CoQ10 [41].
Six clinical trials have been reported testing CoQ10 in association with other agents (e.g., riboflavin and niacin) in patients with advanced malignancies under antineoplastic therapy as supportive or palliative treatments [175,176,177,178,179,180]. Some of these trials reported survival prolongation [177], and an early study by Lockwood et al. [176] reported partial and complete regression of breast cancer in patients treated with CoQ10. A clinical trial by Rusciani et al. [180] tested the effects of CoQ10 and interferon α-2b in melanoma patients, and found significantly decreased recurrence rates vs. patients receiving interferon only.
Four clinical trials tested the CoQ10 in patients with chronic kidney disease or undergoing hemodialysis, or submitted to statin treatment [181,182,183,184]. Sakata et al. [182] reported CoQ10 administration in hemodialysis patients as partially effective for suppressing OS. Shojaei et al. [184] tested CoQ10 and CARN, separately or in association, in hemodialysis patients who were on statin treatment. This study showed that supplementation with CoQ10 and CARN reduced serum levels of lipoprotein(a) in maintenance hemodialysis patients treated with statins. Altogether, the clinical trials testing CoQ10 in patients with kidney diseases failed to report clear-cut beneficial effects (SR = 0.50).
Controversial results were reported from clinical trials aimed at testing CoQ10 in counteracting various dysmetabolic conditions [185,186,187], statin-induced myalgias [188,189,190,191,192], bronchial asthma [193], pre-eclampsia [194], cutaneous infections [195], psoriasis [76,196], cataract surgery [197], and idiopathic infertility [198].
5. l-Carnitine and Acetyl- or Propionyl-Carnitine
Multi-decade long investigations showed the crucial roles of CARN in mitochondrial physiology, along with CARN deficiency in several disorders. As reviewed by Gilbert since 1985 [199], CARN deficiency results in accumulation of neutral lipid deposits within skeletal muscle, myocardium and liver, with mitochondria aggregates in skeletal muscle and myocardium. The efficacy of CARN vs. acetyl-CARN (ALC) was compared both in vitro and in aging rat brain since early studies [200,201]. CARN and ALC were similar in elevating carnitine levels in plasma and brain as well as in increasing ambulatory activity of old rats. However, ALC but not CARN was able to decrease the level of oxidative stress biomarkers in the brain of old rats [201]. Subsequent investigations pointed to a role of ALC in the reactivation of mitochondrial biogenesis in aging through the increased expression of PGC-1α signaling pathway [202,203].
As shown in Table 3, out of 74 evaluated studies, 18 clinical trials (of which 16 controlled trials) tested the effects of CARN in kidney diseases, treating a total of 427 patients with end-stage renal disease under hemodialysis, based on the rationale that CARN levels sharply decrease during hemodialysis, thus impairing response to recombinant human erythropoietin (rHuEPO) [181,184,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220]. One study utilized both CARN and CoQ10—separately or in association—finding positive effects of both supplements, yet without evidence for improvement effects following combined supplementation [184]. Some reports failed to find significant improvements following CARN administration [205,206,207,208,213,220] (overall SR = 0.58). One might note that out of four trials adopting i.v. CARN (or PLC) administration, three resulted in significant improvements in the tested endpoints, such as Medical Outcomes Short Form-36, rHuEPO requirement, hemodynamic flow, endothelial profile and homocysteine levels, or inflammatory status as significant decrease in C-reactive protein [210,211,217].
Table 3.
Clinical studies utilizing l-carnitine (CARN) (or acetyl-CARN (ALC)) aimed at compensating OS/MDF-related pathogenetic mechanisms.
| Diseases/Conditions | No. Studies (Controlled Studies) | No. Treated Patients | Success Ratio | References |
|---|---|---|---|---|
| Kidney diseases | 18 (16) | 427 | 0.58 | [184,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220] |
| Type 1 and Type 2 Diabetes | 13 (9) | 1894 | 1.00 | [221,222,223,224,225,226,227,228,229,230,231,232,233] |
| Heart and vessel diseases | 9 (6) | 359 | 1.00 | [78,114,234,235,236,237,238,239,240] |
| Liver diseases | 6 (2) | 275 | 1.00 | [241,242,243,244,245,246] |
| Neurological diseases | 9 (9) | 384 | 1.00 | [68,247,248,249,250,251,252,253,254] |
| Malignancies | 7 (6) | 699 | 0.70 | [255,256,257,258,259,260,261] |
| Genetic diseases | 6 (4) | 202 | 1.00 | [262,263,264,265,266,267] |
| HIV | 4 (1) | 93 | 1.00 | [97,268,269,270] |
| Other diseases § | 2 (2) | 99 | 1.00 | [271,272] |
| Total | 74 | 4432 |
Unlike renal diseases, all of 13 clinical trials (9 controlled trials) supplementing CARN (or ALC, or PLC) to patients with Type 1 and Type 2 DM to a total of 1894 patients succeeded achieving improvements in disease status [221,222,223,224,225,226,227,228,229,230,231,232,233]. Long-term infusion of CARN or ALC in DM patients was tested by the group of Giancaterini [221,222], resulting in improved insulin sensitivity, and in decreased lactate levels, suggesting activation of pyruvate dehydrogenase, whose activity is depressed in the insulin resistant status. Three independent studies found a long-term treatment with ALC or with PLC effective and well tolerated in improving neurophysiological parameters and in reducing pain [223,226,229]. Derosa et al. reported that CARN significantly lowered the plasma lipoprotein(a) vs. placebo in hypercholesterolemic patients with DM [224]. A clinical trial with PLC in patients with DM and peripheral arterial disease was reported by Ragozzino et al. [225], who found significant increases in maximal walking distance, initial claudication distance, and a decrease in dosage of oral antihyperglycemic agents. Two studies investigated the effects of CARN or ALC treatment on lipidemic profile of DM patients [223,224], failing to detect a decrease of lipidemic profile of CARN alone to this endpoint, yet Solfrizzi et al. [228] found significant effects from combined treatment with CARN and simvastatin in lowering Lp(a) serum levels in patients with DM than with simvastatin alone. A study by Malaguarnera et al. [230] testing CARN in DM patients found significant improvements compared with the placebo group in a number of OS endpoints, proinflammatory markers and lipidemic profile.
A clinical trial was conducted by Ruggenenti et al. [231] by testing the effects of ALC on glucose disposal rate in patients with metabolic syndrome and hypertension, reporting that ALC ameliorated arterial hypertension, insulin resistance, glucose tolerance, and hypoadiponectinemia. Patients with Type 1 DM were treated by Uzun et al. [233] with CARN to evaluate changes in their neuropathy frequency and nerve conduction velocity, and found that CARN treatment in early stages improved neuropathy in Type 1 DM patients.
Altogether, the body of literature of clinical trials testing CARN—or CARN derivatives—in patients with DM provides substantial evidence for the amelioration of multiple DM-related endpoints that encourage the clinical use of CARN—or CARN derivatives—in DM patients. One may note that no report was found of combined treatments of DM patients using CARN with either ALA or CoQ10, despite the body of positive evidence accumulated in clinical trials testing ALA, or CoQ10 in DM patients (as shown Table 1 and Table 2).
A series of 9 clinical trials (6 controlled trials) were reported testing CARN (or ALC, or PLC) in 359 patients with heart and vessel diseases [78,114,234,235,236,237,238,239,240]. A study by McMackin et al. [78] reported on the effects of combined ALC and ALA treatment in patients with coronary artery disease, and found significant improvement as increased brachial artery diameter, a trend to decreased systolic blood pressure for the whole patient group, with a significant effect in the subgroup with blood pressure above the median and in the subgroup with metabolic syndrome. An analogous investigation was reported by Kumar et al. [114], who tested the effects of carni Q-gel (CARN and ubiquinol) in patients with heart failure. Serum concentration of IL-6 was significantly decreased in the intervention group without such changes in the control group. TNF-α, which was comparable at baseline, also showed a greater decline in the carni Q-gel group compared to the placebo group. Serum CoQ10 showed a significant increase in the carni Q-gel group as compared to the control group. The symptom scale indicated that the majority of treated patients showed a significant improvement compared to the placebo group [114]. Altogether, both of these clinical trials using associations of ALC with ALA [78] or CARN with CoQ10 (as ubiquinol) [114] showed definite improvements both in heart and vessel performance and in related laboratory endpoints.
Relevant mechanistic data on PLC treatment in patients with peripheral arterial disease (PAD) were obtained by Loffredo et al. [234], who measured serum levels of nitrite and nitrate (NOx) and 8-hydroxy-2'-deoxyguanosine (8-OHdG), and maximal walking distance (MWD) in PAD patients. Serum levels of 8-OHdG were significantly increased in PAD patients, and serum levels of NOx were significantly decreased. Patients treated with PLC showed a significant increase of MWD and in NOx, while 8-OHdG levels underwent a significant decrease, unlike the patients given placebo [234].
Improved short-term exercise capacity, diastolic function and symptoms, WHO heart functional class were reported in patients receiving CARN [237,238], with improvements in diastolic function and in myocardial hypoxanthine concentration [239,240].
A total of 275 patients affected by various liver disorders were treated with CARN or ALC in 6 clinical trials (of which 2 controlled studies) [241,242,243,244,245,246]. Łapiński and Grzeszczuk [241] tested CARN (or l-ornitine l-aspartate) in patients with liver cirrhosis, and evaluated the outcomes as changes in serum concentrations of ammonia, cholesterol and triglycerides. A significant improvement was observed both in the patients treated with CARN and in those treated with l-ornitine l-aspartate. However, the individuals treated with CARN showed a significant increase of serum cholesterol and triglyceride concentrations.
Patients with non-alcoholic fatty liver disease were treated with CARN by Lim et al. [242]; by measuring a liver function test, peripheral blood mitochondrial DNA and 8-oxo-dG analysis. The results showed a decrease in ALT, AST, and total bilirubin in treated patients, not in controls. In the CARN treated patients, mitochondrial DNA copy number was significantly increased, unlike the control group. 8-oxo-dG levels showed a non-significant decrease in CARN group while it tended to increase in the control group. The group of Malaguarnera et al. has conducted a series of clinical trials testing the effects of CARN in patients with hepatic encephalopathy or with HCV-induced chronic hepatitis [243,244,245,246]. Beneficial effects in CARN-treated patients included significant differences in AST, ALT, viremia, Hb, RBC, WBC and platelets. Also significant improvements were detected in neurologic scores and fatigue symptoms in hepatic encephalopathy following CARN administration [244,245].
Nine controlled clinical trials for CARN—mostly ALC—were conducted in a total of 384 patients with some neurological disorders, including Alzheimer's disease, multiple sclerosis, migraine, sciatica, fibromyalgia, chronic fatigue syndrome, and amyotrophic lateral sclerosis [68,247,248,249,250,251,252,253,254]. A report by Memeo and Loiero [68] compared the effects of treating sciatica patients with either ALA or ALC, and found significant protective effects from both of these agents detected as improvements from baseline in neuropathy on electromyography.
Tomassini et al. [247] tested the efficacy of ALC vs. amantadine, a widely used drug in treating patients with multiple sclerosis (MS)-related fatigue. The results showed significant effects of ALC compared with amantadine for the Fatigue Severity Scale, suggesting that ALC is better tolerated and more effective than amantadine for the treatment of MS-related fatigue.
Patients with Alzheimer’s disease and vascular dementia were treated with ALC by Gavrilova et al. [248], and the outcomes were assessed with MMSE and CGI scales, and a battery of neuropsychological tests. The treatment effect of ALC was significantly higher than in the placebo group.
Tarighat Esfanjani et al. [249] evaluated the effects of magnesium, CARN, and concurrent magnesium-CARN supplementation in migraine prophylaxis. Migrainous patients were randomly assigned into three intervention groups: magnesium oxide vs. CARN vs. Mg-CARN, and a control group. The results showed a significant reduction in all migraine indicators in all studied groups, yet without a clear-cut difference in-between the three supplementation regimens.
Four independent clinical trials tested CARN effects [250,251,252,253] in patients with chronic fatigue syndrome (CFS), or fibromyalgia (FM), or narcolepsy (NL). Pistone et al. [250] treated elderly subjects with onset of fatigue following slight physical activity. Wessely and Powell fatigue scores decreased significantly in subjects taking CARN vs. the placebo group, with significant improvements in total fat mass, total muscle mass, total cholesterol, LDL-C, HDL-C, triglycerides, apoA1, and apoB [250]. Miyagawa et al. [251] tested CARN administration in NL patients and found a significant reduction of excessive daytime sleepiness in CARN-treated NL patients vs. those receiving placebo. A clinical trial testing the effects of ALC in FM patients was reported by Rossini et al. [252]. The “total myalgic score” and the number of positive tender points declined significantly in the ALC-treated patients vs. the placebo group, and a significant difference was observed for depression and musculo-skeletal pain [252]. The effects of ALC and PLC were tested in patients with CFS by Vermeulen and Scholte [253]. This clinical trial compared ALC, PLC, and their combination. Clinical global impression of change after treatment showed considerable improvement in 59% of the patients in the ALC group and 63% in the PLC group, but less in the ALC plus PLC group (37%). In the ALC group the changes in plasma CARN levels correlated with clinical improvement [253].
A clinical trial by Beghi et al. tested the effects of ALC plus riluzole vs. riluzole and placebo on disability and mortality of patients with amyotrophic lateral clerosis [254]. Patients receiving ALC became non-self-sufficient to significantly less extent compared to those receiving placebo, and median survival was significantly extended in ALC-treated patients vs. placebo group [254].
Based on CARN depletion in advanced cancer, palliative treatments by CARN supplementation have been proposed by a series of seven clinical trials, of which six controlled trials [255,256,257,258,259,260,261]. Unfortunately, the outcomes showed a treatment-related increase of CARN serum levels, yet CARN or ALC supplementation did not improve fatigue in patients, or even increased chemotherapy-induced peripheral neuropathy [261]; otherwise, positive results were associated with treatments with multiple agents [258,259], thus casting doubts as to the specific efficacy of CARN supplementation.
A few genetic disorders were the focus of six clinical trials, of which 4 controlled trials, using CARN or ALC [262,263,264,265,266,267]. A clinical trial was carried out by Schöls et al. [262] by testing the administration of CARN vs. creatine in patients with Friedreich’s ataxia. After CARN treatment, patients had a significantly improved phosphocreatine recovery compared to baseline, while creatine effects did not reach significance.
Two independent clinical trials tested the effects of CARN in thalassemic patients, concurring to report improved cardiac function [263,264]. Moreover, Karimi et al. [264] tested the effect of combination therapy of hydroxyurea with CARN and magnesium chloride on hematologic parameters and cardiac function of patients with β-thalassemia intermedia. Patients were randomly divided into four groups: group A (hydroxyurea alone); group B (hydroxyurea and CARN); group C (hydroxyurea and magnesium chloride); and group D (hydroxyurea, CARN and magnesium chloride). In groups B, C, and D, mean Hb and hematocrit significantly increased during 6-month treatment [264].
Three independent studies tested the effects of CARN or ALC in patients with autistic spectrum disorders (ASD) [265,266,267]. Ellaway et al. [265] tested CARN in a group of Rett syndrome females, and found significantly improved sleep efficiency and expressive speech, compared to control Rett syndrome patients. A multi-center clinical trial testing ALC on the attention deficit hyperactivity disorder in fragile X syndrome boys was reported by Torrioli et al. [266]. Patients treated with ALC, compared with the placebo group, showed a stronger reduction of hyperactivity and improvement of social behavior [266]. Geier et al. [267] tested CARN in patients with ASD that were randomly assigned to receive a controlled regimen liquid CARN or placebo for 3 months. Treated patients showed significant improvements in Childhood Autism Rating Scale, modified clinical global impression, and Autism Treatment Evaluation Checklist scores.
Four clinical trials (of which one controlled trial) have tested the CARN, or ALC, aimed at improving different complications of HIV infection [97,268,269,270]. The above-cited report by Milazzo et al. [97] tested ALC vs. a combination of ALA and N-acetylcysteine (NAC) for a number of OS/MDF and other endpoints in HIV-1-infected patients with lipoatrophy, showing that either ALC, or ALA/NAC supplementation exerts a protective role on mitochondrial function in HIV-1-infected patients [97]. Two closely related studies [268,269] tested the effects of ALC in mitigating the neuropathy associated with antiretroviral toxicity in HIV-infected patients. Mean pain intensity score was significantly reduced, while electrophysiological parameters did not significantly change during treatment [269].
A controlled clinical trial was reported by Pignatelli et al. [271] on the effect of CARN on OS and platelet activation after major surgery, whose trauma is associated with an increased ROS production. At baseline and after treatment, OS was evaluated by detection of circulating levels of soluble NOX2-derived peptide, and by analyzing platelet ROS formation. The results showed an increase of OS endpoints in the placebo group compared with the baseline after the surgical intervention, while the CARN-treated group did not significantly differ from the baseline.
Patients with ulcerative colitis (UC) were treated by Mikhailova et al. [272] by PLC testing a clinical/endoscopic response. Patients with mild-to-moderate UC receiving stable oral aminosalicylate or thiopurine therapy were randomised to receive PLC or placebo. A significant response was found in patients receiving PLC as clinical/endoscopic response vs. those receiving placebo [272].
