Abstract
Hepatocellular carcinoma (HCC) is a complex disease with multiple underlying pathogenic mechanisms caused by a variety of etiologic factors. Emerging evidence showed that long non-coding RNAs (lncRNAs), with size larger than 200 nucleotides (nt), play important roles in various types of cancer development and progression. In recent years, some dysregulated lncRNAs in HCC have been revealed and roles for several of them in HCC have been characterized. All these findings point to the potential of lncRNAs as prospective novel therapeutic targets in HCC. In this review, we summarize known dysregulated lncRNAs in HCC, and review potential biological roles and underlying molecular mechanisms of lncRNAs in HCC. Additionally, we discussed prospects of lncRNAs as potential biomarker and therapeutic target for HCC. In conclusion, this paper will help us gain better understanding of molecular mechanisms by which lncRNAs perform their function in HCC and also provide general strategies and directions for future research.
Keywords: long noncoding RNA, hepatocellular carcinoma, dysregulation, biological roles, molecular mechanism
1. Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers in the world as more than 700,000 cases are diagnosed and approximately 600,000 deaths are reported annually, especially in, East and Southeast Asia, Africa and Southern Europe [1,2]. This disease is often associated with an extremely poor prognosis because patients are either diagnosed at a very late stage or experience recurrence and metastasis after surgical resection [3]. It is well known that a variety of risk factors have been associated with the incidence of HCC, such as hepatitis B virus (HBV) and hepatitis C virus (HCV) infection, aflatoxin B1 intake, tobacco smoking, alcoholic cirrhosis, and so on [4]. Poor understanding of the mechanisms underlying the pathogenesis of HCC makes it difficult to be diagnosed and treated at an early stage, thus an urgent needs to elucidate the molecular mechanisms underlying HCC and to identify and provide effective targets for therapy or early detection of HCC. Although significant advances have been made in recent decades, our understanding of the underlying molecular mechanisms of HCC remains limited and these investigations have largely focused on the role of protein-coding genes and some classic epigenetic factors, including microRNAs (miRNAs), DNA methylation, and several types of histone modifications involving histone methylation and acetylation [4,5,6,7,8,9,10].
In recent years, advancements in genome-wide analyses of the mammalian transcriptome have revealed a novel class of transcripts, long noncoding RNAs (lncRNAs), which are pervasively transcribed in the genome [11]. LncRNAs are arbitrarily defined as transcripts of more than 200 nucleotides (nt) in length that lack significant open reading frames (ORF) and can be localized to both the nucleus and cytoplasm [12,13]. Accumulating evidence indicates that lncRNAs are not the “dark matter” of the genome, but that they play significant roles in various biological processes through complicated mechanisms, including X-inactivation, genomic imprinting, cell differentiation, cell apoptosis, stem cell pluripotency, nuclear trafficking, heat shock response, and genome rearrangement [14]. It is noteworthy that an increasing number of studies have demonstrated lncRNAs as a new class of regulatory molecules that are involved in a variety of human disease, especially cancer, through modulating gene expression at transcriptional, post-transcriptional or epigenetic level [15]. Some classical lncRNAs have been found to be dysregulated in a variety of cancers and have been shown to possess clinical potential as diagnostic biomarkers and therapeutic targets due to their aberrant expression is significantly associated with carcinogenesis, metastasis or prognosis, such as H19, HOTAIR, MALAT1, MEG3, and XIST [16,17]. HCC, as the most common type of primary liver cancer, is revealing the potential roles of lncRNAs in hepatocellular carcinoma and is attracting increased attention in cancer research in recent years. Excitingly, some significant and substantial progress has been made toward identifying and functional characterizing HCC-related lncRNAs.
In this review, we focus our attention on lncRNAs that are involved in HCC. Firstly, we summarize known dysregulated lncRNAs in HCC, and then we review potential biological roles and underlying molecular mechanisms of lncRNAs in HCC. Finally, we discuss prospects of lncRNAs as potential biomarker and therapeutic target for HCC.
All in all, this paper will help us gain a better understanding of molecular mechanisms by which lncRNAs perform their function in HCC and also provide general strategies and directions for future research.
2. Dysregulation of Long Non-Coding RNAs (lncRNAs) in Hepatocellular Carcinoma
It has been shown that most lncRNAs are expressed in a tissue/cell type-specific pattern or in a developmental stage specific manner and are transcribed by RNA polymerase II (RNA Pol II), as well as possess a 5'-methyl cap and a polyadenylated tail, similar to mRNAs, indicating these lncRNAs can be tightly regulated and may play specific biological roles in a variety of biological processes and human disease, and can also lead to undesired biological consequences when dysregulated. Indeed, a growing body of evidence has indicated that dysfunctional lncRNAs are implicated in a broad range of cancers, including HCC. So far, a handful of dysregulatd lncRNAs that are associated with HCC have been identified (see Table 1).
Table 1.