6. Treatments with Mitochondrial Nutrient Combinations
Out of 262 clinical trials reviewed here (Table 1, Table 2 and Table 3), only a tiny minority of seven reports referred to the combined use of two MN, ALA and/or CoQ10 and/or CARN [41,68,76,78,81,88,184], as shown in Table 4. Only four clinical trials tested the ALA/CoQ10 combination [41,76,81,88], while three clinical trials tested CARN combined with either ALA or CoQ10 [68,78,184]. Surprisingly, no retrieved report mentioned the combination of the three cofactors in any clinical trial; to the best of our knowledge; only Mattiazzi et al. [273] reported on the combined in vitro use of ALA, CoQ10 and CARN in improving a mitochondrial (mtDNA T8993G (NARP)) mutation on T8993G mutated cells.
Table 4.
Clinical studies utilizing multiple mitochondrial nutrients (α-lipoic acid and/or coenzyme Q10 and/or l-carnitine) aimed at compensating OS/MDF-related pathogenetic mechanisms.
The report by Rodriguez et al. [88] tested the combined use of ALA, CoQ10 and creatine in a group of patients with different mitochondrial diseases; these may be viewed as the “ideal” target for administration of MN, in view of supporting known MDF [6,7,9,14,274,275,276] that involve OS, decreased ATP production, and involvement of anaerobic energetic pathways. The combination therapy resulted in lower resting plasma lactate and OS markers (urinary 8-isoprostanes), and improved energetic performance [88].
Beyond mitochondrial diseases, a substantial body of evidence has been accumulated pointing to the involvement of OS/MDF in a number of heterogeneous diseases [9].
The combined use of ALA and CoQ10 was tested in patients with Type 2 DM [41] and in cardiac surgery patients [81]. One may note that both the report by Palacka et al. [41] and that by Leong et al. [81] fulfilled the expected clinical outcomes, along with the finding of decreased plasma lipid peroxides [41].
A number of clinical trials have been conducted by testing MN in combinations with classical antioxidants and/or herbal compounds and/or disease-specific drugs.
A total of 27 reports were evaluated concerning the therapies utilizing ALA or CoQ10 combinations with various antioxidants and/or herbal compounds and/or drugs (Table 5 and Table 6). As shown in Table 5, a study utilizing ALA and Vit E alone or in combination failed to find changes in the lipid profile or insulin sensitivity of Type 2 DM patients [44]. Supplementation of quercetin plus Vit C or ALA alone did not change the blood biomarkers of inflammation and disease severity of rheumatoid arthritis patients under conventional treatments [64].
Table 5.
Clinical studies utilizing mitochondrial nutrients and antioxidants and/or herbal compounds aimed at compensating OS/MDF-related pathogenetic mechanisms.
| Diseases/Conditions | α-Lipoic Acid + Other Agent(s) (Daily Dose) | References |
|---|---|---|
| Type 1 and Type 2 diabetes | ALA 100 mg, CoQ10 60 mg, Vit E 200 mg | [41] |
| ALA 600 mg, Vit E 800 mg | [44] | |
| ALA 800 mg, pyridoxine 80 mg | [56] | |
| ALA 600 mg, transdermal testosterone 50 mg | [59] | |
| ALA 2 × 600 mg, allopurinol 300 mg, nicotinamide 2 × 750 mg | [60] | |
| Heart surgery | ALA 100 mg, CoQ10 100 mg, Mg orotate 400 mg, ω-3 PUFA 300 mg, Se 200 µg | [81] |
| Metabolic syndrome | ALA 600 mg, Vit E 100 IU | [77] |
| Cancer-related anorexia/cachexia | ALA 300 mg, polyphenols 400 mg, carbocysteine 2.7 g, Vit E 400 mg, Vit A 30,000 IU, Vit C 500 mg, (n-3)-PUFA 2 cans | [94] |
| Alzheimer disease | ALA 900 mg, CoQ10 400 mg, Vit C 500 mg, Vit E 800 IU | [65] |
| Parkinson’s disease | ALA 1200 mg, folate 5 mg, Vit B12 1500 µg | [277] |
| Carpal tunnel syndrome | ALA 600 mg, γ-linolenic acid 360 mg, Vit B6 150 mg, Vit B1 100 mg, Vit B12 500 µg | [67] |
| Down syndrome | ALA 600 mg, Vit E 900 IU, Vit C 200 mg | [86] |
| HCV infection | ALA 300 mg, glycyrrhizin 1 g, Schisandra 1.5 g, silymarin 750 mg, Vit C 6 g, l-glutathione 300 mg, Vit E 800 IU | [72] |
| Psoriasis | ALA 600 mg, CoQ10 50 mg, resveratrol 20 mg, Vit E 36 mg, Krill oil 300 mg, Vitis vinifera seed oil 30 mg, Se 27 mg | [76] |
| Osteoporosis | ALA 2 × 300 mg, Vit C 30 mg, Vit E 5 mg, Se 2.75 mg | [99] |
| Vitiligo | ALA 100 mg, Vit C 100 mg, Vit E 40 mg, PUFA 12% | [98] |
Table 6.
Clinical studies utilizing mitochondrial nutrients and antioxidants and/or herbal compounds aimed at compensating OS/MDF-related pathogenetic mechanisms.
| Diseases/Conditions | Coenzyme Q10 + Other Agent(s) (Daily Dose) | References |
|---|---|---|
| Heart surgery | CoQ10 100 mg, ALA 100 mg, Mg orotate 400 mg, ω-3 PUFA 300 mg, Se 200 µg | [81] |
| Chronic heart failure | CoQ10 150 mg, Ca 250 mg, Mg 150 mg, Zn 15 mg, Cu 1.2 mg, Se 50 µg, Vit A 800 µg, thiamine 200 mg, riboflavin 2 mg, Vit B6 200 mg, folate 5 mg, Vit B12 200 µg, Vit C 500 mg, Vit E 400 mg, Vit D 10 µg | [112,124] |
| Cardiovascular diseases | CoQ10 120 mg, Vit C 1 g, Vit E 400 IU, Se 200 µg | [128] |
| Cardiovascular mortality | CoQ10 200 mg, organic Se 200 μg | [136] |
| Breast cancer | CoQ10 100 mg, riboflavin 10 mg, niacin 50 mg | [175] |
| CoQ10 90 mg, Vit C 2850 mg, Vit E 2500 IU, β-carotene 32.5 IU, Se 387 mg, γ-linolenic acid 1.2 g, n-3 fatty acids 3.5 g | [176] | |
| CoQ10 300 mg, Vit E 300 IU | [177] | |
| Prostate cancer | CoQ10 200 mg, Vit C 750 mg, Vit E 350 mg, Se 200 µg | [179] |
| Friedreich ataxia | CoQ10 400 mg, Vit E 2100 IU | [140] |
| CoQ10 600 mg, Vit E 2100 IU | [141] | |
| Fibromyalgia | CoQ10 200 mg, Gingko biloba extract 200 mg | [160] |
A combined administration with ALA, CoQ10, magnesium orotate, ω-3 polyunsaturated fatty acids (ω-3 PUFA) and selenium was associated by Leong et al. [81] with improved redox status and reduced myocardial damage in patients undergoing heart surgery.
A clinical trial in Alzheimer’s disease patients showed that supplementation with ALA, Vit E and Vit C reduced OS in the brain [65]. The beneficial effects of combination therapy were also observed in Parkinson’s disease (PD) patients receiving homocysteine-lowering therapy (folate and Vit B) and ALA supplementation, suggesting that combination therapy may prevent bone loss in PD patients [277]. A combination therapy of ALA and transdermal testosterone provided amelioration of on erectile dysfunction and quality of life in patients with Type 2 DM [59]. Treatment with ALA and γ-linolenic acid was proposed for controlling symptoms and improving the evolution of carpal tunnel syndrome [67]. Antioxidant supplementation with Vit C, Vit E and ALA was reported as a safe, though ineffective, treatment for dementia in individuals with Down syndrome [86].
Combined supplementations were tested in liver disorders, such as chronic hepatitis virus C infection. Melhem et al. [72] reported on a combination of ALA with antioxidative preparations (glycyrrhizin, Schisandra, silymarin, Vit C, l-glutathione, and Vit E) in patients with chronic hepatitis C, with favorable response rate.
Twelve clinical trials have reported on combination regimens with CoQ10 and various antioxidants (Table 6). Witte et al. [112] tested a combination of CoQ10 and high-dose micronutrients that was found to improve left ventricular function and quality of life in elderly patients with chronic heart failure. Shargorodsky et al. [128] tested a combination therapy with CoQ10, Vit C, Vit E and selenium in patients with multiple cardiovascular risk factors, finding an improvement in glucose and lipid metabolism, and decreased blood pressure.
A clinical trial utilizing CoQ10 and a mixture of antioxidants was reported by Hertz and Lister [175] to improve survival of patients with end-stage cancer. An early report by Lockwood et al. [176] found that supplementation with CoQ10 and antioxidants, γ-linolenic and n-3 fatty acids significantly improved clinical conditions in breast cancer patients. A trial by Premkumar et al. [177], also conducted on breast cancer patients, reported that a supplementation with CoQ10, riboflavin and niacin decreased the levels of pro-angiogenic factors and increased the levels of anti-angiogenic factors, and reduced tumor burden. On the other hand, a combination therapy with CoQ10 and Vit E did not result in improvement of fatigue symptoms in breast cancer patients, according to Lesser et al. [178]. Negative results were obtained by Hoenjet et al. [179] by testing a combined supplement containing CoQ10, Vit E, Vit C and selenium in patients with hormonally untreated carcinoma of the prostate, which did not affect serum level of PSA or hormone levels.
Two studies by Hart and co-workers [140,141] reported on a combined treatment with CoQ10 and Vit E in patients with Friedreich ataxia finding sustained improvement in mitochondrial energy production and a significant improvement in cardiac function. A combination therapy with CoQ10 and Ginkgo biloba extract significantly improved quality-of-life in patients with clinically diagnosed fibromyalgia [160].
Taken together, the literature on clinical trials testing MN and “classical” antioxidants may provide crucial data on the therapeutic efficacy of compounds aimed at improving mitochondrial health and several OS/MDF-related endpoints, suggesting the design of improved treatments of OS/MDF-related diseases.
7. State-of-Art: Critical Remarks
The present survey of published clinical trials testing MN may reflect the current state-of-art across broad-ranging disorders that are usually focused in separate medical disciplines. On the other hand, the clinical trials testing MN published to date have been conducted on some selected diseases, far below the potential scope of investigation, by considering the extensive range of OS/MDF-related disorders [9]. Trying to interpret this delay, one might find one or more of the following explanations: (a) a disease is commonly recognized to rely on other etiologic grounds than OS/MDF: this is the case, e.g., of Fanconi anemia and of other genetic diseases [8,278]; (b) very rare diseases discourage patient recruitment in view of clinical trials as, e.g., progerias [8,279]; (c) the specialist community may be committed in established therapeutic strategies, thus disregarding potential adjuvant interventions.
Other two remarks may be addressed to the state-of-art in the literature of clinical trials using MN. First, the reviewed studies were mostly oriented to clinical endpoints of the investigated disorders, by underscoring any relevant mechanistic aspects enabling to elucidate trial’s results. Second, and most relevant, the vast majority of the reviewed trials tested one MN only, by disregarding: (a) the complementary roles of these cofactors in mitochondrial functions; (b) their different physico-chemical behaviors in water- vs. lipid-phase; and (c) the scarce, if any, knowledge as to specific MDF in a given disorder and the appropriate requirements of ALA, or CoQ10, or CARN (or its derivatives) supplementation. Thus, the as yet prevailing design of trials using only one MN may fail to achieve the desired outcomes of OS/MDF compensation and hence of improving clinical endpoints. This limitation, along with some possible shortage in dosages and/or in trial duration might have contributed to some unsuccessful trial outcomes. One may note that supplement combinations of more than one MN, or the use of ALA or CoQ10 with classical antioxidants may have contributed to the overall success of these trials (see Table 4, Table 5 and Table 6). Thus, one may anticipate the optimization of future clinical trials in OS/MDF-related disorders by means of combined MN treatments, possibly associated with “classical” antioxidants.
The choice of MN formulations may have critical implications on the expected success of MN-based clinical trials. An outstanding aspect in the design of a MN-based clinical trial is represented by the type of formulation chosen. The oxidative state of molecules, chirality, and the vehiculants used in the formulation may have dramatic implications in terms of bioavailability and interaction with the recipient biological system. In the case of CoQ10, bioavailability is known to be influenced by modality of administration, with higher plasma levels reached by administration of the same amount divided in multiple doses, and by vehiculant type with soft gels containing triglyceride dispersing medium showing a better bioavailability in comparison with the crystalline form [280]. Another issue, also linked with bioavailability, is the oxidative state of the active compound in the formulation. Most of the studies cited used ubiquinone, however recently also the reduced form of CoQ10 (ubiquinol) has been used in several clinical trials [113,114,198]. Direct use of ubiquinol displays the advantages of improved absorption and consequently enhanced bioavailability, and direct exposure of the reduced (and active) antioxidant form to digestive epithelial tissue; finally, ubiquinol may influence the ubiquinone/total CoQ10 ratio in conditions where reductive systems are sub-optimal as it has been shown in the aging process [281].
α-Lipoic acid is characterized by an enhanced bioavailability compared to CoQ10, with absorption rates around 30%–40% of an oral dose of ALA [282]. Moreover, while ALA being synthethized by biological systems is exclusively in the R-lipoic acid chiral form, formulations are often (R/S) or (+/−) ALA, composed by a 50/50 mixture of R- and S-ALA. It has been shown that bioavailability of the two isomers is different, R-lipoic acid showing peak plasma concentrations in pharmacokinetic studies 40%–50% higher than S-LA, suggesting that R-LA is better absorbed than S-LA [282].
Regarding CARN and its derivatives, especially ALC, the state-of-art provides extensive information as to the efficacy of CARN, ALC and PLC. The advantages of using ALC vs. CARN have been provided [200,201,202,203], along with successful use of ALC [68,78,222,223,226,231,246,247,248,252,253,254,261,266,267,268] or PLC [210,225,235,239,253,272] in a number of clinical trials. Acetyl-l-carnitine probably acts as an acetylating agent and its involvement in epigenetic modifications of proteins might explain its success in aging and in OS/MDF-related disorders [283,284,285]. Future research on CARN/ALC/PLC supplementation should address the correlation of supplement dosage, changes and maintenance of tissue concentrations, and metabolic and functional changes and outcomes [286,287]. An analogous research strategy was highlighted by Camp et al. [288] on nutritional interventions for inborn errors of metabolism, suggesting combined strategies in improving the clinical management of an extensive number of disorders.
8. Prospects of Clinical Trials in OS/MDF-Related Disorders
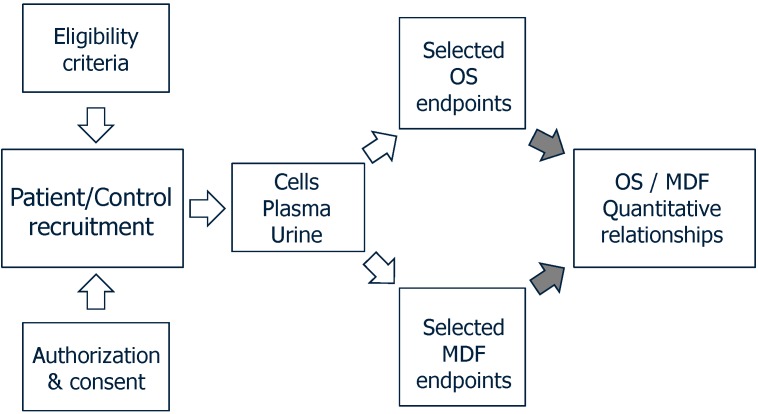
The scheme depicted in Figure 1 points to the prerequisite of identifying the OS and MDF endpoints that are altered in a given disorder, often incompletely elucidated. A choice of OS endpoints should include some established parameters including oxidative damage to DNA, lipids, carbohydrates and proteins, and changes in the glutathione system. A few selected MDF endpoints should be addressed to verify the deficiency, if any, of ALA (to date almost unexplored), CoQ10, and CARN. Other relevant endpoints in assessing an in vivo MDF should include mitochondrial DNA content, ATP production, OXPHOS activities, lactate/pyruvate ratio, and expression of mitochondrial antioxidant activities (MnSOD and peroxiredoxin 3). This set of measurements should clarify the quantitative aspects of the disorder to be focused in a clinical trial design. Once the characterization of OS/MDF endpoints is accomplished, the target outcomes of the clinical trials may be defined.
Figure 1.
Preliminary database to be achieved in design of mitochondrial nutrients (MN)-based clinical trials.
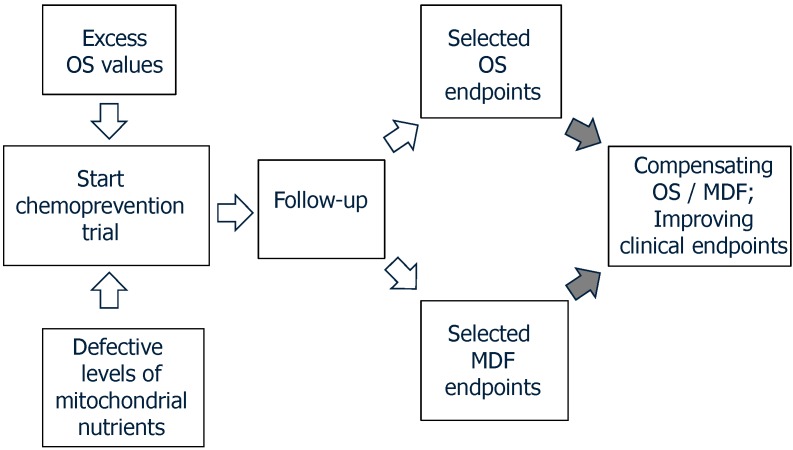
As shown in Figure 2, the OS/MDF endpoints for a given disease, found as abnormal in the preliminary step, shall be the grounds for choosing the best appropriate supplements to be administered to patients. These supplements may include one or—more reasonably—more MN, as discussed above. The follow-up period will be of paramount relevance; indeed, short admnistration regimens may offer hints for efficacy and safety, yet they may conceal long-run effects. Moreover, in diseases with multi-year-long progression, expecting the desired outcomes may be a quite elusive task. Thus, follow-up ought to be designed for a reasonably long duration with, e.g., six-month intervals in results analysis, allowing us to monitor the outcomes of multi-year-long administration. The ultimate goal will be achieved by compensating OS/MDF endpoints, along with the amelioration of disease progression.
Figure 2.
Follow-up procedures combining evaluation of MDF and OS endpoints, along with disease specific clinical endpoints.
Materializing the above prospect may have dramatic consequences in the success of to-be clinical trials for OS/MDF-related disorders, trespassing from “hopes”, or from single-parameter information, toward a rational approach that shall foresee properly targeted interventions, then with rational expectations, rather than attempts.
Acknowledgments
Sandra Petrović was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant No. 173046).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Richter C.C., Kass G.E. Oxidative stress in mitochondria: its relationship to cellular Ca2+ homeostasis, cell death, proliferation, and differentiation. Chem. Biol. Interact. 1991;77:1–23. doi: 10.1016/0009-2797(91)90002-O. [DOI] [PubMed] [Google Scholar]
- 2.Sohal R.S., Brunk U.T. Mitochondrial production of pro-oxidants and cellular senescence. Mutat. Res./DNAging. 1992;275:295–304. doi: 10.1016/0921-8734(92)90033-L. [DOI] [PubMed] [Google Scholar]
- 3.Di Monte D.A., Chan P., Sandy M.S. Glutathione in Parkinson’s disease: A link between oxidative stress and mitochondrial damage? Ann. Neurol. 1992;32:S111–S115. doi: 10.1002/ana.410320719. [DOI] [PubMed] [Google Scholar]
- 4.Beal M.F. Therapeutic approaches to mitochondrial dysfunction in Parkinson’s disease. Parkinsonism Relat. Disord. 2009;15:S189–S194. doi: 10.1016/S1353-8020(09)70812-0. [DOI] [PubMed] [Google Scholar]
- 5.Mischley L.K., Allen J., Bradley R. Coenzyme Q10 deficiency in patients with Parkinson’s disease. J. Neurol. Sci. 2012;318:72–75. doi: 10.1016/j.jns.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parikh S., Goldstein A., Koenig M.K., Scaglia F., Enns G.M., Saneto R., Mitochondrial Medicine Society Clinical Directors Working Group. Clinical Director’s Work Group Practice patterns of mitochondrial disease physicians in North America. Part 2: Treatment, care and management. Mitochondrion. 2013;13:681–687. doi: 10.1016/j.mito.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Tarnopolsky M.A. The mitochondrial cocktail: Rationale for combined nutraceutical therapy in mitochondrial cytopathies. Adv. Drug Deliv. Rev. 2008;60:1561–1567. doi: 10.1016/j.addr.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Pallardó F.V., Lloret A., Lebel M., d’Ischia M., Cogger V.C., le Couteur D.G., Gadaleta M.N., Castello G., Pagano G. Mitochondrial dysfunction in some oxidative stress-related genetic diseases: Ataxia-Telangiectasia, Down Syndrome, Fanconi Anaemia and Werner Syndrome. Biogerontology. 2010;11:401–419. doi: 10.1007/s10522-010-9269-4. [DOI] [PubMed] [Google Scholar]
- 9.Pagano G., Aiello Talamanca A., Castello G., Cordero M.D., d’Ischia M., Gadaleta M.N., Pallardó F.V., Petrović S., Tiano L., Zatterale A. Oxidative stress and mitochondrial dysfunction across broad-ranging pathologies: Toward a rational design of chemoprevention strategies by means of mitochondrial nutrients. Oxid. Med. Cell. Longev. 2014;2014:541230. doi: 10.1155/2014/541230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall A.W., Graul R.S., Morgan M.Y., Sherlock S. Treatment of alcohol-related liver disease with thioctic acid: A six month randomised double-blind trial. Gut. 1982;23:1088–1093. doi: 10.1136/gut.23.12.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka J., Tominaga R., Yoshitoshi M., Matsui K., Komori M., Sese A., Yasui H., Tokunaga K. Coenzyme Q10: The prophylactic effect on low cardiac output following cardiac valve replacement. Ann. Thorac. Surg. 1982;33:145–151. doi: 10.1016/S0003-4975(10)61900-5. [DOI] [PubMed] [Google Scholar]
- 12.Langsjoen P.H., Vadhanavikit S., Folkers K. Response of patients in classes III and IV of cardiomyopathy to therapy in a blind and crossover trial with coenzyme Q10. Proc. Natl. Acad. Sci. USA. 1985;82:4240–4244. doi: 10.1073/pnas.82.12.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogasahara S., Nishikawa Y., Yorifuji S., Soga F., Nakamura Y., Takahashi M., Hashimoto S., Kono N., Tarui S. Treatment of Kearns–Sayre syndrome with coenzyme Q10. Neurology. 1986;36:45–53. doi: 10.1212/WNL.36.1.45. [DOI] [PubMed] [Google Scholar]
- 14.Scarlato G.N., Bresolin N.I., Moroni I., Doriguzzi C., Castelli E., Comi G., Angelini C., Carenzi A. Multicenter trial with ubidecarenone: Treatment of 44 patients with mitochondrial myopathies. Rev. Neurol. 1991;147:542–548. [PubMed] [Google Scholar]
- 15.Mayr J.A., Zimmermann F.A., Fauth C., Bergheim C., Meierhofer D., Radmayr D., Zschocke J., Koch J., Sperl W. Lipoic acid synthetase deficiency causes neonatal-onset epilepsy, defective mitochondrial energy metabolism, and glycine elevation. Am. J. Hum. Genet. 2011;89:792–797. doi: 10.1016/j.ajhg.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J. The effects and mechanisms of mitochondrial nutrient alpha-lipoic acid on improving age-associated mitochondrial and cognitive dysfunction: An overview. Neurochem. Res. 2008;33:194–203. doi: 10.1007/s11064-007-9403-0. [DOI] [PubMed] [Google Scholar]
- 17.Maes M., Mihaylova I., Kubera M., Uytterhoeven M., Vrydags N., Bosmans E. Coenzyme Q10 deficiency in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is related to fatigue, autonomic and neurocognitive symptoms and is another risk factor explaining the early mortality in ME/CFS due to cardiovascular disorder. Neuro Endocrinol. Lett. 2009;30:470–476. [PubMed] [Google Scholar]
- 18.Tiano L., Busciglio J. Mitochondrial dysfunction and Down’s syndrome: Is there a role for coenzyme Q10? Biofactors. 2011;37:386–392. doi: 10.1002/biof.184. [DOI] [PubMed] [Google Scholar]
- 19.Hardas S.S., Sultana R., Clark A.M., Beckett T.L., Szweda L.I., Murphy M.P., Butterfield D.A. Oxidative modification of lipoic acid by HNE in Alzheimer disease brain. Redox Biol. 2013;1:80–85. doi: 10.1016/j.redox.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kähler W., Kuklinski B., Rühlmann C., Plötz C. Diabetes mellitus—A free radical-associated disease. Results of adjuvant antioxidant supplementation. Z. Gesamte Inn. Med. 1993;48:223–232. [PubMed] [Google Scholar]
- 21.Jacob S., Henriksen E.J., Schiemann A.L., Simon I., Clancy D.E., Tritschler H.J., Jung W.I., Augustin H.J., Dietze G.J. Enhancement of glucose disposal in patients with type 2 diabetes by alpha-lipoic acid. Arzneimittelforschung. 1995;45:872–874. [PubMed] [Google Scholar]
- 22.Ziegler D., Hanefeld M., Ruhnau K.J., Meissner H.P., Lobisch M., Schütte K., Gries F.A. Treatment of symptomatic diabetic peripheral neuropathy with the anti-oxidant alpha-lipoic acid. A 3-week multicentre randomized controlled trial (ALADIN Study) Diabetologia. 1995;38:1425–1433. doi: 10.1007/BF00400603. [DOI] [PubMed] [Google Scholar]
- 23.Ziegler D., Schatz H., Conrad F., Gries F.A., Ulrich H., Reichel G. Effects of treatment with the antioxidant alpha-lipoic acid on cardiac autonomic neuropathy in NIDDM patients. A 4-month randomized controlled multicenter trial (DEKAN Study). Deutsche Kardiale Autonome Neuropathie. Diabetes Care. 1997;20:369–373. doi: 10.2337/diacare.20.3.369. [DOI] [PubMed] [Google Scholar]
- 24.Strokov I.A., Kozlova N.A., Mozolevskiĭ IuV, Miasoedov S.P., Iakhno N.N. The efficacy of the intravenous administration of the trometamol salt of thioctic (alpha-lipoic) acid in diabetic neuropathy. Zh. Nevrol. Psikhiatr. Im. S. S. Korsakova. 1999;99:18–22. [PubMed] [Google Scholar]
- 25.Jacob S.P., Ruus P.R., Hermann R., Tritschler H.J., Maerker E., Renn W., Augustin H.J., Dietze G.J., Rett K. Oral administration of RAC-alpha-lipoic acid modulates insulin sensitivity in patients with type-2 diabetes mellitus: A placebo-controlled pilot trial. Free Radic. Biol. Med. 1999;27:309–314. doi: 10.1016/S0891-5849(99)00089-1. [DOI] [PubMed] [Google Scholar]
- 26.Ziegler D., Hanefeld M., Ruhnau K.J., Hasche H., Lobisch M., Schütte K., Kerum G., Malessa R. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: A 7-month multicenter randomized controlled trial (ALADIN III study). ALADIN III Study Group. Alpha-lipoic acid in diabetic neuropathy. Diabetes Care. 1999;22:1296–1301. doi: 10.2337/diacare.22.8.1296. [DOI] [PubMed] [Google Scholar]
- 27.Reljanovic M.G., Reichel G.K., Rett K., Lobisch M., Schuette K., Möller W., Tritschler H.J., Mehnert H. Treatment of diabetic polyneuropathy with the antioxidant thioctic acid (alpha-lipoic acid): A two year multicenter randomized double-blind placebo-controlled trial (ALADIN II). Alpha lipoic acid in diabetic neuropathy. Free Radic. Res. 1999;31:171–179. doi: 10.1080/10715769900300721. [DOI] [PubMed] [Google Scholar]
- 28.Ruhnau K.J., Meissner H.P., Finn J.R., Reljanovic M., Lobisch M., Schütte K., Nehrdich D., Tritschler H.J., Mehnert H., Ziegler D. Effects of 3-week oral treatment with the antioxidant thioctic acid (alpha-lipoic acid) in symptomatic diabetic polyneuropathy. Diabet. Med. 1999;16:1040–1043. doi: 10.1046/j.1464-5491.1999.00190.x. [DOI] [PubMed] [Google Scholar]
- 29.Negrişanu G., Roşu M., Bolte B., Lefter D., Dabelea D. Effects of 3-month treatment with the antioxidant alpha-lipoic acid in diabetic peripheral neuropathy. Rom. J. Intern. Med. 1999;37:297–306. [PubMed] [Google Scholar]
- 30.Androne L., Gavan N.A., Veresiu I.A., Orasan R. In vivo effect of lipoic acid on lipid peroxidation in patients with diabetic neuropathy. In Vivo. 2000;14:327–330. [PubMed] [Google Scholar]
- 31.Haak E., Usadel K.H., Kusterer K., Amini P., Frommeyer R., Tritschler H.J., Haak T. Effects of alpha-lipoic acid on microcirculation in patients with peripheral diabetic neuropathy. Exp. Clin. Endocrinol. Diabetes. 2000;108:168–174. doi: 10.1055/s-2000-7739. [DOI] [PubMed] [Google Scholar]
- 32.Evans J.L., Heymann C.J., Goldfine I.D., Gavin L.A. Pharmacokinetics, tolerability, and fructosamine-lowering effect of a novel, controlled-release formulation of alpha-lipoic acid. Endocr. Pract. 2002;8:29–35. doi: 10.4158/EP.8.1.29. [DOI] [PubMed] [Google Scholar]
- 33.Ametov A.S., Barinov A., Dyck P.J., Hermann R., Kozlova N., Litchy W.J., Low P.A., Nehrdich D., Novosadova M., O’Brien P.C., et al. The sensory symptoms of diabetic polyneuropathy are improved with alpha-lipoic acid: The SYDNEY trial. Diabetes Care. 2003;26:770–776. doi: 10.2337/diacare.26.3.770. [DOI] [PubMed] [Google Scholar]
- 34.Hahm J.R., Kim B.J., Kim K.W. Clinical experience with thioctacid (thioctic acid) in the treatment of distal symmetric polyneuropathy in Korean diabetic patients. J. Diabetes Complicat. 2004;18:79–85. doi: 10.1016/S1056-8727(03)00033-3. [DOI] [PubMed] [Google Scholar]
- 35.Ziegler D., Ametov A., Barinov A., Dyck P.J., Gurieva I., Low P.A., Munzel U., Yakhno N., Raz I., Novosadova M., et al. Oral treatment with alpha-lipoic acid improves symptomatic diabetic polyneuropathy: The SYDNEY 2 trial. Diabetes Care. 2006;29:2365–2370. doi: 10.2337/dc06-1216. [DOI] [PubMed] [Google Scholar]
- 36.Kamenova P. Improvement of insulin sensitivity in patients with type 2 diabetes mellitus after oral administration of alpha-lipoic acid. Hormones. 2006;5:251–258. doi: 10.14310/horm.2002.11191. [DOI] [PubMed] [Google Scholar]
- 37.Liu F., Zhang Y., Yang M., Liu B., Shen Y.D., Jia W.P., Xiang K.S. Curative effect of alpha-lipoic acid on peripheral neuropathy in type 2 diabetes: A clinical study. Zhonghua Yi Xue Za Zhi. 2007;87:2706–2709. [PubMed] [Google Scholar]
- 38.Xiang G.D., Sun H.L., Zhao L.S., Hou J., Yue L., Xu L. The antioxidant alpha-lipoic acid improves endothelial dysfunction induced by acute hyperglycaemia during OGTT in impaired glucose tolerance. Clin. Endocrinol. 2008;68:716–723. doi: 10.1111/j.1365-2265.2007.03099.x. [DOI] [PubMed] [Google Scholar]
- 39.Bureković A., Terzić M., Alajbegović S., Vukojević Z., Hadzić N. The role of alpha-lipoic acid in diabetic polyneuropathy treatment. Bosn. J. Basic Med. Sci. 2008;8:341–245. doi: 10.17305/bjbms.2008.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heinisch B.B., Francesconi M., Mittermayer F., Schaller G., Gouya G., Wolzt M., Pleiner J. Alpha-lipoic acid improves vascular endothelial function in patients with type 2 diabetes: A placebo-controlled randomized trial. Eur. J. Clin. Investig. 2010;40:148–154. doi: 10.1111/j.1365-2362.2009.02236.x. [DOI] [PubMed] [Google Scholar]
- 41.Palacka P., Kucharska J., Murin J., Dostalova K., Okkelova A., Cizova M., Waczulikova I., Moricova S., Gvozdjakova A. Complementary therapy in diabetic patients with chronic complications: A pilot study. Bratisl. Lek. Listy. 2010;111:205–211. [PubMed] [Google Scholar]
- 42.Gu X.M., Zhang S.S., Wu J.C., Tang Z.Y., Lu Z.Q., Li H., Liu C., Chen L., Ning G. Efficacy and safety of high-dose α-lipoic acid in the treatment of diabetic polyneuropathy. Zhonghua Yi Xue Za Zhi. 2010;90:2473–2476. [PubMed] [Google Scholar]
- 43.Xiang G., Pu J., Yue L., Hou J., Sun H. α-Lipoic acid can improve endothelial dysfunction in subjects with impaired fasting glucose. Metabolism. 2011;60:480–485. doi: 10.1016/j.metabol.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 44.De Oliveira A.M., Rondó P.H., Luzia L.A., D'Abronzo F.H., Illison V.K. The effects of lipoic acid and α-tocopherol supplementation on the lipid profile and insulin sensitivity of patients with type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled trial. Diabetes Res. Clin. Pract. 2011;92:253–260. doi: 10.1016/j.diabres.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 45.Ansar H., Mazloom Z., Kazemi F., Hejazi N. Effect of alpha-lipoic acid on blood glucose, insulin resistance and glutathione peroxidase of type 2 diabetic patients. Saudi Med. J. 2011;32:584–588. [PubMed] [Google Scholar]
- 46.Ziegler D., Low P.A., Litchy W.J., Boulton A.J., Vinik A.I., Freeman R., Samigull R., Tritschler H., Munzel U., Maus J., et al. Efficacy and safety of antioxidant treatment with α-lipoic acid over 4 years in diabetic polyneuropathy: The NATHAN 1 trial. Diabetes Care. 2011;34:2054–2060. doi: 10.2337/dc11-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haritoglou C., Gerss J., Hammes H.P., Kampik A., Ulbig M.W., RETIPON Study Group Alpha-lipoic acid for the prevention of diabetic macular edema. Ophthalmologica. 2011;226:127–137. doi: 10.1159/000329470. [DOI] [PubMed] [Google Scholar]
- 48.Rahman S.T., Merchant N., Haque T., Wahi J., Bhaheetharan S., Ferdinand K.C., Khan B.V. The impact of lipoic acid on endothelial function and proteinuria in quinapril-treated diabetic patients with stage I hypertension: Results from the QUALITY study. J. Cardiovasc. Pharmacol. Ther. 2012;17:139–145. doi: 10.1177/1074248411413282. [DOI] [PubMed] [Google Scholar]