Dysregulatd long non-coding RNAs (lncRNAs) that are associated with HCC.
| LncRNA | Dysregulation | Biological Functions in HCC | Molecular Mechanism | Reference |
|---|---|---|---|---|
| H19 | Up-regulated | Promote HCC growth | Competitively bind to the let-7; interact with EZH2 and repress the expression of E-cadherin | [18,19,20,21,22,23,24,25] |
| HOTAIR | Up-regulated | Promote HCC growth | Competitively bind to the miR-331-3p and depress the expression of HER2; interact with EZH2 and LSD1 | [27,28,52,53] |
| HOTTIP | Up-regulated | Promote proliferation of HCC cells | Interact with WDR5/MLL and drive the H3K4me3 | [29,30] |
| HULC | Up-regulated | Promote HCC growth | Competitively bind to the miR-372 | [31,32,33] |
| KCNQ1OT1 | Up-regulated | Promote HCC progression | Interact with Ehmt2 and PRC2 complex and drive the H3K9 and H3K27 methylation | [34,54] |
| Linc-ROR | Up-regulated | Promote cell survival during hypoxic stress | Competitively bind to the miR-145 Competitively bind to | [37] |
| MALAT1 | Up-regulated | Promote invasion | miR-9 targets MALAT1 in the nucleus and regulates the MALAT1 in an AGO2-dependent manner; competitively bind to miR-125b | [38,39,55,56] |
| MEG3 | Down-regulated | HCC growth control | Interact with PRC2 complex | [41,42,57] |
| PCNA-AS1 | Up-regulated | Promote HCC growth | Form RNA hybridization to increase PCNA mRNA stability | [43] |
| LncRNA-DREH | Down-regulated | Inhibit growth and metastasis | Interact with vimentin protein and repress its expression | [44] |
| LncRNA-HEIH | Up-regulated | Promote HCC growth | Interact with EZH2 and repress the expression of p15, p16, p21, p57 | [45] |
| LncRNA-MVIH | Up-regulated | Promote HCC growth, microvascular invasion and intrahepatic metastasis | Interact with PGK1 and inhibit its secretion | [46] |
| LncRNA-LET | Down-regulated | Inhibit hypoxia-induced HCC cell invasion | Interact with NF90 protein and increases its degradation | [47] |
| LncRNA-ATB | Up-regulated | Induced by TGF-β and promotes EMT, HCC cell invasion and metastasis | Competitively bind to the miR-200 family; binding IL-11 mRNA | [48] |
| LncRNA-hPVT1 | Up-regulated | Promotes HCC growth | Interact with NOP2 protein and enhance its stability | [49] |
Abbreviations: HER2, human epidermal growth factor receptor 2; Ehmt2, histone-lysine N-methyltransferase EHMT2; EZH2, enhancer of zeste homolog 2; ERK, extracellular signal-regulated kinases; IL-11, Interleukin 11; LDS1, lysine-specific demethylase 1; MAPK, mitogen-activated protein kinase; MLL, mixed lineage leukemia; PGK1, phosphoglycerate kinase 1; PRC2, polycomb repressive complex 2; WDR5, WD repeat domain 5; ZAK, mitogen-activated protein kinase kinase kinase MLT.
H19, a 2.3-kb lncRNA, is a famous paternally imprinted (maternally expressed) gene and is highly expressed from the early stages of embryogenesis to fetal life in many organs but is almost entirely down-regulated postnatally and it plays important roles in embryonic development and growth control [18]. Emerging evidence showed that erasure of H19 imprinting and subsequent high expression level of H19 was associated with tumor growth, metastasis and invasion of several types of cancer. [19,20,21]. Kim and Lee (1997) firstly found that the expression of H19 usually shift from monoallelic to biallelic in HCC and it might play a causal role in the epigenetic mechanism involved in tumor development and/or process [22]. Later, H19 RNA level was shown to be up-regulated in HBV-associated HCC [23]. Additionally, Matouk et al. (2007 and 2010) revealed that hypoxia could strongly up-regulated the level of H19 RNA in the HCC cell line [24,25].
HOTAIR (Homeobox antisense intergenic RNA), an ncRNA with a length of 2158 bp, is transcribed from the antisense strand of homeobox C gene locus in chromosome 12. A large number of studies have shown that HOTAIR is up-regulated in various cancers and correlates with carcinogenesis and metastasis, as well as poor prognosis [26]. A previous study from Geng et al. reported that HOTAIR expression was significantly higher in hepatocellular carcinoma (HCC) tissue than that in adjacent noncancerous tissues [27]. A recent study performed in HCC also found that HOTAIR was overexpressed in HCC patients and was associated with a worse prognosis and an increased risk of metastasis in these patients [28].
HOTTIP (HOXA transcript at the distal tip), an lncRNA transcribed from the 5' end of the HOXA locus that regulates the activation of some HOXA genes in vivo [29]. A recent study from Quagliata et al. reported that HOTTIP was significantly up-regulated in HCC specimens and its high expression level was associated with metastasis formation and poor patient survival in HCC [30].
HULC (highly up-regulated in liver cancer), a 500 bp spliced lncRNA, was first identified as a novel mRNA-like noncoding RNA that up-regulated remarkably in HCC by Panzitt et al. [31]. HULC expression has been reported to be regulated by the transcription factor CREB (cyclic adenosine monophosphate responsive element binding protein) in Hep3B cells [32]. Interestingly, the expression level of HULC is positively associated with those of hepatitis B virus X protein (HBx) in clinical HCC tissues. Moreover, HBx could up-regulate HULC expression level in L-O2 cells (a human immortalized normal liver cell line) and HepG2 cells (a human hepatoma cell line) [33].
KCNQ1OT1 (potassium voltage-gated channel, KQT-like subfamily, member 1 overlapping transcript 1), a maternally imprinted lncRNA transcribed from KCNQ1 locus and responsible for transcriptional silencing a bunch of genes at KCNQ1 locus in cis by modulating histone methylation, has been found to be involved in various types of cancers [34]. A recent study showed that a short tandem repeat (STR) polymorphism (rs35622507) within the KCNQ1OT1 coding region was identified as the risk conferring polymorphism for HCC in the Chinese population and a significant genotype–phenotype correlation in which the protective genotypes (heterozygote and non-10) of the STR polymorphism confer increased KCNQ1OT1 expression and partially decreased CDKN1C expression in vitro [34].
Linc-RoR (long non-coding RNA regulator of reprogramming), a large intergenic noncoding RNA with a length of 2.6-kb, was previously identified as a key reprogramming regulator and whose expression is connected to pluripotency via regulating the key pluripotency transcription factors (TFs) including Oct4, Sox2, and Nanog as a competing endogenous RNA (ceRNA) [35,36]. Interestingly, Takahashi et al. reveled that expression of linc-RoR was up-regulated in malignant cells compared to non-malignant hepatocytes and increased in responses to hypoxia [37].
MALAT1 (metastasis-associated lung adenocarcinoma transcript1), an lncRNA originally identified to be overexpressed in patients at high risk for metastasis of non-small cell lung tumors (NSCLC), was up-regulated in many solid tumors and associated with cancer metastasis and recurrence [38]. MALAT1 has been shown to be up-regulated in HCC cell lines and clinical tissue samples [38,39].
MEG3 is a maternal imprinted gene highly expressed in the human pituitary. It is able to interact with cyclic AMP, p53 (Tumor protein p53), and growth differentiation factor 15 (GDF15) and plays an important role in cell proliferation control. Decrease of MEG3 expression has been observed in several types of cancer [40]. Huang et al. (2007) found that MEG3 is down-regulated in HCC compared to normal liver tissues [41]. A later study also showed that MEG3 expression was markedly reduced in four human HCC cell lines compared with normal hepatocytes, and overexpression of MEG3 in HCC cells dramatically inhibited HCC cell growth, as well as MEG3 expression could be regulated by microRNA-29 [42].
PCNA-AS1 (proliferating cell nuclear antigen antisense RNA 1), an antisense long noncoding RNAs located on the opposite strand of gene proliferating cell nuclear antigen (PCNA), was recently found to be significantly up-regulated in HCC compared with peritumoral tissues by Yuan et al. [43].
In particular, the unprecedented advances in high-throughput screening technologies, such as microarrays and transcriptome sequencing, facilitate large-scale identification and characterization of novel disease-related genes, including lncRNAs. Excitingly, some papers have revealed the lncRNA expression profiles in HCC samples and paired non-tumor samples using microarray, and a set of HCC-related lncRNAs have been identified. For example, lncRNA-DREH (down-regulated expression by HBx), an lncRNA differentially expressed between livers of HBx transgenic mice and wild-type mice, was identified by microarray and the expression level of its human ortholog RNA, hDREH, was frequently down-regulated in HBV-related HCC tissues in comparison with the adjacent noncancerous hepatic tissues, and its decrease significantly, dramatically, associated with poor survival in HCC patients [44]. By comparing the lncRNA expression profiles of HBV-related HCC and paired peritumoral tissue, Yang et al. found lncRNA-HEIH (high expression in HCC), one of differentially expressed lncRNA, was highly expressed in HBV-related HCC and was significantly correlated with recurrence [45]. LncRNA-MVIH (microvascular invasion in HCC), an lncRNA derived from microarray data that used for identification of lncRNA-HEIH, was also shown to be up-regulated in HCC [46]. LncRNA-LET (low expression in tumor), an lncRNA also derived from the same microarray data that used for identification of lncRNA-HEIH, was shown to be down-regulated in HCC [47]. By comparing lncRNA expression levels between TGF-β treated and untreated SMMC-7721 hepatoma cells using microarray, Yuan et al. found lncRNA-ATB (lncRNA activated by TGF-β), was highly expressed in HCC and associated with poor prognosis in HCC [48]. Additionally, a recent study revealed lncRNA-hPVT1 (human plasmacytoma variant translocation 1), the human ortholog of lncRNA-mPVT1 (mouse plasmacytoma variant translocation 1) that was a fetal liver-specific lncRNAs identified by microarray analysis, is significantly up-regulated in HCC tissues and high hPVT1 expression is associated with poor prognosis in HCC patients [49].
uc002mbe.2, a TSA (Trichostatin A)-induced lncRNA, was strongly expressed in TSA-treated Huh7 cells. Yang et al. found that uc002mbe.2 had more than 300-folds induction upon TSA treatment and its expression level was significantly lower in the HCC cell lines and liver cancer tissue compared with normal human hepatocytes and adjacent noncancerous tissues [50].
URHC (up-regulated in hepatocellular carcinoma), an lncRNA was highly expressed in hepatoma cells and HCC tissues and was originally identified by comparing lncRNA expression profiling of three HCC cell lines and normal hepatocytes using lncRNA microarray. Xu et al. revealed that the higher expression of URHC was correlated with poor overall survival [51].
3. Biological Roles of lncRNAs in Hepatocellular Carcinoma
3.1. Hepatocellular carcinoma (HCC) Growth
To date, many lncRNAs dysregulated in HCC have been demonstrated to play important roles in HCC growth in vitro or in vivo. In the study conducted by Matouk et al., the authors found that ablations of tumorigenicity of HCC in vivo was seen by H19 knockdown which also significantly abrogated anchorage-independent growth after hypoxia recovery [24]. In vitro assays in the HCC cell line Bel7402 demonstrated that knockdown of HOTAIR lincRNA could reduce cell proliferation [27]. Du et al. demonstrated that HULC could promote cell proliferation by MTT, colony formation assay, and tumorigenicity assay [33]. Quagliata et al. demonstrated that knockdown of HOTTIP could significantly reduce cell proliferation of HuH-6 and HuH-7 cell lines [30]. Yang et al. revealed that knockdown of lncRNA-HEIH could inhibit the proliferation of HCC cell by affecting cell cycle and the growth of tumors from lncRNA-HEIH-down-regulated xenografts were significantly inhibited when compared with that of tumors formed from control xenografts [45]. Yang et al. also demonstrated that lncRNA-MVIH could promote HCC growth both in vitro and in vivo [46]. Huang et al. revealed that suppression of cellular lncRNA-DREH could enhance the cell proliferation effect in vitro and its overexpression could repress the growth of tumor in vivo [44]. Recently, Takahashi et al. uncovered that knockdown of linc-RoR, a hypoxia-responsive lncRNA, could decrease cell viability in HCC cells during hypoxia [37]. Yuan et al. demonstrated lncRNA PCNA-AS1 could dramatically promote tumor growth in vitro and in vivo [43]. A recent study revealed lncRNA-hPVT1 could promote cell proliferation, cell cycling and stem cell-like phenotype of HCC cells in vitro and promote HCC growth in vivo [49]. Additionally, another recent study demonstrated that URHC inhibition could reduce the proliferation of HCC cells [51].