- 49.Bertolotto F., Massone A. Combination of alpha lipoic acid and superoxide dismutase leads to physiological and symptomatic improvements in diabetic neuropathy. Drugs R&D. 2012;12:29–34. doi: 10.2165/11599200-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Porasuphatana S., Suddee S., Nartnampong A., Konsil J., Harnwong B., Santaweesuk A. Glycemic and oxidative status of patients with type 2 diabetes mellitus following oral administration of alpha-lipoic acid: A randomized double-blinded placebo-controlled study. Asia Pac. J. Clin. Nutr. 2012;21:12–21. [PubMed] [Google Scholar]
- 51.Ibrahimpasic K. Alpha lipoic acid and glycaemic control in diabetic neuropathies at type 2 diabetes treatment. Med. Arch. 2013;67:7–9. doi: 10.5455/medarh.2013.67.7-9. [DOI] [PubMed] [Google Scholar]
- 52.Tankova T., Koev D., Dakovska L. Alpha-lipoic acid in the treatment of autonomic diabetic neuropathy (controlled, randomized, open-label study) Rom. J. Intern. Med. 2004;42:457–464. [PubMed] [Google Scholar]
- 53.Huang E.A., Gitelman S.E. The effect of oral alpha-lipoic acid on oxidative stress in adolescents with type 1 diabetes mellitus. Pediat. Diabetes. 2008;9:69–73. doi: 10.1111/j.1399-5448.2007.00342.x. [DOI] [PubMed] [Google Scholar]
- 54.Mollo R., Zaccardi F., Scalone G., Scavone G., Rizzo P., Navarese E.P., Manto A., Pitocco D., Lanza G.A., Ghirlanda G., et al. Effect of α-lipoic acid on platelet reactivity in type 1 diabetic patients. Diabetes Care. 2012;35:196–197. doi: 10.2337/dc11-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morcos M., Borcea V., Isermann B., Gehrke S., Ehret T., Henkels M., Schiekofer S., Hofmann M., Amiral J., Tritschler H., et al. Effect of alpha-lipoic acid on the progression of endothelial cell damage and albuminuria in patients with diabetes mellitus: An exploratory study. Diabetes Res. Clin. Pract. 2001;5:175–183. doi: 10.1016/S0168-8227(01)00223-6. [DOI] [PubMed] [Google Scholar]
- 56.Noori N., Tabibi H., Hosseinpanah F., Hedayati M., Nafar M. Effects of combined lipoic acid and pyridoxine on albuminuria, advanced glycation end-products, and blood pressure in diabetic nephropathy. Int. J. Vitam. Nutr. Res. 2013;83:77–85. doi: 10.1024/0300-9831/a000147. [DOI] [PubMed] [Google Scholar]
- 57.Hegazy S.K., Tolba O.A., Mostafa T.M., Eid M.A., el-Afify D.R. Alpha-lipoic acid improves subclinical left ventricular dysfunction in asymptomatic patients with type 1 diabetes. Rev. Diabet. Stud. 2013;10:58–67. doi: 10.1900/RDS.2013.10.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang Z., Wan X., Liu J., Deng W., Chen A., Liu L., Liu J., Wei G., Li H., Fang D., et al. Short-term continuous subcutaneous insulin infusion combined with insulin sensitizers rosiglitazone, metformin, or antioxidant α-lipoic acid in patients with newly diagnosed type 2 diabetes mellitus. Diabetes Technol. Ther. 2013;15:859–869. doi: 10.1089/dia.2013.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mitkov M.D., Aleksandrova I.Y., Orbetzova M.M. Effect of transdermal testosterone or alpha-lipoic acid on erectile dysfunction and quality of life in patients with type 2 diabetes mellitus. Folia Med. 2013;55:55–63. doi: 10.2478/folmed-2013-0006. [DOI] [PubMed] [Google Scholar]
- 60.Pop-Busui R., Stevens M.J., Raffel D.M., White E.A., Mehta M., Plunkett C.D., Brown M.B., Feldman E.L. Effects of triple antioxidant therapy on measures of cardiovascular autonomic neuropathy and on myocardial blood flow in type 1 diabetes: A randomized controlled trial. Diabetologia. 2013;56:1835–1844. doi: 10.1007/s00125-013-2942-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang X., Zhang Y., Gao X., Wu J., Jiao X., Zhao J., Lv X. Investigating the role of backward walking therapy in alleviating plantar pressure of patients with diabetic peripheral neuropathy. Arch. Phys. Med. Rehabil. 2014;95:832–839. doi: 10.1016/j.apmr.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 62.Yadav V., Marracci G., Lovera J., Woodward W., Bogardus K., Marquardt W., Shinto L., Morris C., Bourdette D. Lipoic acid in multiple sclerosis: A pilot study. Mult. Sclerosis. 2005;11:159–165. doi: 10.1191/1352458505ms1143oa. [DOI] [PubMed] [Google Scholar]
- 63.Khalili M., Azimi A., Izadi V., Eghtesadi S., Mirshafiey A., Sahraian M.A., Motevalian A., Norouzi A., Sanoobar M., Eskandari G., et al. Does lipoic acid consumption affect the cytokine profile in multiple sclerosis patients: A double-blind, placebo-controlled, randomized clinical trial. Neuroimmunomodulation. 2014;21:291–296. doi: 10.1159/000356145. [DOI] [PubMed] [Google Scholar]
- 64.Bae S.C., Jung W.J., Lee E.J., Yu R., Sung M.K. Effects of antioxidant supplements intervention on the level of plasma inflammatory molecules and disease severity of rheumatoid arthritis patients. J. Am. Coll. Nutr. 2009;28:56–62. doi: 10.1080/07315724.2009.10719762. [DOI] [PubMed] [Google Scholar]
- 65.Galasko D.R., Peskind E., Clark C.M., Quinn J.F., Ringman J.M., Jicha G.A., Cotman C., Cottrell B., Montine T.J., Thomas R.G., et al. Alzheimer’s disease cooperative study. Antioxidants for Alzheimer disease: A randomized clinical trial with cerebrospinal fluid biomarker measures. Arch. Neurol. 2012;69:836–841. doi: 10.1001/archneurol.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Magis D., Ambrosini A., Sándor P., Jacquy J., Laloux P., Schoenen J. A randomized double-blind placebo-controlled trial of thioctic acid in migraine prophylaxis. Headache. 2007;47:52–57. doi: 10.1111/j.1526-4610.2006.00626.x. [DOI] [PubMed] [Google Scholar]
- 67.Di Geronimo G., Caccese A.F., Caruso L., Soldati A., Passaretti U. Treatment of carpal tunnel syndrome with alpha-lipoic acid. Eur. Rev. Med. Pharmacol. Sci. 2009;13:133–139. [PubMed] [Google Scholar]
- 68.Memeo A., Loiero M. Thioctic acid and acetyl-l-carnitine in the treatment of sciatic pain caused by a herniated disc: A randomized, double-blind, comparative study. Clin. Drug Investig. 2008;28:495–500. doi: 10.2165/00044011-200828080-00004. [DOI] [PubMed] [Google Scholar]
- 69.Ranieri M., Sciuscio M., Cortese A.M., Santamato A., di Teo L., Ianieri G., Bellomo R.G., Stasi M., Megna M. The use of alpha-lipoic acid (ALA), gamma linolenic acid (GLA) and rehabilitation in the treatment of back pain: Effect on health-related quality of life. Int. J. Immunopathol. Pharmacol. 2009;22:45–50. doi: 10.1177/03946320090220S309. [DOI] [PubMed] [Google Scholar]
- 70.Shinto L., Quinn J., Montine T., Dodge H.H., Woodward W., Baldauf-Wagner S., Waichunas D., Bumgarner L., Bourdette D., Silbert L., et al. A randomized placebo-controlled pilot trial of omega-3 fatty acids and alpha lipoic acid in Alzheimer’s disease. J. Alzheimers Dis. 2014;38:111–120. doi: 10.3233/JAD-130722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dünschede F., Erbes K., Kircher A., Westermann S., Seifert J., Schad A., Oliver K., Kiemer A.K., Theodor J. Reduction of ischemia reperfusion injury after liver resection and hepatic inflow occlusion by alpha-lipoic acid in humans. World J. Gastroenterol. 2006;12:6812–6817. doi: 10.3748/wjg.v12.i42.6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Melhem A., Stern M., Shibolet O., Israeli E., Ackerman Z., Pappo O., Hemed N., Rowe M., Ohana H., Zabrecky G., et al. Treatment of chronic hepatitis C virus infection via antioxidants: Results of a phase I clinical trial. J. Clin. Gastroenterol. 2005;39:737–742. doi: 10.1097/01.mcg.0000174023.73472.29. [DOI] [PubMed] [Google Scholar]
- 73.Koh E.H., Lee W.J., Lee S.A., Kim E.H., Cho E.H., Jeong E., Kim D.W., Kim M.S., Park J.Y., Park K.G., et al. Effects of alpha-lipoic acid on body weight in obese subjects. Am. J. Med. 2011;124:85.e1-8. doi: 10.1016/j.amjmed.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 74.McNeilly A.M., Davison G.W., Murphy M.H., Nadeem N., Trinick T., Duly E., Novials A., McEneny J. Effect of α-lipoic acid and exercise training on cardiovascular disease risk in obesity with impaired glucose tolerance. Lipids Health Dis. 2011;10:217. doi: 10.1186/1476-511X-10-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Y., Han P., Wu N., He B., Lu Y., Li S., Liu Y., Zhao S., Liu L., Li Y. Amelioration of lipid abnormalities by α-lipoic acid through antioxidative and anti-inflammatory effects. Obesity. 2011;19:1647–1653. doi: 10.1038/oby.2011.121. [DOI] [PubMed] [Google Scholar]
- 76.Skroza N., Proietti I., Bernardini N., la Viola G., Nicolucci F., Pampena R., Tolino E., Zuber S., Mancini M.T., Soccodato V., et al. Efficacy of food supplement to improve metabolic syndrome parameters in patients affected by moderate to severe psoriasis during anti-TNFα treatment. G. Ital. Dermatol. Venereol. 2013;148:661–665. [PubMed] [Google Scholar]
- 77.Manning P.J., Sutherland W.H., Williams S.M., Walker R.J., Berry E.A., de Jong S.A., Ryalls A.R. The effect of lipoic acid and vitamin E therapies in individuals with the metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2013;23:543–549. doi: 10.1016/j.numecd.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 78.McMackin C.J., Widlansky M.E., Hamburg N.M. Effect of combined treatment with alpha-lipoic acid and acetyl-l-carnitine on vascular function and blood pressure in patients with coronary artery disease. J. Clin. Hypertens. 2007;9:249–255. doi: 10.1111/j.1524-6175.2007.06052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li R.J., Ji W.Q., Pang J.J., Wang J.L., Chen Y.G., Zhang Y. Alpha-lipoic acid ameliorates oxidative stress by increasing aldehyde dehydrogenase-2 activity in patients with acute coronary syndrome. Tohoku J. Exp. Med. 2013;229:45–51. doi: 10.1620/tjem.229.45. [DOI] [PubMed] [Google Scholar]
- 80.Vincent H.K., Bourguignon C.M., Vincent K.R., Taylor A.G. Effects of alpha-lipoic acid supplementation in peripheral arterial disease: A pilot study. J. Altern. Complement. Med. 2007;13:577–584. doi: 10.1089/acm.2007.6177. [DOI] [PubMed] [Google Scholar]
- 81.Leong J.Y., van der Merwe J., Pepe S., Bailey M., Perkins A., Lymbury R., Esmore D., Marasco S., Rosenfeldt F. Perioperative metabolic therapy improves redox status and outcomes in cardiac surgery patients: A randomised trial. Heart Lung Circ. 2010;19:584–591. doi: 10.1016/j.hlc.2010.06.659. [DOI] [PubMed] [Google Scholar]
- 82.Chang J.W., Lee E.K., Kim T.H., Min W.K., Chun S., Lee K.U., Kim S.B., Park J.S. Effects of alpha-lipoic acid on the plasma levels of asymmetric dimethylarginine in diabetic end-stage renal disease patients on hemodialysis: A pilot study. Am. J. Nephrol. 2007;27:70–74. doi: 10.1159/000099035. [DOI] [PubMed] [Google Scholar]
- 83.Ramos L.F., Kane J., McMonagle E., Le P., Wu P., Shintani A., Ikizler T.A., Himmelfarb J. Effects of combination tocopherols and alpha lipoic acid therapy on oxidative stress and inflammatory biomarkers in chronic kidney disease. J. Ren. Nutr. 2011;21:211–218. doi: 10.1053/j.jrn.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khabbazi T., Mahdavi R., Safa J., Pour-Abdollahi P. Effects of alpha-lipoic acid supplementation on inflammation, oxidative stress, and serum lipid profile levels in patients with end-stage renal disease on hemodialysis. J. Ren. Nutr. 2012;22:244–250. doi: 10.1053/j.jrn.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 85.Himmelfarb J., Ikizler T.A., Ellis C., Wu P., Shintani A., Dalal S., Kaplan M., Chonchol M., Hakim R.M. Provision of antioxidant therapy in hemodialysis (PATH): A randomized clinical trial. J. Am. Soc. Nephrol. 2014;25:623–633. doi: 10.1681/ASN.2013050545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lott I.T., Doran E., Nguyen V.Q., Tournay A., Head E., Gillen D.L. Down syndrome and dementia: A randomized, controlled trial of antioxidant supplementation. Am. J. Med. Genet. A. 2011;155A:1939–1948. doi: 10.1002/ajmg.a.34114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Martins V.D., Manfredini V., Peralba M.C., Benfato M.S. Alpha-lipoic acid modifies oxidative stress parameters in sickle cell trait subjects and sickle cell patients. Clin. Nutr. 2009;28:192–197. doi: 10.1016/j.clnu.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 88.Rodriguez M.C., MacDonald J.R., Mahoney D.J., Parise G., Beal M.F., Tarnopolsky M.A. Beneficial effects of creatine, CoQ10, and lipoic acid in mitochondrial disorders. Muscle Nerve. 2007;35:235–242. doi: 10.1002/mus.20688. [DOI] [PubMed] [Google Scholar]
- 89.Femiano F., Gombos F., Scully C., Busciolano M., de Luca P. Burning mouth syndrome (BMS): Controlled open trial of the efficacy of alpha-lipoic acid (thioctic acid) on symptomatology. Oral Dis. 2000;6:274–277. doi: 10.1111/j.1601-0825.2000.tb00138.x. [DOI] [PubMed] [Google Scholar]
- 90.Carbone M., Pentenero M., Carrozzo M., Ippolito A., Gandolfo S. Lack of efficacy of alpha-lipoic acid in burning mouth syndrome: A double-blind, randomized, placebo-controlled study. Eur. J. Pain. 2009;13:492–496. doi: 10.1016/j.ejpain.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 91.López-Jornet P., Camacho-Alonso F., Leon-Espinosa S. Efficacy of alpha lipoic acid in burning mouth syndrome: A randomized, placebo-treatment study. J. Oral. Rehabil. 2009;36:52–57. doi: 10.1111/j.1365-2842.2008.01914.x. [DOI] [PubMed] [Google Scholar]
- 92.Marino R., Torretta S., Capaccio P., Pignataro L., Spadari F. Different therapeutic strategies for burning mouth syndrome: Preliminary data. J. Oral Pathol. Med. 2010;39:611–616. doi: 10.1111/j.1600-0714.2010.00922.x. [DOI] [PubMed] [Google Scholar]
- 93.López-D’Alessandro E., Escovich L. Combination of alpha lipoic acid and gabapentin, its efficacy in the treatment of Burning Mouth Syndrome: A randomized, double-blind, placebo controlled trial. Med. Oral Patol. Oral Cir. Bucal. 2011;16:e635–e640. doi: 10.4317/medoral.16942. [DOI] [PubMed] [Google Scholar]
- 94.Mantovani G., Macciò A., Madeddu C., Gramignano G., Lusso M.R., Serpe R., Massa E., Astara G., Deiana L. A phase II study with antioxidants, both in the diet and supplemented, pharmaconutritional support, progestagen, and anti-cyclooxygenase-2 showing efficacy and safety in patients with cancer-related anorexia/cachexia and oxidative stress. Cancer Epidemiol. Biomark. Prev. 2006;15:1030–1034. doi: 10.1158/1055-9965.EPI-05-0538. [DOI] [PubMed] [Google Scholar]
- 95.Mantovani G., Macciò A., Madeddu C., Gramignano G., Serpe R., Massa E., Dessì M., Tanca F.M., Sanna E., Deiana L., et al. Randomized phase III clinical trial of five different arms of treatment for patients with cancer cachexia: Interim results. Nutrition. 2008;24:305–313. doi: 10.1016/j.nut.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 96.Jariwalla R.J., Lalezari J., Cenko D., Mansour S.E., Kumar A., Gangapurkar B., Nakamura D. Restoration of blood total glutathione status and lymphocyte function following alpha-lipoic acid supplementation in patients with HIV infection. J. Altern. Complement. Med. 2008;14:139–146. doi: 10.1089/acm.2006.6397. [DOI] [PubMed] [Google Scholar]