3.2. HCC Invasion and Metastasis
It is well known that the poor prognosis and high recurrence rate of HCC is largely due to the high incidence of intrahepatic and extrahepatic metastases [58]. Thus, the inhibition of invasion and metastasis is of great importance in HCC therapies. There is now increasing evidence that lncRNAs play important roles in invasion and metastasis of HCC. For example, Huang et al. demonstrated that overexpression of lncRNA-Dreh could inhibit tumor metastasis in vivo by establishing orthotopic liver implanted metastatic models and peripheral intravascular implanted metastatic models [44]. Yuan et al. revealed that lncRNA-MVIH overexpression resulted in significantly frequent intrahepatic metastasis by establishing the liver metastasis tumor model [46]. Lai et al. found that inhibition of MALAT1 in HepG2 cells could effectively reduce cell motility and invasiveness [39]. Yang et al. found that the low expression of lncRNA-LET is involved in cell invasion under hypoxic or normoxic conditions and its overexpression can inhibit the metastasis of HCC in vivo [47]. Additionally, a recent study revealed lncRNA-ATB, an lncRNA activated by TGF-β, can induce EMT and cell invasion in vitro and promote the invasion-metastasis cascade of HCC cells in vivo [48].
3.3. HCC Apoptosis
Some studies have demonstrated that lncRNAs are involved in HCC via acting on cell apoptosis. Braconi et al. revealed that MEG3 expression was markedly reduced in four human HCC cell lines, compared with normal hepatocytes, and enforced expression of MEG3 in HCC cells significantly decreased both anchorage-dependent and -independent cell growth, and induced apoptosis [42]. Yang et al. found that the TSA-induced uc002mbe.2 expression was positively correlated with the apoptotic effect of TSA in HCC cells and knockdown the expression of uc002mbe.2 significantly reduced TSA-induced apoptosis of Huh7 cells [50]. In addition, Xu et al. demonstrated that knockdown of the expression of URHC could promote apoptosis of HCC cells [51].
4. Molecular Mechanisms of LncRNAs in Hepatocellular Carcinoma
4.1. LncRNA-Protein Interaction
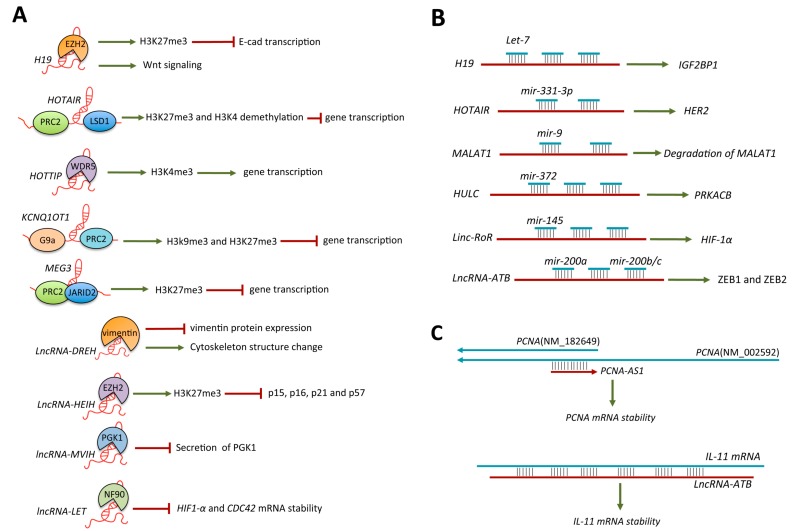
A large number of studies have revealed that many lncRNAs exert their function through interaction with proteins or protein complexes, especially with epigenetic complexes, such as polycomb repressive complex 1 (PRC1) and polycomb repressive complex 2 (PRC2) [59]. Some HCC-related lncRNA have been demonstrated to play roles in tumorigenesis via forming ribonucleoprotein (RNP) (Figure 1A). For example, it has been found that H19 can specifically associate with enhancer of zeste homolog 2 (EZH2), a key subunit of the PRC2 complex, and inhibit E-cad expression by directly suppressing E-cad transcription and by indirectly activating Wnt signaling [21]. Tsai et al. demonstrated that HOTAIR served as a scaffold for two distinct histone modification complexes, PRC2 and LSD1/CoREST/REST complex. The ability to tether two distinct complexes enables RNA-mediated assembly of PRC2 and LSD1 and coordinates targeting of PRC2 and LSD1 to chromatin for coupled H3K27 methylation and H3K4 demethylation [53]. Wang et al. revealed HOTTIP RNA could bind the adaptor protein WDR5 directly and targets WDR5/MLL complexes across HOXA, driving H3K4 trimethylation and gene transcription [29]. Pandey et al. found that KCNQ1OT1 could interact with chromatin and with the H3K9- and H3K27-specific histone methyltransferases G9a and the PRC2 complex in a lineage-specific manner [54]. Kaneko et al. uncovered MEG3 interacted with PRC2 mainly through the RBR of JARID2 and MEG3 acts in trans on PRC2 and JARID2 by facilitating their recruitment to a subset of target genes [57]. It was found that LncRNA-DREH could specifically associate with protein vimentin, a type III intermediate filament (IF) and the major cytoskeletal component of mesenchymal cells [44]. A recent study found LncRNA-HEIH also can associate with EZH2, and this association is required for the repression of EZH2 target genes in HCC, involving p15, p16, p21 and p57 [45]. Yuan et al. demonstrated that lncRNA-MVIH could activate angiogenesis by interacting with PGK1, a protein secreted by tumor cells and inhibit angiogenesis, and inhibiting its secretion [46]. In addition, another study revealed that lncRNA-LET could bind to NF90, a double-stranded RNA-binding protein that has been implicated in the stabilization, transport, and translational control of many target mRNAs, and decreases HIF1-α and CDC42 mRNA stability through its association with NF90 under hypoxic and normoxic conditions, respectively [47].
Figure 1.
Overview of the molecular mechanisms of lncRNAs in HCC. (A) LncRNA-protein interaction; (B) LncRNA–microRNA interaction; and (C) LncRNA–mRNA interaction.
4.2. LncRNA-MicroRNA Interaction
Interestingly, several recent reports have provided a model that suggests that lncRNA may function as competing endogenous RNA (ceRNA) in modulating the concentration and biological functions of microRNAs. These lncRNAs act as miRNA “sponges” generally share microRNA response elements (MREs) with the transcripts of several important genes and inhibiting normal miRNA targeting activity on mRNA. Several HCC-related lncRNA have been identified as miRNA “sponges” (Figure 1B). For example, it is found that vertebrate H19 harbors both canonical and non-canonical binding sites for the let-7 family of microRNAs, which plays key roles in development and cancer. Kallen et al. demonstrate that H19 modulates let-7 availability by acting as a molecular sponge using H19 knockdown and overexpression, as well as in vivo crosslinking and genome-wide transcriptome analysis [18]. Liu et al. revealed that HOTAIR could function as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer [52]. Leucci et al. demonstrated that miR-9 could directly bind MALAT1 RNA in vivo and regulate the MALAT1 in the nucleus in an AGO2-dependent manner [55]. Han et al. found miR-125b could directly bind MALAT1 and down-regulated the MALAT1 in bladder cancer [56]. Wang et al. demonstrated that the lncRNA-HULC acts as ceRNA of the protein coding gene PRKACB and induces its increased translation by controlling the expression and activity of miR-372 in HCC. Takahashi et al. revealed that linc-RoR functioned as miRNA sponge to limit endogenous miR-145 that can modulate the expression of key effectors of the hypoxia response such as HIF-1α expression in HCC. In addition, Yuan et al. found that lncRNA-ATB up-regulated ZEB1 and ZEB2 by competitively binding the miR-200 family and then induced EMT and invasion of HCC [48]. However, it is noteworthy that a recent study from Bartel and Stoffel lab found target derepression of ceRNAs was in a threshold-like manner at high target site abundance and this threshold was insensitive to the effective levels of the miRNA via quantitating miRNA and target abundance. Strikingly, they concluded that modulation of miRNA target abundance is unlikely to cause significant effects on gene expression and metabolism through a ceRNA effect in vivo, supporting that endogenous lncRNAs might actually function as ceRNA is highly unlikely [60].
4.3. LncRNA-mRNA Interaction
Accumulating evidence indicates that lncRNA can influence mRNA processing and post-transcriptional regulation via forming lncRNA-mRNA duplex depends on complementary base pairing, involving control of splicing, translation, and mRNA stability [61]. Some HCC-related lncRNA have been demonstrated that they can directly bind to target mRNA to exert post-transcriptional regulation (Figure 1C). For example, PCNA-AS1, antisense to PCNA, could increase PCNA mRNA stability via forming lncRNA–mRNA hybridization in HCC [43]. Additionally, Yuan et al. found that lncRNA-ATB specially increased the stability of IL-11 mRNA, which depends on the binding of IL-11 mRNA in HCC [48].
5. Conclusions and Perspectives
Hepatocellular carcinoma is a complex disease with multiple underlying pathogenic mechanisms caused by a variety of risk factors, and a better understanding of molecular mechanisms will help to identify potential molecular targets for diagnosis and therapy. In recent years, long noncoding RNA are gaining the attention of researchers in many fields, particularly in cancer and a large number of lncRNAs have been identified and there is an exponential growth of studies on the biological functions of lncRNAs in human cancers, including HCC.
LncRNAs often exhibit spatially and temporally-regulated expression patterns that that are expressed from specific tissue/cell types [62]. Their specificity makes them accurate biomarkers for cancer diagnostics. Furthermore, it has been demonstrated that cancer-specific lncRNAs can be detectable in plasma and urine of patients [63,64,65]. For example, HULC, an lncRNA highly up-regulated in liver cancer and positively related with Edmondson histological grades or with hepatitis B (HBV)-positive status, could be detected in the plasma of HCC patients compared to healthy controls and its higher detection rates were observed in the plasma of patients with higher Edmondson grades or with HBV-positive status [63]. The novel potential biomarkers can be discovered through certain types of highly expressed cancer-associated lncRNAs.