- 97.Milazzo L., Menzaghi B., Caramma I., Nasi M., Sangaletti O., Cesari M., Zanone Poma B., Cossarizza A., Antinori S., Galli M. Effect of antioxidants on mitochondrial function in HIV-1-related lipoatrophy: A pilot study. AIDS Res. Hum. Retroviruses. 2010;26:1207–1214. doi: 10.1089/aid.2010.0024. [DOI] [PubMed] [Google Scholar]
- 98.Dell’Anna M.L., Mastrofrancesco A., Sala R., Venturini M., Ottaviani M., Vidolin A.P., Leone G., Calzavara P.G., Westerhof W., Picardo M. Antioxidants and narrow band-UVB in the treatment of vitiligo: A double-blind placebo controlled trial. Clin. Exp. Dermatol. 2007;32:631–636. doi: 10.1111/j.1365-2230.2007.02514.x. [DOI] [PubMed] [Google Scholar]
- 99.Mainini G., Rotondi M., di Nola K., Pezzella M.T., Iervolino S.A., Seguino E., D’Eufemia D., Iannicelli I., Torella M. Oral supplementation with antioxidant agents containing alpha lipoic acid: Effects on postmenopausal bone mass. Clin. Exp. Obstet. Gynecol. 2012;39:489–493. [PubMed] [Google Scholar]
- 100.Garrido-Maraver J., Cordero M.D., Oropesa-Avila M., Oropesa-Avila M., Vega A.F., de la Mata M., Pavon A.D., Alcocer-Gomez E., Calero C.P., Paz M.V., et al. Clinical applications of coenzyme Q10. Front. Biosci. 2014;19:619–633. doi: 10.2741/4231. [DOI] [PubMed] [Google Scholar]
- 101.Singh R.B., Niaz M.A. Serum concentration of lipoprotein(a) decreases on treatment with hydrosoluble coenzyme Q10 in patients with coronary artery disease: Discovery of a new role. Int. J. Cardiol. 1999;68:23–29. doi: 10.1016/S0167-5273(98)00323-4. [DOI] [PubMed] [Google Scholar]
- 102.Singh R.B., Niaz M.A., Rastogi S.S., Shukla P.K., Thakur A.S. Effect of hydrosoluble coenzyme Q10 on blood pressures and insulin resistance in hypertensive patients with coronary artery disease. J. Hum. Hypertens. 1999;13:203–208. doi: 10.1038/sj.jhh.1000778. [DOI] [PubMed] [Google Scholar]
- 103.Tiano L., Belardinelli R., Carnevali P., Principi F., Seddaiu G., Littarru G.P. Effect of coenzyme Q10 administration on endothelial function and extracellular superoxide dismutase in patients with ischaemic heart disease: A double-blind, randomized controlled study. Eur. Heart J. 2007;28:2249–2255. doi: 10.1093/eurheartj/ehm267. [DOI] [PubMed] [Google Scholar]
- 104.Belardinelli R., Muçaj A., Lacalaprice F., Solenghi M., Principi F., Tiano L., Littarru G.P. Coenzyme Q10 improves contractility of dysfunctional myocardium in chronic heart failure. Biofactors. 2005;25:137–145. doi: 10.1002/biof.5520250115. [DOI] [PubMed] [Google Scholar]
- 105.Dai Y.L., Luk T.H., Yiu K.H., Wang M., Yip P.M., Lee S.W., Li S.W., Tam S., Fong B., Lau C.P., et al. Reversal of mitochondrial dysfunction by coenzyme Q10 supplement improves endothelial function in patients with ischaemic left ventricular systolic dysfunction: A randomized controlled trial. Atherosclerosis. 2011;216:395–401. doi: 10.1016/j.atherosclerosis.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 106.Lee B.J., Huang Y.C., Chen S.J., Lin P.T. Coenzyme Q10 supplementation reduces oxidative stress and increases antioxidant enzyme activity in patients with coronary artery disease. Nutrition. 2012;28:250–255. doi: 10.1016/j.nut.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 107.Hofman-Bang C., Rehnqvist N., Swedberg K., Wiklund I., Aström H. Coenzyme Q10 as an adjunctive in the treatment of chronic congestive heart failure. The Q10 Study Group. J. Card. Fail. 1995;1:101–107. doi: 10.1016/1071-9164(95)90011-X. [DOI] [PubMed] [Google Scholar]
- 108.Munkholm H., Hansen H.H., Rasmussen K. Coenzyme Q10 treatment in serious heart failure. Biofactors. 1999;9:285–289. doi: 10.1002/biof.5520090225. [DOI] [PubMed] [Google Scholar]
- 109.Khatta M., Alexander B.S., Krichten C.M., Fisher M.L., Freudenberger R., Robinson S.W., Gottlieb S.S. The effect of coenzyme Q10 in patients with congestive heart failure. Ann. Intern. Med. 2000;132:636–640. doi: 10.7326/0003-4819-132-8-200004180-00006. [DOI] [PubMed] [Google Scholar]
- 110.Berman M., Erman A., Ben-Gal T., Dvir D., Georghiou G.P., Stamler A., Vered Y., Vidne B.A., Aravot D. Coenzyme Q10 in patients with end-stage heart failure awaiting cardiac transplantation: A randomized, placebo-controlled study. Clin. Cardiol. 2004;27:295–299. doi: 10.1002/clc.4960270512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Damian M.S., Ellenberg D., Gildemeister R., Lauermann J., Simonis G., Sauter W., Georgi C. Coenzyme Q10 combined with mild hypothermia after cardiac arrest: A preliminary study. Circulation. 2004;110:3011–3016. doi: 10.1161/01.CIR.0000146894.45533.C2. [DOI] [PubMed] [Google Scholar]
- 112.Witte K.K., Nikitin N.P., Parker A.C., von Haehling S., Volk H.D., Anker S.D., Clark A.L., Cleland J.G. The effect of micronutrient supplementation on quality-of-life and left ventricular function in elderly patients with chronic heart failure. Eur. Heart J. 2005;26:2238–2244. doi: 10.1093/eurheartj/ehi442. [DOI] [PubMed] [Google Scholar]
- 113.Langsjoen P.H., Langsjoen A.M. Supplemental ubiquinol in patients with advanced congestive heart failure. Biofactors. 2008;32:119–128. doi: 10.1002/biof.5520320114. [DOI] [PubMed] [Google Scholar]
- 114.Kumar A., Singh R.B., Saxena M., Niaz M.A., Josh S.R., Chattopadhyay P., Mechirova V., Pella D., Fedacko J. Effect of carni Q-gel (ubiquinol and carnitine) on cytokines in patients with heart failure in the Tishcon study. Acta Cardiol. 2007;62:349–354. doi: 10.2143/AC.62.4.2022278. [DOI] [PubMed] [Google Scholar]
- 115.Belcaro G., Cesarone M.R., Dugall M., Hosoi M., Ippolito E., Bavera P., Grossi M.G. Investigation of pycnogenol in combination with coenzyme Q10 in heart failure patients (NYHA II/III) Panminerva Med. 2010;52:21–25. [PubMed] [Google Scholar]
- 116.Fumagalli S., Fattirolli F., Guarducci L., Cellai T., Baldasseroni S., Tarantini F., di Bari M., Masotti G., Marchionni N. Coenzyme Q10 terclatrate and creatine in chronic heart failure: A randomized, placebo-controlled, double-blind study. Clin. Cardiol. 2011;34:211–217. doi: 10.1002/clc.20846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kuklinski B., Weissenbacher E., Fähnrich A. Coenzyme Q10 and antioxidants in acute myocardial infarction. Mol. Aspects Med. 1994;15:s143–s147. doi: 10.1016/0098-2997(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 118.Singh R.B., Wander G.S., Rastogi A., Shukla P.K., Mittal A., Sharma J.P., Mehrotra S.K., Kapoor R., Chopra R.K. Randomized, double-blind placebo-controlled trial of coenzyme Q10 in patients with acute myocardial infarction. Cardiovasc. Drugs Ther. 1998;12:347–353. doi: 10.1023/A:1007764616025. [DOI] [PubMed] [Google Scholar]
- 119.Singh R.B., Neki N.S., Kartikey K., Pella D., Kumar A., Niaz M.A., Thakur A.S. Effect of coenzyme Q10 on risk of atherosclerosis in patients with recent myocardial infarction. Mol. Cell. Biochem. 2003;246:75–82. doi: 10.1023/A:1023408031111. [DOI] [PubMed] [Google Scholar]
- 120.Permanetter B., Rössy W., Klein G., Weingartner F., Seidl K.F., Blömer H. Ubiquinone (coenzyme Q10) in the long-term treatment of idiopathic dilated cardiomyopathy. Eur. Heart J. 1992;13:1528–1533. doi: 10.1093/oxfordjournals.eurheartj.a060096. [DOI] [PubMed] [Google Scholar]
- 121.Langsjoen P.H., Folkers K., Lyson K., Muratsu K., Lyson T., Langsjoen P. Pronounced increase of survival of patients with cardiomyopathy when treated with coenzyme Q10 and conventional therapy. Int. J. Tissue React. 1990;12:163–168. [PubMed] [Google Scholar]
- 122.Morisco C., Trimarco B., Condorelli M. Effect of coenzyme Q10 therapy in patients with congestive heart failure: A long-term multicenter randomized study. Clin. Investig. 1993;71:S134–S136. doi: 10.1007/BF00226854. [DOI] [PubMed] [Google Scholar]
- 123.Ma A.W., Zhang W., Liu Z. Effect of protection and repair of injury of mitochondrial membrane-phospholipid on prognosis in patients with dilated cardiomyopathy. Blood Press. Suppl. 1. 1996;3:53–55. [PubMed] [Google Scholar]
- 124.Sacher H.L., Sacher M.L., Landau S.W., Kersten R., Dooley F., Sacher A., Sacher M., Dietrick K., Ichkhan K. The clinical and hemodynamic effects of coenzyme Q10 in congestive cardiomyopathy. Am. J. Ther. 1997;4:66–72. doi: 10.1097/00045391-199702000-00003. [DOI] [PubMed] [Google Scholar]
- 125.Soongswang J., Sangtawesin C., Durongpisitkul K., Laohaprasitiporn D., Nana A., Punlee K., Kangkagate C. The effect of coenzyme Q10 on idiopathic chronic dilated cardiomyopathy in children. Pediatr. Cardiol. 2005;26:361–366. doi: 10.1007/s00246-004-0742-1. [DOI] [PubMed] [Google Scholar]
- 126.Caso G., Kelly P., McNurlan M.A., Lawson W.E. Effect of coenzyme Q10 on myopathic symptoms in patients treated with statins. Am. J. Cardiol. 2007;99:1409–1412. doi: 10.1016/j.amjcard.2006.12.063. [DOI] [PubMed] [Google Scholar]
- 127.Kocharian A., Shabanian R., Rafiei-Khorgami M., Kiani A., Heidari-Bateni G. Coenzyme Q10 improves diastolic function in children with idiopathic dilated cardiomyopathy. Cardiol. Young. 2009;19:501–506. doi: 10.1017/S1047951109990795. [DOI] [PubMed] [Google Scholar]
- 128.Shargorodsky M., Debby O., Matas Z., Zimlichman R. Effect of long-term treatment with antioxidants (vitamin C, vitamin E, coenzyme Q10 and selenium) on arterial compliance, humoral factors and inflammatory markers in patients with multiple cardiovascular risk factors. Nutr. Metab. 2010;7:55. doi: 10.1186/1743-7075-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Judy W.V., Stogsdill W.W., Folkers K. Myocardial preservation by therapy with coenzyme Q10 during heart surgery. Clin. Investig. 1993;71:S155–S161. doi: 10.1007/BF00226859. [DOI] [PubMed] [Google Scholar]
- 130.Taggart D.P., Jenkins M., Hooper J., Hadjinikolas L., Kemp M., Hue D., Bennett G. Effects of short-term supplementation with coenzyme Q10 on myocardial protection during cardiac operations. Ann. Thorac. Surg. 1996;61:829–833. doi: 10.1016/0003-4975(95)01120-X. [DOI] [PubMed] [Google Scholar]
- 131.Chello M., Mastroroberto P., Romano R., Castaldo P., Bevacqua E., Marchese A.R. Protection by coenzyme Q10 of tissue reperfusion injury during abdominal aortic cross-clamping. J. Cardiovasc. Surg. 1996;37:229–235. [PubMed] [Google Scholar]
- 132.Zhou M., Zhi Q., Tang Y., Yu D., Han J. Effects of coenzyme Q10 on myocardial protection during cardiac valve replacement and scavenging free radical activity in vitro. J. Cardiovasc. Surg. 1999;40:355–361. [PubMed] [Google Scholar]
- 133.Rosenfeldt F., Marasco S., Lyon W., Wowk M., Sheeran F., Bailey M., Esmore D., Davis B., Pick A., Rabinov M., et al. Coenzyme Q10 therapy before cardiac surgery improves mitochondrial function and in vitro contractility of myocardial tissue. J. Thorac. Cardiovasc. Surg. 2005;129:25–32. doi: 10.1016/j.jtcvs.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 134.Keith M., Mazer C.D., Mikhail P., Jeejeebhoy F., Briet F., Errett L. Coenzyme Q10 in patients undergoing (coronary artery bypass graft) CABG: Effect of statins and nutritional supplementation. Nutr. Metab. Cardiovasc. Dis. 2008;18:105–111. doi: 10.1016/j.numecd.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 135.Makhija N., Sendasgupta C., Kiran U., Lakshmy R., Hote M.P., Choudhary S.K., Airan B., Abraham R. The role of oral coenzyme Q10 in patients undergoing coronary artery bypass graft surgery. J. Cardiothorac. Vasc. Anesth. 2008;22:832–839. doi: 10.1053/j.jvca.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 136.Alehagen U., Johansson P., Björnstedt M., Rosén A., Dahlström U. Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: A 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Int. J. Cardiol. 2013;167:1860–1866. doi: 10.1016/j.ijcard.2012.04.156. [DOI] [PubMed] [Google Scholar]
- 137.Miles M.V., Patterson B.J., Chalfonte-Evans M.L., Horn P.S., Hickey F.J., Schapiro M.B., Steele P.E., Tang P.H., Hotze S.L. Coenzyme Q10 (ubiquinol-10) supplementation improves oxidative imbalance in children with trisomy 21. Pediatr. Neurol. 2007;37:398–403. doi: 10.1016/j.pediatrneurol.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 138.Tiano L., Padella L., Santoro L., Carnevali P., Principi F., Brugè F., Gabrielli O., Littarru G.P. Prolonged coenzyme Q10 treatment in Down syndrome patients: Effect on DNA oxidation. Neurobiol. Aging. 2012;33:626.e1-8. doi: 10.1016/j.neurobiolaging.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 139.Sparaco M., Gaeta L.M., Santorelli F.M., Passarelli C., Tozzi G., Bertini E., Simonati A., Scaravilli F., Taroni F., Duyckaerts C., et al. Friedreich’s ataxia: Oxidative stress and cytoskeletal abnormalities. J. Neurol. Sci. 2009;287:111–118. doi: 10.1016/j.jns.2009.08.052. [DOI] [PubMed] [Google Scholar]
- 140.Cooper J.M., Korlipara L.V., Hart P.E., Bradley J.L., Schapira A.H. Coenzyme Q10 and vitamin E deficiency in Friedreich’s ataxia: Predictor of efficacy of vitamin E and coenzyme Q10 therapy. Eur. J. Neurol. 2008;15:1371–1379. doi: 10.1111/j.1468-1331.2008.02318.x. [DOI] [PubMed] [Google Scholar]
- 141.Hart P.E., Lodi R., Rajagopalan B., Bradley J.L., Crilley J.G., Turner C., Blamire A.M., Manners D., Styles P., Schapira A.H., et al. Antioxidant treatment of patients with Friedreich ataxia: Four-year follow-up. Arch. Neurol. 2005;62:621–626. doi: 10.1001/archneur.62.4.621. [DOI] [PubMed] [Google Scholar]
- 142.Barbiroli B., Frassineti C., Martinelli P., Iotti S., Lodi R., Cortelli P., Montagna P. Coenzyme Q10 improves mitochondrial respiration in patients with mitochondrial cytopathies. An in vivo study on brain and skeletal muscle by phosphorous magnetic resonance spectroscopy. Cell. Mol. Biol. 1997;43:741–749. [PubMed] [Google Scholar]
- 143.Abe K., Matsuo Y., Kadekawa J., Inoue S., Yanagihara T. Effect of coenzyme Q10 in patients with mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS): Evaluation by noninvasive tissue oximetry. J. Neurol. Sci. 1999;162:65–68. doi: 10.1016/S0022-510X(98)00296-2. [DOI] [PubMed] [Google Scholar]
- 144.Hanisch F., Zierz S. Only transient increase of serum CoQ subset 10 during long-term CoQ10 therapy in mitochondrial ophthalmoplegia. Eur. J. Med. Res. 2003;8:485–491. [PubMed] [Google Scholar]
- 145.Glover E.I., Martin J., Maher A., Thornhill R.E., Moran G.R., Tarnopolsky M.A. A randomized trial of coenzyme Q10 in mitochondrial disorders. Muscle Nerve. 2010;42:739–748. doi: 10.1002/mus.21758. [DOI] [PubMed] [Google Scholar]