Therapeutic benefit can be obtained through RNA-based therapeutic strategies, such as siRNA and microRNA, or using small molecule compounds designed specifically to interact with target lncRNAs or ribonucleoprotein complexes. The nucleotides drugs can be effectively delivered to the liver using viral and non-viral systems. For viral mediated delivery, several types of viral vectors can be used, such as adenoviral and retroviral vector. Viral vector-mediated RNA delivery to liver can be achieved via the hepatic artery, portal vein, or bile duct or by direct injection to the liver [66]. For non-viral approaches, a suite of synthetic delivery carriers for liver targeting has been developed, such as galactosylated liposomes [67], poly-l-glutamic acid-coated liposomes [68], octaarginine (R8)-modified lipid nanoparticles [69], pH-triggered and PEGylated nanoparticles [70].
Although the roles played by lncRNAs in HCC have just begun to be revealed, with rapid development of high throughput detection technologies, such as microarray and RNA-sequencing and available bioinformatics tools for lncRNAs functional analysis, an increasing number of HCC-related lncRNAs will be identified and characterized. This will provide new insights into the complicated lncRNAs regulatory network, and ultimately provide novel strategies for HCC clinical diagnosis and treatment.
Acknowledgments
This work was supported by the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT1171), the National Key Technology R&D Program in the 11th Five-Year Plan of China (No. 2007BAI07A00), the National Natural Science Foundation of China (No. 81201925) and the Special Foundation for High-tech Industry development Program of Shaan Xi Province (No. 2012-874).
Author Contributions
Zongfang Li and Jin Sun designed the manuscript; Jin Sun and Beibei Bie wrote the manuscript; Zongfang Li, Shu Zhang and Jun Yang revised the manuscript. All authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Forner A., Llovet J.M., Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA: A Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Villanueva A., Minguez B., Forner A., Reig M., Llovet J.M. Hepatocellular carcinoma: Novel molecular approaches for diagnosis, prognosis, and therapy. Ann. Rev. Med. 2010;61:317–328. doi: 10.1146/annurev.med.080608.100623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aravalli R.N., Steer C.J., Cressman E.N. Molecular mechanisms of hepatocellular carcinoma. Hepatology. 2008;48:2047–2063. doi: 10.1002/hep.22580. [DOI] [PubMed] [Google Scholar]
- 5.Mah W.C., Lee C.G. DNA methylation: Potential biomarker in hepatocellular carcinoma. Biomark. Res. 2014;2:5. doi: 10.1186/2050-7771-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar M., Zhao X., Wang X.W. Molecular carcinogenesis of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: One step closer to personalized medicine? Cell Biosci. 2011;1:5. doi: 10.1186/2045-3701-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gramantieri L., Fornari F., Callegari E., Sabbioni S., Lanza G., Croce C.M., Bolondi L., Negrini M. MicroRNA involvement in hepatocellular carcinoma. J. Cell. Mol. Med. 2008;12:2189–2204. doi: 10.1111/j.1582-4934.2008.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saito Y., Hibino S., Saito H. Alterations of epigenetics and microRNA in hepatocellular carcinoma. Hepatol. Res. 2014;44:31–42. doi: 10.1111/hepr.12147. [DOI] [PubMed] [Google Scholar]
- 9.Chervona Y., Costa M. Histone modifications and cancer: Biomarkers of prognosis? Am. J. Cancer Res. 2012;2:589–597. [PMC free article] [PubMed] [Google Scholar]
- 10.Ma L., Chua M.S., Andrisani O., So S. Epigenetics in hepatocellular carcinoma: An update and future therapy perspectives. World J. Gastroenterol. 2014;20:333–345. doi: 10.3748/wjg.v20.i2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carninci P., Kasukawa T., Katayama S., Gough J., Frith M.C., Maeda N., Oyama R., Ravasi T., Lenhard B., Wells C., et al. The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–1563. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 12.Kapranov P., Cheng J., Dike S., Nix D.A., Duttagupta R., Willingham A.T., Stadler P.F., Hertel J., Hackermuller J., Hofacker I.L., et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science. 2007;316:1484–1488. doi: 10.1126/science.1138341. [DOI] [PubMed] [Google Scholar]
- 13.Van Heesch S., van Iterson M., Jacobi J., Boymans S., Essers P.B., de Bruijn E., Hao W., Macinnes A.W., Cuppen E., Simonis M. Extensive localization of long noncoding RNAs to the cytosol and mono- and polyribosomal complexes. Genome Biol. 2014;15:R6. doi: 10.1186/gb-2014-15-1-r6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun J., Lin Y., Wu J. Long non-coding RNA expression profiling of mouse testis during postnatal development. PLoS One. 2013;8:e75750. doi: 10.1371/journal.pone.0075750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wapinski O., Chang H.Y. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21:354–361. doi: 10.1016/j.tcb.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Li C.H., Chen Y. Targeting long non-coding RNAs in cancers: Progress and prospects. Int. J. Biochem. Cell Biol. 2013;45:1895–1910. doi: 10.1016/j.biocel.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 17.Gutschner T., Diederichs S. The hallmarks of cancer: A long non-coding RNA point of view. RNA Biol. 2012;9:703–719. doi: 10.4161/rna.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kallen A.N., Zhou X.B., Xu J., Qiao C., Ma J., Yan L., Lu L., Liu C., Yi J.S., Zhang H., et al. The imprinted H19 lncRNA antagonizes let-7 microRNAs. Mol. Cell. 2013;52:101–112. doi: 10.1016/j.molcel.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsang W.P., Ng E.K., Ng S.S., Jin H., Yu J., Sung J.J., Kwok T.T. Oncofetal H19-derived miR-675 regulates tumor suppressor RB in human colorectal cancer. Carcinogenesis. 2010;31:350–358. doi: 10.1093/carcin/bgp181. [DOI] [PubMed] [Google Scholar]
- 20.Shi Y., Wang Y., Luan W., Wang P., Tao T., Zhang J., Qian J., Liu N., You Y. Long non-coding RNA H19 promotes glioma cell invasion by deriving miR-675. PLoS One. 2014;9:e86295. doi: 10.1371/journal.pone.0086295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo M., Li Z., Wang W., Zeng Y., Liu Z., Qiu J. Long non-coding RNA H19 increases bladder cancer metastasis by associating with EZH2 and inhibiting E-cadherin expression. Cancer Lett. 2013;333:213–221. doi: 10.1016/j.canlet.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 22.Kim K.S., Lee Y.I. Biallelic expression of the H19 and IGF2 genes in hepatocellular carcinoma. Cancer Lett. 1997;119:143–148. doi: 10.1016/s0304-3835(97)00264-4. [DOI] [PubMed] [Google Scholar]
- 23.Iizuka N., Oka M., Yamada-Okabe H., Mori N., Tamesa T., Okada T., Takemoto N., Tangoku A., Hamada K., Nakayama H. Comparison of gene expression profiles between hepatitis B virus-and hepatitis C virus-infected hepatocellular carcinoma by oligonucleotide microarray data on the basis of a supervised learning method. Cancer Res. 2002;62:3939–3944. [PubMed] [Google Scholar]
- 24.Matouk I.J., DeGroot N., Mezan S., Ayesh S., Abu-lail R., Hochberg A., Galun E. The H19 non-coding RNA is essential for human tumor growth. PLoS One. 2007;2:e845. doi: 10.1371/journal.pone.0000845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matouk I.J., Mezan S., Mizrahi A., Ohana P., Abu-Lail R., Fellig Y., Degroot N., Galun E., Hochberg A. The oncofetal H19 RNA connection: Hypoxia, p53 and cancer. Biochim. Biophys. Acta. 2010;1803:443–451. doi: 10.1016/j.bbamcr.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J., Zhang P., Wang L., Piao H.L., Ma L. Long non-coding RNA HOTAIR in carcinogenesis and metastasis. Acta Biochim. Biophys. Sin. 2014;46:1–5. doi: 10.1093/abbs/gmt117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geng Y., Xie S., Li Q., Ma J., Wang G. Large intervening non-coding RNA HOTAIR is associated with hepatocellular carcinoma progression. J. Int. Med. Res. 2011;39:2119–2128. doi: 10.1177/147323001103900608. [DOI] [PubMed] [Google Scholar]
- 28.Ishibashi M., Kogo R., Shibata K., Sawada G., Takahashi Y., Kurashige J., Akiyoshi S., Sasaki S., Iwaya T., Sudo T., et al. Clinical significance of the expression of long non-coding RNA HOTAIR in primary hepatocellular carcinoma. Oncol. Rep. 2013;29:946–950. doi: 10.3892/or.2012.2219. [DOI] [PubMed] [Google Scholar]
- 29.Wang K.C., Yang Y.W., Liu B., Sanyal A., Corces-Zimmerman R., Chen Y., Lajoie B.R., Protacio A., Flynn R.A., Gupta R.A., et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature. 2011;472:120–124. doi: 10.1038/nature09819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quagliata L., Matter M.S., Piscuoglio S., Arabi L., Ruiz C., Procino A., Kovac M., Moretti F., Makowska Z., Boldanova T. Long noncoding RNA HOTTIP/HOXA13 expression is associated with disease progression and predicts outcome in hepatocellular carcinoma patients. Hepatology. 2014;59:911–923. doi: 10.1002/hep.26740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panzitt K., Tschernatsch M.M., Guelly C., Moustafa T., Stradner M., Strohmaier H.M., Buck C.R., Denk H., Schroeder R., Trauner M. Characterization of HULC, a novel gene with striking up-regulation in hepatocellular carcinoma, as noncoding RNA. Gastroenterology. 2007;132:330–342. doi: 10.1053/j.gastro.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 32.Wang J., Liu X., Wu H., Ni P., Gu Z., Qiao Y., Chen N., Sun F., Fan Q. CREB up-regulates long non-coding RNA, HULC expression through interaction with microRNA-372 in liver cancer. Nucleic Acids Res. 2010;38:5366–5383. doi: 10.1093/nar/gkq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du Y., Kong G., You X., Zhang S., Zhang T., Gao Y., Ye L., Zhang X. Elevation of highly up-regulated in liver cancer (HULC) by hepatitis B virus X protein promotes hepatoma cell proliferation via down-regulating p18. J. Biol. Chem. 2012;287:26302–26311. doi: 10.1074/jbc.M112.342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wan J., Huang M., Zhao H., Wang C., Zhao X., Jiang X., Bian S., He Y., Gao Y. A novel tetranucleotide repeat polymorphism within KCNQ1OT1 confers risk for hepatocellular carcinoma. DNA Cell Biol. 2013;32:628–634. doi: 10.1089/dna.2013.2118. [DOI] [PubMed] [Google Scholar]