- 146.Huntington Study Group A randomized, placebo-controlled trial of coenzyme Q10 and remacemide in Huntington’s disease. Neurology. 2001;57:397–404. doi: 10.1212/wnl.57.3.397. [DOI] [PubMed] [Google Scholar]
- 147.Huntington Study Group Pre2CARE Investigators. Hyson H.C., Kieburtz K., Shoulson I., McDermott M., Ravina B., de Blieck E.A., Cudkowicz M.E., Ferrante R.J., Como P., et al. Safety and tolerability of high-dosage coenzyme Q10 in Huntington’s disease and healthy subjects. Mov. Disord. 2010;25:1924–1928. doi: 10.1002/mds.22408. [DOI] [PubMed] [Google Scholar]
- 148.Spurney C.F., Rocha C.T., Henricson E., Florence J., Mayhew J., Gorni K., Pasquali L., Pestronk A., Martin G.R., Hu F., et al. CINRG pilot trial of coenzyme Q10 in steroid-treated Duchenne muscular dystrophy. Muscle Nerve. 2011;44:174–178. doi: 10.1002/mus.22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Folkers K., Wolaniuk J., Simonsen R., Morishita M., Vadhanavikit S. Biochemical rationale and the cardiac response of patients with muscle disease to therapy with coenzyme Q10. Proc. Natl. Acad. Sci. USA. 1985;82:4513–4516. doi: 10.1073/pnas.82.13.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Folkers K., Simonsen R. Two successful double-blind trials with coenzyme Q10 (vitamin Q10) on muscular dystrophies and neurogenic atrophies. Biochim. Biophys. Acta. 1995;1271:281–286. doi: 10.1016/0925-4439(95)00040-B. [DOI] [PubMed] [Google Scholar]
- 151.Linnane A.W., Kopsidas G., Zhang C., Yarovaya N., Kovalenko S., Papakostopoulos P., Eastwood H., Graves S., Richardson M. Cellular redox activity of coenzyme Q10: Effect of CoQ10 supplementation on human skeletal muscle. Free Radic. Res. 2002;36:445–453. doi: 10.1080/10715760290021306. [DOI] [PubMed] [Google Scholar]
- 152.Shults C.W., Oakes D., Kieburtz K., Beal M.F., Haas R., Plumb S., Juncos J.L., Nutt J., Shoulson I., Carter J., et al. Effects of coenzyme Q10 in early Parkinson disease: Evidence of slowing of the functional decline. Arch. Neurol. 2002;59:1541–1550. doi: 10.1001/archneur.59.10.1541. [DOI] [PubMed] [Google Scholar]
- 153.Müller T., Büttner T., Gholipour A.F., Kuhn W. Coenzyme Q10 supplementation provides mild symptomatic benefit in patients with Parkinson’s disease. Neurosci. Lett. 2003;341:201–204. doi: 10.1016/S0304-3940(03)00185-X. [DOI] [PubMed] [Google Scholar]
- 154.Storch A., Jost W.H., Vieregge P., Spiegel J., Greulich W., Durner J., Müller T., Kupsch A., Henningsen H., Oertel W.H., et al. Randomized, double-blind, placebo-controlled trial on symptomatic effects of coenzyme Q10 in Parkinson disease. Arch. Neurol. 2007;64:938–944. doi: 10.1001/archneur.64.7.nct60005. [DOI] [PubMed] [Google Scholar]
- 155.Sanoobar M., Eghtesadi S., Azimi A., Khalili M., Jazayeri S., Reza Gohari M. Coenzyme Q10 supplementation reduces oxidative stress and increases antioxidant enzyme activity in patients with relapsing-remitting multiple sclerosis. Int. J. Neurosci. 2013;123:776–782. doi: 10.3109/00207454.2013.801844. [DOI] [PubMed] [Google Scholar]
- 156.Sanoobar M., Eghtesadi S., Azimi A., Khalili M., Khodadadi B., Jazayeri S., Gohari M.R., Aryaeian N. Coenzyme Q10 supplementation ameliorates inflammatory markers in patients with multiple sclerosis: A double blind, placebo, controlled randomized clinical trial. Nutr. Neurosci. 2014 doi: 10.1179/1476830513Y.0000000106. [DOI] [PubMed] [Google Scholar]
- 157.Cordero M.D., Cano-García F.J., Alcocer-Gómez E., de Miguel M., Sánchez-Alcázar J.A. Oxidative stress correlates with headache symptoms in fibromyalgia: Coenzyme Q10 effect on clinical improvement. PLoS One. 2012;7:e35677. doi: 10.1371/journal.pone.0035677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cordero M.D., Alcocer-Gómez E., de Miguel M., Culic O., Carrión A.M., Alvarez-Suarez J.M., Bullón P., Battino M., Fernández-Rodríguez A., Sánchez-Alcazar J.A. Can coenzyme q10 improve clinical and molecular parameters in fibromyalgia? Antioxid. Redox Signal. 2013;19:1356–1361. doi: 10.1089/ars.2013.5260. [DOI] [PubMed] [Google Scholar]
- 159.Miyamae T., Seki M., Naga T., Uchino S., Asazuma H., Yoshida T., Iizuka Y., Kikuchi M., Imagawa T., Natsumeda Y., et al. Increased oxidative stress and coenzyme Q10 deficiency in juvenile fibromyalgia: Amelioration of hypercholesterolemia and fatigue by ubiquinol-10 supplementation. Redox Rep. 2013;18:12–19. doi: 10.1179/1351000212Y.0000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lister R.E. An open, pilot study to evaluate the potential benefits of coenzyme Q10 combined with Ginkgo biloba extract in fibromyalgia syndrome. J. Int. Med. Res. 2002;30:195–199. doi: 10.1177/147323000203000213. [DOI] [PubMed] [Google Scholar]
- 161.Kaufmann P., Thompson J.L., Levy G., Buchsbaum R., Shefner J., Krivickas L.S., Katz J., Rollins Y., Barohn R.J., Jackson C.E., et al. Phase II trial of CoQ10 for ALS finds insufficient evidence to justify phase III. Ann. Neurol. 2009;66:235–244. doi: 10.1002/ana.21743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rozen T.D., Oshinsky M.L., Gebeline C.A., Bradley K.C., Young W.B., Shechter A.L., Silberstein S.D. Open label trial of coenzyme Q10 as a migraine preventive. Cephalalgia. 2002;22:137–141. doi: 10.1046/j.1468-2982.2002.00335.x. [DOI] [PubMed] [Google Scholar]
- 163.Hershey A.D., Powers S.W., Vockell A.L., Lecates S.L., Ellinor P.L., Segers A., Burdine D., Manning P., Kabbouche M.A. Coenzyme Q10 deficiency and response to supplementation in pediatric and adolescent migraine. Headache. 2007;47:73–80. doi: 10.1111/j.1526-4610.2007.00652.x. [DOI] [PubMed] [Google Scholar]
- 164.Slater S.K., Nelson T.D., Kabbouche M.A., LeCates S.L., Horn P., Segers A., Manning P., Powers S.W., Hershey A.D. A randomized, double-blinded, placebo-controlled, crossover, add-on study of CoEnzyme Q10 in the prevention of pediatric and adolescent migraine. Cephalalgia. 2011;31:897–905. doi: 10.1177/0333102411406755. [DOI] [PubMed] [Google Scholar]
- 165.Forester B.P., Zuo C.S., Ravichandran C., Harper D.G., Du F., Kim S., Cohen B.M., Renshaw P.F. Coenzyme Q10 effects on creatine kinase activity and mood in geriatric bipolar depression. J. Geriatr. Psychiatry Neurol. 2012;25:43–50. doi: 10.1177/0891988712436688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Stamelou M., Reuss A., Pilatus U., Magerkurth J., Niklowitz P., Eggert K.M., Krisp A., Menke T., Schade-Brittinger C., Oertel W.H., et al. Short-term effects of coenzyme Q10 in progressive supranuclear palsy: A randomized, placebo-controlled trial. Mov. Disord. 2008;23:942–949. doi: 10.1002/mds.22023. [DOI] [PubMed] [Google Scholar]
- 167.Watts G.F., Playford D.A., Croft K.D., Ward N.C., Mori T.A., Burke V. Coenzyme Q10 improves endothelial dysfunction of the brachial artery in type II diabetes mellitus. Diabetologia. 2002;45:420–426. doi: 10.1007/s00125-001-0760-y. [DOI] [PubMed] [Google Scholar]
- 168.Hodgson J.M., Watts G.F., Playford D.A., Burke V., Croft K.D. Coenzyme Q10 improves blood pressure and glycaemic control: A controlled trial in subjects with type 2 diabetes. Eur. J. Clin. Nutr. 2002;56:1137–1142. doi: 10.1038/sj.ejcn.1601464. [DOI] [PubMed] [Google Scholar]
- 169.Playford D.A., Watts G.F., Croft K.D., Burke V. Combined effect of coenzyme Q10 and fenofibrate on forearm microcirculatory function in type 2 diabetes. Atherosclerosis. 2003;168:169–179. doi: 10.1016/S0021-9150(02)00417-3. [DOI] [PubMed] [Google Scholar]
- 170.Chew G.T., Watts G.F., Davis T.M., Stuckey B.G., Beilin L.J., Thompson P.L., Burke V., Currie P.J. Hemodynamic effects of fenofibrate and coenzyme Q10 in type 2 diabetic subjects with left ventricular diastolic dysfunction. Diabetes Care. 2008;31:1502–1509. doi: 10.2337/dc08-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hamilton S.J., Chew G.T., Watts G.F. Coenzyme Q10 improves endothelial dysfunction in statin-treated type 2 diabetic patients. Diabetes Care. 2009;32:810–812. doi: 10.2337/dc08-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kolahdouz Mohammadi R., Hosseinzadeh-Attar M.J., Eshraghian M.R., Nakhjavani M., Khorami E., Esteghamati A. The effect of coenzyme Q10 supplementation on metabolic status of type 2 diabetic patients. Minerva Gastroenterol. Dietol. 2013;59:231–236. [PubMed] [Google Scholar]
- 173.Suzuki S., Hinokio Y., Ohtomo M., Hirai M., Hirai A., Chiba M., Kasuga S., Satoh Y., Akai H., Toyota T. The effects of coenzyme Q10 treatment on maternally inherited diabetes mellitus and deafness, and mitochondrial DNA 3243 (A to G) mutation. Diabetologia. 1998;41:584–588. doi: 10.1007/s001250050950. [DOI] [PubMed] [Google Scholar]
- 174.Henriksen J.E., Andersen C.B., Hother-Nielsen O., Vaag A., Mortensen S.A., Beck-Nielsen H. Impact of ubiquinone (coenzyme Q10) treatment on glycaemic control, insulin requirement and well-being in patients with type 1 diabetes mellitus. Diabet. Med. 1999;16:312–318. doi: 10.1046/j.1464-5491.1999.00064.x. [DOI] [PubMed] [Google Scholar]
- 175.Hertz N., Lister R.E. Improved survival in patients with end-stage cancer treated with coenzyme Q10 and other antioxidants: A pilot study. J. Int. Med. Res. 2009;37:1961–1971. doi: 10.1177/147323000903700634. [DOI] [PubMed] [Google Scholar]
- 176.Lockwood K., Moesgaard S., Folkers K. Partial and complete regression of breast cancer in patients in relation to dosage of coenzyme Q10. Biochem. Biophys. Res. Commun. 1994;199:1504–1508. doi: 10.1006/bbrc.1994.1401. [DOI] [PubMed] [Google Scholar]
- 177.Premkumar V.G., Yuvaraj S., Sathish S., Shanthi P., Sachdanandam P. Anti-angiogenic potential of Coenzyme Q10, riboflavin and niacin in breast cancer patients undergoing tamoxifen therapy. Vascul. Pharmacol. 2008;48:191–201. doi: 10.1016/j.vph.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 178.Lesser G.J., Case D., Stark N., Williford S., Giguere J., Garino L.A., Naughton M.J., Vitolins M.Z., Lively M.O., Shaw E.G., et al. A randomized, double-blind, placebo-controlled study of oral coenzyme Q10 to relieve self-reported treatment-related fatigue in newly diagnosed patients with breast cancer. J. Support Oncol. 2013;11:31–42. doi: 10.1016/j.suponc.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hoenjet K.M., Dagnelie P.C., Delaere K.P., Wijckmans N.E., Zambon J.V., Oosterhof G.O. Effect of a nutritional supplement containing vitamin E, selenium, vitamin c and coenzyme Q10 on serum PSA in patients with hormonally untreated carcinoma of the prostate: A randomised placebo-controlled study. Eur. Urol. 2005;47:433–439. doi: 10.1016/j.eururo.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 180.Rusciani L., Proietti I., Paradisi A., Rusciani A., Guerriero G., Mammone A., de Gaetano A., Lippa S. Recombinant interferon alpha-2b and coenzyme Q10 as a postsurgical adjuvant therapy for melanoma: A 3-year trial with recombinant interferon-alpha and 5-year follow-up. Melanoma Res. 2007;17:177–183. doi: 10.1097/CMR.0b013e32818867a0. [DOI] [PubMed] [Google Scholar]
- 181.Gazdíková K., Gvozdjáková A., Kucharská J., Spustová V., Braunová Z., Dzúrik R. Effect of coenzyme Q10 in patients with kidney diseases. Cas. Lek. Cesk. 2001;140:307–310. [PubMed] [Google Scholar]
- 182.Sakata T., Furuya R., Shimazu T., Odamaki M., Ohkawa S., Kumagai H. Coenzyme Q10 administration suppresses both oxidative and antioxidative markers in hemodialysis patients. Blood Purif. 2008;26:371–378. doi: 10.1159/000135605. [DOI] [PubMed] [Google Scholar]
- 183.Mori T.A., Burke V., Puddey I., Irish A., Cowpland C.A., Beilin L., Dogra G., Watts G.F. The effects of omega-3 fatty acids and coenzyme Q10 on blood pressure and heart rate in chronic kidney disease: A randomized controlled trial. J. Hypertens. 2009;27:1863–1872. doi: 10.1097/HJH.0b013e32832e1bd9. [DOI] [PubMed] [Google Scholar]
- 184.Shojaei M., Djalali M., Khatami M., Siassi F., Eshraghian M. Effects of carnitine and coenzyme Q10 on lipid profile and serum levels of lipoprotein(a) in maintenance hemodialysis patients on statin therapy. Iran J. Kidney Dis. 2011;5:114–118. [PubMed] [Google Scholar]
- 185.Kaikkonen J., Nyyssönen K., Tomasi A., Iannone A., Tuomainen T.P., Porkkala-Sarataho E., Salonen J.T. Antioxidative efficacy of parallel and combined supplementation with coenzyme Q10 and d-alpha-tocopherol in mildly hypercholesterolemic subjects: A randomized placebo-controlled clinical study. Free Radic. Res. 2000;33:329–340. doi: 10.1080/10715760000301501. [DOI] [PubMed] [Google Scholar]
- 186.Lee Y.J., Cho W.J., Kim J.K., Lee D.C. Effects of coenzyme Q10 on arterial stiffness, metabolic parameters, and fatigue in obese subjects: A double-blind randomized controlled study. J. Med. Food. 2011;14:386–390. doi: 10.1089/jmf.2010.1202. [DOI] [PubMed] [Google Scholar]
- 187.Young J.M., Florkowski C.M., Molyneux S.L., McEwan R.G., Frampton C.M., Nicholls M.G., Scott R.S., George P.M. A randomized, double-blind, placebo-controlled crossover study of coenzyme Q10 therapy in hypertensive patients with the metabolic syndrome. Am. J. Hypertens. 2012;25:261–270. doi: 10.1038/ajh.2011.209. [DOI] [PubMed] [Google Scholar]
- 188.Mabuchi H., Nohara A., Kobayashi J., Kawashiri M.A., Katsuda S., Inazu A., Koizumi J., Hokuriku Lipid Research Group Effects of CoQ10 supplementation on plasma lipoprotein lipid, CoQ10 and liver and muscle enzyme levels in hypercholesterolemic patients treated with atorvastatin: A randomized double-blind study. Atherosclerosis. 2007;195:e182–e189. doi: 10.1016/j.atherosclerosis.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 189.Young J.M., Florkowski C.M., Molyneux S.L., McEwan R.G., Frampton C.M., George P.M., Scott R.S. Effect of coenzyme Q10 supplementation on simvastatin-induced myalgia. Am. J. Cardiol. 2007;100:1400–1403. doi: 10.1016/j.amjcard.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 190.Bookstaver D.A., Burkhalter N.A., Hatzigeorgiou C. Effect of coenzyme Q10 supplementation on statin-induced myalgias. Am. J. Cardiol. 2012;110:526–529. doi: 10.1016/j.amjcard.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 191.Zlatohlavek L., Vrablik M., Grauova B., Motykova E., Ceska R. The effect of coenzyme Q10 in statin myopathy. Neuro Endocrinol. Lett. 2012;33:98–101. [PubMed] [Google Scholar]
- 192.Fedacko J., Pella D., Fedackova P., Hänninen O., Tuomainen P., Jarcuska P., Lopuchovsky T., Jedlickova L., Merkovska L., Littarru G.P. Coenzyme Q10 and selenium in statin-associated myopathy treatment. Can. J. Physiol. Pharmacol. 2013;91:165–170. doi: 10.1139/cjpp-2012-0118. [DOI] [PubMed] [Google Scholar]
- 193.Gvozdjáková A., Kucharská J., Bartkovjaková M., Gazdíková K., Gazdík F.E. Coenzyme Q10 supplementation reduces corticosteroids dosage in patients with bronchial asthma. Biofactors. 2005;25:235–240. doi: 10.1002/biof.5520250129. [DOI] [PubMed] [Google Scholar]
- 194.Teran E., Hernandez I., Nieto B., Tavara R., Ocampo J.E., Calle A. Coenzyme Q10 supplementation during pregnancy reduces the risk of pre-eclampsia. Int. J. Gynaecol. Obstet. 2009;105:43–45. doi: 10.1016/j.ijgo.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 195.De Luca C., Kharaeva Z., Raskovic D., Pastore P., Luci A., Korkina L. Coenzyme Q10, vitamin E, selenium, and methionine in the treatment of chronic recurrent viral mucocutaneous infections. Nutrition. 2012;28:509–514. doi: 10.1016/j.nut.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 196.Kharaeva Z., Gostova E., de Luca C., Raskovic D., Korkina L. Clinical and biochemical effects of coenzyme Q10, vitamin E, and selenium supplementation to psoriasis patients. Nutrition. 2009;25:295–302. doi: 10.1016/j.nut.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 197.Fogagnolo P., Sacchi M., Ceresara G., Paderni R., Lapadula P., Orzalesi N., Rossetti L. The effects of topical coenzyme Q10 and vitamin E d-α-tocopheryl polyethylene glycol 1000 succinate after cataract surgery: A clinical and in vivo confocal study. Ophthalmologica. 2013;229:26–31. doi: 10.1159/000342196. [DOI] [PubMed] [Google Scholar]
- 198.Safarinejad M.R., Safarinejad S., Shafiei N., Safarinejad S. Effects of the reduced form of coenzyme Q10 (ubiquinol) on semen parameters in men with idiopathic infertility: A double-blind, placebo controlled, randomized study. J. Urol. 2012;188:526–531. doi: 10.1016/j.juro.2012.03.131. [DOI] [PubMed] [Google Scholar]
- 199.Gilbert E.F. Carnitine deficiency. Pathology. 1985;17:161–171. doi: 10.3109/00313028509063752. [DOI] [PubMed] [Google Scholar]
- 200.Siliprandi N., Siliprandi D., Ciman M. Stimulation of oxidation of mitochondrial fatty acids and of acetate by acetylcarnitine. Biochem. J. 1965;96:777–780. doi: 10.1042/bj0960777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Liu J., Head E., Kuratsune H., Cotman C.W., Ames B.N. Comparison of the effects of l-carnitine and acetyl-l-carnitine on carnitine levels, ambulatory activity, and oxidative stress biomarkers in the brain of old rats. Ann. N. Y. Acad. Sci. 2004;1033:117–131. doi: 10.1196/annals.1320.011. [DOI] [PubMed] [Google Scholar]
- 202.Musicco C., Capelli V., Pesce V., Timperio A.M., Calvani M., Mosconi L., Cantatore P., Gadaleta M.N. Rat liver mitochondrial proteome: Changes associated with aging and acetyl-l-carnitine treatment. J. Proteomics. 2011;74:2536–2547. doi: 10.1016/j.jprot.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 203.Pesce V., Nicassio L., Fracasso F., Musicco C., Cantatore P., Gadaleta M.N. Acetyl-l-carnitine activates the peroxisome proliferator-activated receptor-γ coactivators PGC-1α/PGC-1β-dependent signaling cascade of mitochondrial biogenesis and decreases the oxidized peroxiredoxins content in old rat liver. Rejuvenation Res. 2012;15:136–139. doi: 10.1089/rej.2011.1255. [DOI] [PubMed] [Google Scholar]
- 204.Bertoli M., Battistella P.A., Vergani L., Naso A., Gasparotto M.L., Romagnoli G.F., Angelini C. Carnitine deficiency induced during hemodialysis and hyperlipidemia: Effect of replacement therapy. Am. J. Clin. Nutr. 1981;34:1496–1500. doi: 10.1093/ajcn/34.8.1496. [DOI] [PubMed] [Google Scholar]
- 205.Kletzmayr J., Mayer G., Legenstein E., Heinz-Peer G., Leitha T., Hörl W.H., Kovarik J. Anemia and carnitine supplementation in hemodialyzed patients. Kidney Int. Suppl. 1999;69:S93–S106. doi: 10.1038/sj.ki.4490857. [DOI] [PubMed] [Google Scholar]
- 206.Thomas S., Fischer F.P., Mettang T., Pauli-Magnus C., Weber J., Kuhlmann U. Effects of l-carnitine on leukocyte function and viability in hemodialysis patients: A double-blind randomized trial. Am. J. Kidney Dis. 1999;34:678–687. doi: 10.1016/S0272-6386(99)70393-8. [DOI] [PubMed] [Google Scholar]
- 207.Chazot C., Blanc C., Hurot J.M., Charra B., Jean G., Laurent G. Nutritional effects of carnitine supplementation in hemodialysis patients. Clin. Nephrol. 2003;59:24–30. doi: 10.5414/CNP59024. [DOI] [PubMed] [Google Scholar]
- 208.Vaux E.C., Taylor D.J., Altmann P., Rajagopalan B., Graham K., Cooper R., Bonomo Y., Styles P. Effects of carnitine supplementation on muscle metabolism by the use of magnetic resonance spectroscopy and near-infrared spectroscopy in end-stage renal disease. Nephron Clin. Pract. 2004;97:c41–c48. doi: 10.1159/000078399. [DOI] [PubMed] [Google Scholar]
- 209.Steiber A.L., Davis A.T., Spry L., Strong J., Buss M.L., Ratkiewicz M.M., Weatherspoon L.J. Carnitine treatment improved quality-of-life measure in a sample of Midwestern hemodialysis patients. J. Parenter. Enteral. Nutr. 2006;30:10–15. doi: 10.1177/014860710603000110. [DOI] [PubMed] [Google Scholar]
- 210.Signorelli S.S., Fatuzzo P., Rapisarda F., Neri S., Ferrante M., Oliveri Conti G., Fallico R., di Pino L., Pennisi G., Celotta G., et al. A randomised, controlled clinical trial evaluating changes in therapeutic efficacy and oxidative parameters after treatment with propionyl l-carnitine in patients with peripheral arterial disease requiring haemodialysis. Drugs Aging. 2006;23:263–270. doi: 10.2165/00002512-200623030-00008. [DOI] [PubMed] [Google Scholar]
- 211.Rathod R., Baig M.S., Khandelwal P.N., Kulkarni S.G., Gade P.R., Siddiqui S. Results of a single blind, randomized, placebo-controlled clinical trial to study the effect of intravenous l-carnitine supplementation on health-related quality of life in Indian patients on maintenance hemodialysis. Indian J. Med. Sci. 2006;60:143–153. doi: 10.4103/0019-5359.24678. [DOI] [PubMed] [Google Scholar]
- 212.Duranay M., Akay H., Yilmaz F.M., Senes M., Tekeli N., Yücel D. Effects of l-carnitine infusions on inflammatory and nutritional markers in haemodialysis patients. Nephrol. Dial. Transplant. 2006;21:3211–3214. doi: 10.1093/ndt/gfl356. [DOI] [PubMed] [Google Scholar]
- 213.Verrina E., Caruso U., Calevo M.G., Emma F., Sorino P., de Palo T., Lavoratti G., Turrini Dertenois L., Cassanello M., Cerone R., et al. Effect of carnitine supplementation on lipid profile and anemia in children on chronic dialysis. Pediatr. Nephrol. 2007;22:727–733. doi: 10.1007/s00467-006-0408-8. [DOI] [PubMed] [Google Scholar]
- 214.Fatouros I.G., Douroudos I., Panagoutsos S., Pasadakis P., Nikolaidis M.G., Chatzinikolaou A., Sovatzidis A., Michailidis Y., Jamurtas A.Z., Mandalidis D., et al. Effects of l-carnitine on oxidative stress responses in patients with renal disease. Med. Sci. Sports Exerc. 2010;42:1809–1818. doi: 10.1249/MSS.0b013e3181dbacab. [DOI] [PubMed] [Google Scholar]
- 215.Hakeshzadeh F., Tabibi H., Ahmadinejad M., Malakoutian T., Hedayati M. Effects of l-carnitine supplement on plasma coagulation and anticoagulation factors in hemodialysis patients. Renal Failure. 2010;32:1109–1114. doi: 10.3109/0886022X.2010.510617. [DOI] [PubMed] [Google Scholar]
- 216.Tabibi H., Hakeshzadeh F., Hedayati M., Malakoutian T. Effects of l-carnitine supplement on serum amyloid A and vascular inflammation markers in hemodialysis patients: A randomized controlled trial. J. Ren. Nutr. 2011;21:485–491. doi: 10.1053/j.jrn.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 217.Suchitra M.M., Ashalatha V.L., Sailaja E., Rao A.M., Reddy V.S., Bitla A.R., Sivakumar V., Rao P.V. The effect of l-carnitine supplementation on lipid parameters, inflammatory and nutritional markers in maintenance hemodialysis patients. Saudi J. Kidney Dis. Transpl. 2011;22:1155–1159. [PubMed] [Google Scholar]
- 218.Naini A.E., Sadeghi M., Mortazavi M., Moghadasi M., Harandi A.A. Oral carnitine supplementation for dyslipidemia in chronic hemodialysis patients. Saudi J. Kidney Dis. Transpl. 2012;23:484–488. [PubMed] [Google Scholar]
- 219.Sgambat K., Frank L., Ellini A., Sable C., Moudgil A. Carnitine supplementation improves cardiac strain rate in children on chronic hemodialysis. Pediatr. Nephrol. 2012;27:1381–1387. doi: 10.1007/s00467-012-2144-6. [DOI] [PubMed] [Google Scholar]
- 220.Mercadal L., Coudert M., Vassault A., Pieroni L., Debure A., Ouziala M., Depreneuf H., Fumeron C., Servais A., Bassilios N., et al. l-Carnitine treatment in incident hemodialysis patients: The multicenter, randomized, double-blinded, placebo-controlled CARNIDIAL trial. Clin. J. Am. Soc. Nephrol. 2012;7:1836–1842. doi: 10.2215/CJN.12431211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Mingrone G., Greco A.V., Capristo E., Benedetti G., Giancaterini A., de Gaetano A., Gasbarrini G. l-Carnitine improves glucose disposal in type 2 diabetic patients. J. Am. Coll. Nutr. 1999;18:77–82. doi: 10.1080/07315724.1999.10718830. [DOI] [PubMed] [Google Scholar]
- 222.Giancaterini A., de Gaetano A., Mingrone G., Gniuli D., Liverani E., Capristo E., Greco A.V. Acetyl-l-carnitine infusion increases glucose disposal in type 2 diabetic patients. Metabolism. 2000;49:704–708. doi: 10.1053/meta.2000.6250. [DOI] [PubMed] [Google Scholar]
- 223.De Grandis D., Minardi C. Acetyl-l-carnitine (levacecarnine) in the treatment of diabetic neuropathy. A long-term, randomised, double-blind, placebo-controlled study. Drugs R&D. 2002;3:223–231. doi: 10.2165/00126839-200203040-00001. [DOI] [PubMed] [Google Scholar]
- 224.Derosa G., Cicero A.F., Gaddi A., Mugellini A., Ciccarelli L., Fogari R. The effect of l-carnitine on plasma lipoprotein(a) levels in hypercholesterolemic patients with type 2 diabetes mellitus. Clin. Ther. 2003;25:1429–1439. doi: 10.1016/S0149-2918(03)80130-3. [DOI] [PubMed] [Google Scholar]
- 225.Ragozzino G., Mattera E., Madrid E., Salomone P., Fasano C., Gioia F., Acerra G., del Guercio R., Federico P. Effects of propionyl-carnitine in patients with type 2 diabetes and peripheral vascular disease: Results of a pilot trial. Drugs R&D. 2004;5:185–190. doi: 10.2165/00126839-200405040-00001. [DOI] [PubMed] [Google Scholar]
- 226.Sima A.A., Calvani M., Mehra M., Amato A. Acetyl-l-Carnitine Study Group. Acetyl-l-carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: An analysis of two randomized placebo-controlled trials. Diabetes Care. 2005;28:89–94. doi: 10.2337/diacare.28.1.89. [DOI] [PubMed] [Google Scholar]
- 227.Rahbar A.R., Shakerhosseini R., Saadat N., Taleban F., Pordal A., Gollestan B. Effect of l-carnitine on plasma glycemic and lipidemic profile in patients with type II diabetes mellitus. Eur. J. Clin. Nutr. 2005;59:592–596. doi: 10.1038/sj.ejcn.1602109. [DOI] [PubMed] [Google Scholar]
- 228.Solfrizzi V., Capurso C., Colacicco A.M., D’Introno A., Fontana C., Capurso S.A., Torres F., Gadaleta A.M., Koverech A., Capurso A., et al. Efficacy and tolerability of combined treatment with l-carnitine and simvastatin in lowering lipoprotein(a) serum levels in patients with type 2 diabetes mellitus. Atherosclerosis. 2006;188:455–461. doi: 10.1016/j.atherosclerosis.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 229.Signorelli S.S., Neri S., di Pino L., Marchese G., Ferrante M., Oliveri Conti G., Fallico R., Celotta G., Pennisi G., Anzaldi M. Effect of PLC on functional parameters and oxidative profile in type 2 diabetes-associated PAD. Diabetes Res. Clin. Pract. 2006;72:231–237. doi: 10.1016/j.diabres.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 230.Malaguarnera M., Vacante M., Avitabile T., Malaguarnera M., Cammalleri L., Motta M. l-Carnitine supplementation reduces oxidized LDL cholesterol in patients with diabetes. Am. J. Clin. Nutr. 2009;89:71–76. doi: 10.3945/ajcn.2008.26251. [DOI] [PubMed] [Google Scholar]
- 231.Ruggenenti P., Cattaneo D., Loriga G., Ledda F., Motterlini N., Gherardi G., Orisio S., Remuzzi G. Ameliorating hypertension and insulin resistance in subjects at increased cardiovascular risk: Effects of acetyl-l-carnitine therapy. Hypertension. 2009;54:567–574. doi: 10.1161/HYPERTENSIONAHA.109.132522. [DOI] [PubMed] [Google Scholar]
- 232.Molfino A., Cascino A., Conte C., Ramaccini C., Rossi Fanelli F., Laviano A. Caloric restriction and l-carnitine administration improves insulin sensitivity in patients with impaired glucose metabolism. J. Parenter. Enteral. Nutr. 2010;34:295–299. doi: 10.1177/0148607109353440. [DOI] [PubMed] [Google Scholar]
- 233.Uzun N., Sarikaya S., Uluduz D., Aydin A. Peripheric and automatic neuropathy in children with type 1 diabetes mellitus: The effect of l-carnitine treatment on the peripheral and autonomic nervous system. Electromyogr. Clin. Neurophysiol. 2005;45:343–351. [PubMed] [Google Scholar]
- 234.Loffredo L., Pignatelli P., Cangemi R., Andreozzi P., Panico M.A., Meloni V., Violi F. Imbalance between nitric oxide generation and oxidative stress in patients with peripheral arterial disease: Effect of an antioxidant treatment. J. Vasc. Surg. 2006;44:525–530. doi: 10.1016/j.jvs.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 235.De Marchi S., Zecchetto S., Rigoni A., Prior M., Fondrieschi L., Scuro A., Rulfo F., Arosio E. Propionyl-l-carnitine improves endothelial function, microcirculation and pain management in critical limb ischemia. Cardiovasc. Drugs Ther. 2012;26:401–408. doi: 10.1007/s10557-012-6408-y. [DOI] [PubMed] [Google Scholar]
- 236.Goldenberg N.A., Krantz M.J., Hiatt W.R. l-Carnitine plus cilostazol versus cilostazol alone for the treatment of claudication in patients with peripheral artery disease: A multicenter, randomized, double-blind, placebo-controlled trial. Vasc. Med. 2012;17:145–154. doi: 10.1177/1358863X12442264. [DOI] [PubMed] [Google Scholar]
- 237.Xu X.Q., Jing Z.C., Jiang X., Zhao Q.H., He J., Dai L.Z., Wu W.H., Li Y., Yao J. Clinical efficacy of intravenous l-carnitine in patients with right-sided heart failure induced by pulmonary arterial hypertension. Zhonghua Xin Xue Guan Bing Za Zhi. 2010;38:152–155. [PubMed] [Google Scholar]
- 238.Serati A.R., Motamedi M.R., Emami S., Varedi P., Movahed M.R. l-Carnitine treatment in patients with mild diastolic heart failure is associated with improvement in diastolic function and symptoms. Cardiology. 2010;116:178–182. doi: 10.1159/000318810. [DOI] [PubMed] [Google Scholar]
- 239.Lango R., Smoleński R.T., Rogowski J., Siebert J., Wujtewicz M., Słomińska E.M., Lysiak-Szydłowska W., Yacoub M.H. Propionyl-l-carnitine improves hemodynamics and metabolic markers of cardiac perfusion during coronary surgery in diabetic patients. Cardiovasc. Drugs Ther. 2005;19:267–275. doi: 10.1007/s10557-005-3349-8. [DOI] [PubMed] [Google Scholar]
- 240.Xiang D., Sun Z., Xia J., Dong N., Du X., Chen X. Effect of l-carnitine on cardiomyocyte apoptosis and cardiac function in patients undergoing heart valve replacement operation. J. Huazhong Univ. Sci. Technol. Med. Sci. 2005;25:501–504. doi: 10.1007/BF02895999. [DOI] [PubMed] [Google Scholar]
- 241.Łapiński T.W., Grzeszczuk A. The impact of carnitine on serum ammonia concentration and lipid metabolism in patients with alcoholic liver cirrhosis. Pol. Merkur. Lekarski. 2003;15:38–41. [PubMed] [Google Scholar]
- 242.Lim C.Y., Jun D.W., Jang S.S., Cho W.K., Chae J.D., Jun J.H. Effects of carnitine on peripheral blood mitochondrial DNA copy number and liver function in non-alcoholic fatty liver disease. Korean J. Gastroenterol. 2010;55:384–389. doi: 10.4166/kjg.2010.55.6.384. [DOI] [PubMed] [Google Scholar]
- 243.Malaguarnera M., Pistone G., Astuto M., dell’Arte S., Finocchiaro G., lo Giudice E., Pennisi G. l-Carnitine in the treatment of mild or moderate hepatic encephalopathy. Dig. Dis. 2003;21:271–275. doi: 10.1159/000073347. [DOI] [PubMed] [Google Scholar]
- 244.Malaguarnera M., Vacante M., Giordano M., Motta M., Bertino G., Pennisi M., Neri S., Malaguarnera M., Li Volti G., Galvano F. l-Carnitine supplementation improves hematological pattern in patients affected by HCV treated with Peg interferon-α 2b plus ribavirin. World J. Gastroenterol. 2011;17:4414–4420. doi: 10.3748/wjg.v17.i39.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Neri S., Pistone G., Saraceno B., Pennisi G., Luca S., Malaguarnera M. l-Carnitine decreases severity and type of fatigue induced by interferon-alpha in the treatment of patients with hepatitis C. Neuropsychobiology. 2003;47:94–97. doi: 10.1159/000070016. [DOI] [PubMed] [Google Scholar]
- 246.Malaguarnera M., Vacante M., Giordano M., Pennisi G., Bella R., Rampello L., Malaguarnera M., Li Volti G., Galvano F. Oral acetyl-l-carnitine therapy reduces fatigue in overt hepatic encephalopathy: A randomized, double-blind, placebo-controlled study. Am. J. Clin. Nutr. 2011;93:799–808. doi: 10.3945/ajcn.110.007393. [DOI] [PubMed] [Google Scholar]
- 247.Tomassini V., Pozzilli C., Onesti E., Pasqualetti P., Marinelli F., Pisani A., Fieschi C. Comparison of the effects of acetyl l-carnitine and amantadine for the treatment of fatigue in multiple sclerosis: Results of a pilot, randomised, double-blind, crossover trial. J. Neurol. Sci. 2004;218:103–108. doi: 10.1016/j.jns.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 248.Gavrilova S.I., Kalyn IaB., Kolykhalov I.V., Roshchina I.F., Selezneva N.D. Acetyl-l-carnitine (carnicetine) in the treatment of early stages of Alzheimer's disease and vascular dementia. Zh. Nevrol. Psikhiatr. Im. S. S. Korsakova. 2011;111:16–22. [PubMed] [Google Scholar]
- 249.Tarighat Esfanjani A., Mahdavi R., Ebrahimi Mameghani M., Talebi M., Nikniaz Z., Safaiyan A. The effects of magnesium, l-carnitine, and concurrent magnesium-l-carnitine supplementation in migraine prophylaxis. Biol. Trace Elem. Res. 2012;150:42–48. doi: 10.1007/s12011-012-9487-5. [DOI] [PubMed] [Google Scholar]
- 250.Pistone G., Marino A., Leotta C., dell’Arte S., Finocchiaro G., Malaguarnera M. Levocarnitine administration in elderly subjects with rapid muscle fatigue: Effect on body composition, lipid profile and fatigue. Drugs Aging. 2003;20:761–767. doi: 10.2165/00002512-200320100-00004. [DOI] [PubMed] [Google Scholar]
- 251.Miyagawa T., Kawamura H., Obuchi M., Ikesaki A., Ozaki A., Tokunaga K., Inoue Y., Honda M. Effects of oral l-carnitine administration in narcolepsy patients: A randomized, double-blind, cross-over and placebo-controlled trial. PLoS One. 2013;8:e53707. doi: 10.1371/journal.pone.0053707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Rossini M., di Munno O., Valentini G., Bianchi G., Biasi G., Cacace E., Malesci D., la Montagna G., Viapiana O., Adami S. Double-blind, multicenter trial comparing acetyl l-carnitine with placebo in the treatment of fibromyalgia patients. Clin. Exp. Rheumatol. 2007;25:182–188. [PubMed] [Google Scholar]
- 253.Vermeulen R.C., Scholte H.R. Exploratory open label, randomized study of acetyl- and propionyl-carnitine in chronic fatigue syndrome. Psychosom. Med. 2004;66:276–282. doi: 10.1097/01.psy.0000116249.60477.e9. [DOI] [PubMed] [Google Scholar]
- 254.Beghi E., Pupillo E., Bonito V., Buzzi P., Caponnetto C., Chiò A., Corbo M., Giannini F., Inghilleri M., Bella V.L., et al. Randomized double-blind placebo-controlled trial of acetyl-l-carnitine for ALS. Amyotroph. Lateral Scler. Frontotemporal Degener. 2013;14:397–405. doi: 10.3109/21678421.2013.764568. [DOI] [PubMed] [Google Scholar]
- 255.Cruciani R.A., Dvorkin E., Homel P., Malamud S., Culliney B., Lapin J., Portenoy R.K., Esteban-Cruciani N. Safety, tolerability and symptom outcomes associated with l-carnitine supplementation in patients with cancer, fatigue, and carnitine deficiency: A phase I/II study. J. Pain Symptom Manag. 2006;32:551–559. doi: 10.1016/j.jpainsymman.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 256.Cruciani R.A., Dvorkin E., Homel P., Culliney B., Malamud S., Lapin J., Portenoy R.K., Esteban-Cruciani N. l-Carnitine supplementation in patients with advanced cancer and carnitine deficiency: A double-blind, placebo-controlled study. J. Pain Symptom Manag. 2009;37:622–631. doi: 10.1016/j.jpainsymman.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 257.Cruciani R.A., Zhang J.J., Manola J., Cella D., Ansari B., Fisch M.J. l-Carnitine supplementation for the management of fatigue in patients with cancer: An eastern cooperative oncology group phase III, randomized, double-blind, placebo-controlled trial. J. Clin. Oncol. 2012;30:3864–3869. doi: 10.1200/JCO.2011.40.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Mantovani G. Randomised phase III clinical trial of 5 different arms of treatment on 332 patients with cancer cachexia. Eur. Rev. Med. Pharmacol. Sci. 2010;14:292–301. [PubMed] [Google Scholar]
- 259.Madeddu C., Dessì M., Panzone F., Serpe R., Antoni G., Cau M.C., Montaldo L., Mela Q., Mura M., Astara G., et al. Randomized phase III clinical trial of a combined treatment with carnitine + celecoxib ± megestrol acetate for patients with cancer-related anorexia/cachexia syndrome. Clin. Nutr. 2012;31:176–182. doi: 10.1016/j.clnu.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 260.Kraft M., Kraft K., Gärtner S., Mayerle J., Simon P., Weber E., Schütte K., Stieler J., Koula-Jenik H., Holzhauer P., et al. l-Carnitine-supplementation in advanced pancreatic cancer (CARPAN) a randomized multicentre trial. Nutr. J. 2012;11:52. doi: 10.1186/1475-2891-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Hershman D.L., Unger J.M., Crew K.D., Minasian L.M., Awad D., Moinpour C.M., Hansen L., Lew D.L., Greenlee H., Fehrenbacher L., et al. Randomized double-blind placebo-controlled trial of acetyl-l-carnitine for the prevention of taxane-induced neuropathy in women undergoing adjuvant breast cancer therapy. J. Clin. Oncol. 2013;31:2627–2633. doi: 10.1200/JCO.2012.44.8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Schöls L., Zange J., Abele M., Schillings M., Skipka G., Kuntz-Hehner S., van Beekvelt M.C., Colier W.N., Müller K., Klockgether T., et al. l-Carnitine and creatine in Friedreich’s ataxia. A randomized, placebo-controlled crossover trial. J. Neural Transm. 2005;112:789–796. doi: 10.1007/s00702-004-0216-x. [DOI] [PubMed] [Google Scholar]
- 263.El-Beshlawy A., Ragab L., Fattah A.A., Ibrahim I.Y., Hamdy M., Makhlouf A., Aoun E., Hoffbrand V., Taher A. Improvement of cardiac function in thalassemia major treated with l-carnitine. Acta Haematol. 2004;111:143–148. doi: 10.1159/000076522. [DOI] [PubMed] [Google Scholar]
- 264.Karimi M., Mohammadi F., Behmanesh F., Samani S.M., Borzouee M., Amoozgar H., Haghpanah S. Effect of combination therapy of hydroxyurea with l-carnitine and magnesium chloride on hematologic parameters and cardiac function of patients with beta-thalassemia intermedia. Eur. J. Haematol. 2010;84:52–58. doi: 10.1111/j.1600-0609.2009.01356.x. [DOI] [PubMed] [Google Scholar]
- 265.Ellaway C.J., Peat J., Williams K., Leonard H., Christodoulou J. Medium-term open label trial of l-carnitine in Rett syndrome. Brain Dev. 2001;23:S85–S89. doi: 10.1016/S0387-7604(01)00346-1. [DOI] [PubMed] [Google Scholar]
- 266.Torrioli M.G., Vernacotola S., Peruzzi L., Tabolacci E., Mila M., Militerni R., Musumeci S., Ramos F.J., Frontera M., Sorge G., et al. A double-blind, parallel, multicenter comparison of l-acetylcarnitine with placebo on the attention deficit hyperactivity disorder in fragile X syndrome boys. Am. J. Med. Genet. A. 2008;146:803–812. doi: 10.1002/ajmg.a.32268. [DOI] [PubMed] [Google Scholar]
- 267.Geier D.A., Kern J.K., Davis G., King P.G., Adams J.B., Young J.L., Geier M.R. A prospective double-blind, randomized clinical trial of levocarnitine to treat autism spectrum disorders. Med. Sci. Monit. 2011;17:PI15–PI23. doi: 10.12659/MSM.881792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Osio M., Muscia F., Zampini L., Nascimbene C., Mailland E., Cargnel A., Mariani C. Acetyl-l-carnitine in the treatment of painful antiretroviral toxic neuropathy in human immunodeficiency virus patients: An open label study. J. Peripher. Nerv. Syst. 2006;11:72–76. doi: 10.1111/j.1085-9489.2006.00066.x. [DOI] [PubMed] [Google Scholar]
- 269.Youle M., Osio M., ALCAR Study Group A double-blind, parallel-group, placebo-controlled, multicentre study of acetyl l-carnitine in the symptomatic treatment of antiretroviral toxic neuropathy in patients with HIV-1 infection. HIV Med. 2007;8:241–250. doi: 10.1111/j.1468-1293.2007.00467.x. [DOI] [PubMed] [Google Scholar]
- 270.Benedini S., Perseghin G., Terruzzi I., Scifo P., Invernizzi P.L., del Maschio A., Lazzarin A., Luzi L. Effect of l-acetylcarnitine on body composition in HIV-related lipodystrophy. Horm. Metab. Res. 2009;41:840–845. doi: 10.1055/s-0029-1225625. [DOI] [PubMed] [Google Scholar]
- 271.Pignatelli P., Tellan G., Marandola M., Carnevale R., Loffredo L., Schillizzi M., Proietti M., Violi F., Chirletti P., Delogu G. Effect of l-carnitine on oxidative stress and platelet activation after major surgery. Acta Anaesthesiol. Scand. 2011;55:1022–1028. doi: 10.1111/j.1399-6576.2011.02487.x. [DOI] [PubMed] [Google Scholar]
- 272.Mikhailova T.L., Sishkova E., Poniewierka E., Zhidkov K.P., Bakulin I.G., Kupcinskas L., Lesniakowski K., Grinevich V.B., Malecka-Panas E., Ardizzone S., et al. Randomised clinical trial: The efficacy and safety of propionyl-l-carnitine therapy in patients with ulcerative colitis receiving stable oral treatment. Aliment. Pharmacol. Ther. 2011;34:1088–1097. doi: 10.1111/j.1365-2036.2011.04844.x. [DOI] [PubMed] [Google Scholar]
- 273.Mattiazzi M., Vijayvergiya C., Gajewski C.D., deVivo D.C., Lenaz G., Wiedmann M., Manfredi G. The mtDNA T8993G (NARP) mutation results in an impairment of oxidative phosphorylation that can be improved by antioxidants. Hum. Mol. Genet. 2004;13:869–879. doi: 10.1093/hmg/ddh103. [DOI] [PubMed] [Google Scholar]
- 274.Chinnery P.F. Mitochondrial Disorders Overview. Synonyms: Mitochondrial Encephalomyopathies, Mitochondrial Myopathies, Oxidative Phosphorylation Disorders, Respiratory Chain Disorders. [(accessed on 3 November 2014)]; Available online: http://www.ncbi.nlm.nih.gov/books/NBK1224.
- 275.Di Mauro S., Mancuso M. Mitochondrial diseases: Therapeutic approaches. Biosci. Rep. 2007;27:125–137. doi: 10.1007/s10540-007-9041-4. [DOI] [PubMed] [Google Scholar]
- 276.Smith R.A., Murphey M.P. Mitochondria-targeted antioxidants as therapies. Discov. Med. 2011;11:106–114. [PubMed] [Google Scholar]
- 277.Lee S.H., Kim M.J., Kim B.J., Kim S.R., Chun S., Ryu J.S., Kim G.S., Lee M.C., Koh J.M., Chung S.J. Homocysteine-lowering therapy or antioxidant therapy for bone loss in Parkinson’s disease. Mov. Disord. 2010;25:332–340. doi: 10.1002/mds.22866. [DOI] [PubMed] [Google Scholar]
- 278.Pagano G., Aiello Talamanca A., Castello G., d’Ischia M., Pallardó F.V., Petrović S., Porto B., Tiano L., Zatterale A. From clinical description to in vitro and animal studies, and backwards to patients: Oxidative stress and mitochondrial dysfunction in Fanconi anaemia. Free Radic. Biol. Med. 2013;58:118–125. doi: 10.1016/j.freeradbiomed.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 279.Seco-Cervera M., Spis M., García-Giménez J.L. Oxidative stress and antioxidant response in fibroblasts from Werner and Atypical Werner Syndromes. Aging. 2014;6:231–245. doi: 10.18632/aging.100649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Bhagavan H.N., Chopra R.K. Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. Mitochondrion. 2007;7:S78–S88. doi: 10.1016/j.mito.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 281.Roginsky V.A., Tashlitsky V.N., Skulachev V.P. Chain-breaking antioxidant activity of reduced forms of mitochondria-targeted quinones, a novel type of geroprotectors. Aging. 2009;1:481–489. doi: 10.18632/aging.100049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Teichert J., Hermann R., Ruus P., Preiss R. Plasma kinetics, metabolism, and urinary excretion of alpha-lipoic acid following oral administration in healthy volunteers. J. Clin. Pharmacol. 2003;43:1257–1267. doi: 10.1177/0091270003258654. [DOI] [PubMed] [Google Scholar]
- 283.Pettegrew J.W., Levine J., McClure R.J. Acetyl-l-carnitine physical-chemical, metabolic and therapeutic properties: Relevance for its mode of action in Alzheimer’disease and geriatric depression. Mol. Psychiatry. 2000;5:616–632. doi: 10.1038/sj.mp.4000805. [DOI] [PubMed] [Google Scholar]
- 284.Madiraju P., Pande S.V., Prentki M., Murthy Madiraju S.R. Mitochondrial acetylcarnitine provides acetyl groups for nuclear histone acetylation. Epigenetics. 2009;4:4–6. doi: 10.4161/epi.4.6.9767. [DOI] [PubMed] [Google Scholar]
- 285.Rosca M.G., Lemieux H., Hoppel C.L. Mitochondria in the ederly: Is acetylcarnitine a rejuvinator? Adv. Drug Deliv. Rev. 2009;61:1332–1342. doi: 10.1016/j.addr.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Breithaupt-Grögler K., Niebch G., Schneider E., Erb K., Hermann R., Blume H.H., Schug B.S., Belz G.G. Dose-proportionality of oral thioctic acid—Coincidence of assessments via pooled plasma and individual data. Eur. J. Pharm. Sci. 1999;8:57–65. doi: 10.1016/S0928-0987(98)00061-X. [DOI] [PubMed] [Google Scholar]
- 287.Rebouche C.J. Kinetics, pharmacokinetics, and regulation of l-carnitine and acetyl-l-carnitine metabolism. Ann. N. Y. Acad. Sci. 2004;1033:30–41. doi: 10.1196/annals.1320.003. [DOI] [PubMed] [Google Scholar]
- 288.Camp K.M., Lloyd-Puryear M.A., Yao L., Groft S.C., Parisi M.A., Mulberg A., Gopal-Srivastava R., Cederbaum S., Enns G.M., Ershow A.G., et al. Expanding research to provide an evidence base for nutritional interventions for the management of inborn errors of metabolism. Mol. Genet. Metab. 2013;109:319–328. doi: 10.1016/j.ymgme.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]