- 35.Loewer S., Cabili M.N., Guttman M., Loh Y.H., Thomas K., Park I.H., Garber M., Curran M., Onder T., Agarwal S., et al. Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nat. Genet. 2010;42:1113–1117. doi: 10.1038/ng.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y., Xu Z., Jiang J., Xu C., Kang J., Xiao L., Wu M., Xiong J., Guo X., Liu H. Endogenous miRNA sponge lincRNA-RoR regulates Oct4, Nanog, and Sox2 in human embryonic stem cell self-renewal. Dev. Cell. 2013;25:69–80. doi: 10.1016/j.devcel.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi K., Yan I.K., Haga H., Patel T. Modulation of hypoxia-signaling pathways by extracellular long non-coding RNA regulator of reprogramming. J. Cell Sci. 2014;127:1585–1594. doi: 10.1242/jcs.141069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin R., Maeda S., Liu C., Karin M., Edgington T. A large noncoding RNA is a marker for murine hepatocellular carcinomas and a spectrum of human carcinomas. Oncogene. 2007;26:851–858. doi: 10.1038/sj.onc.1209846. [DOI] [PubMed] [Google Scholar]
- 39.Lai M.C., Yang Z., Zhou L., Zhu Q.Q., Xie H.Y., Zhang F., Wu L.M., Chen L.M., Zheng S.S. Long non-coding RNA MALAT-1 overexpression predicts tumor recurrence of hepatocellular carcinoma after liver transplantation. Med. Oncol. 2012;29:1810–1816. doi: 10.1007/s12032-011-0004-z. [DOI] [PubMed] [Google Scholar]
- 40.Benetatos L., Vartholomatos G., Hatzimichael E. MEG3 imprinted gene contribution in tumorigenesis. Int. J. Cancer. 2011;129:773–779. doi: 10.1002/ijc.26052. [DOI] [PubMed] [Google Scholar]
- 41.Huang J., Zhang X., Zhang M., Zhu J.-D., Zhang Y.-L., Lin Y., Wang K.-S., Qi X.-F., Zhang Q., Liu G.-Z. Up-regulation of DLK1 as an imprinted gene could contribute to human hepatocellular carcinoma. Carcinogenesis. 2007;28:1094–1103. doi: 10.1093/carcin/bgl215. [DOI] [PubMed] [Google Scholar]
- 42.Braconi C., Kogure T., Valeri N., Huang N., Nuovo G., Costinean S., Negrini M., Miotto E., Croce C., Patel T. MicroRNA-29 can regulate expression of the long non-coding RNA gene MEG3 in hepatocellular cancer. Oncogene. 2011;30:4750–4756. doi: 10.1038/onc.2011.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan S.X., Tao Q.F., Wang J., Yang F., Liu L., Wang L.L., Zhang J., Yang Y., Liu H., Wang F., et al. Antisense long non-coding RNA PCNA-AS1 promotes tumor growth by regulating proliferating cell nuclear antigen in hepatocellular carcinoma. Cancer Lett. 2014;349:87–94. doi: 10.1016/j.canlet.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 44.Huang J.F., Guo Y.J., Zhao C.X., Yuan S.X., Wang Y., Tang G.N., Zhou W.P., Sun S.H. Hepatitis B virus X protein (HBx)-related long noncoding RNA (lncRNA) down-regulated expression by HBx (Dreh) inhibits hepatocellular carcinoma metastasis by targeting the intermediate filament protein vimentin. Hepatology. 2013;57:1882–1892. doi: 10.1002/hep.26195. [DOI] [PubMed] [Google Scholar]
- 45.Yang F., Zhang L., Huo X.S., Yuan J.H., Xu D., Yuan S.X., Zhu N., Zhou W.P., Yang G.S., Wang Y.Z. Long noncoding RNA high expression in hepatocellular carcinoma facilitates tumor growth through enhancer of zeste homolog 2 in humans. Hepatology. 2011;54:1679–1689. doi: 10.1002/hep.24563. [DOI] [PubMed] [Google Scholar]
- 46.Yuan S.X., Yang F., Yang Y., Tao Q.F., Zhang J., Huang G., Yang Y., Wang R.Y., Yang S., Huo X.S., et al. Long noncoding RNA associated with microvascular invasion in hepatocellular carcinoma promotes angiogenesis and serves as a predictor for hepatocellular carcinoma patients’ poor recurrence-free survival after hepatectomy. Hepatology. 2012;56:2231–2241. doi: 10.1002/hep.25895. [DOI] [PubMed] [Google Scholar]
- 47.Yang F., Huo X.S., Yuan S.X., Zhang L., Zhou W.P., Wang F., Sun S.H. Repression of the long noncoding RNA-LET by histone deacetylase 3 contributes to hypoxia-mediated metastasis. Mol. Cell. 2013;49:1083–1096. doi: 10.1016/j.molcel.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 48.Yuan J.H., Yang F., Wang F., Ma J.Z., Guo Y.J., Tao Q.F., Liu F., Pan W., Wang T.T., Zhou C.C., et al. A long noncoding RNA activated by TGF-β promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25:666–681. doi: 10.1016/j.ccr.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 49.Wang F., Yuan J.H., Wang S.H., Yang F., Yuan S.X., Ye C., Yang N., Zhou W.P., Li W.L., Li W., et al. Oncofetal long noncoding RNA PVT1 promotes proliferation and stem cell-like property of hepatocellular carcinoma cells by stabilizing NOP2. Hepatology. 2014;60:1278–1290. doi: 10.1002/hep.27239. [DOI] [PubMed] [Google Scholar]
- 50.Yang H., Zhong Y., Xie H., Lai X., Xu M., Nie Y., Liu S., Wan Y.J. Induction of the liver cancer-down-regulated long noncoding RNA uc002mbe.2 mediates trichostatin-induced apoptosis of liver cancer cells. Biochem. Pharmacol. 2013;85:1761–1769. doi: 10.1016/j.bcp.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu W.-H., Zhang J.-B., Dang Z., Li X., Zhou T., Liu J., Wang D.-S., Song W.-J., Dou K.-F. Long non-coding RNA URHC regulates cell proliferation and apoptosis via ZAK through the ERK/MAPK signaling pathway in hepatocellular carcinoma. Int. J. Biol. Sci. 2014;10:664. doi: 10.7150/ijbs.8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu X.-H., Sun M., Nie F.-Q., Ge Y.-B., Zhang E.-B., Yin D.-D., Kong R., Xia R., Lu K.-H., Li J.-H. LncRNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331–3p in gastric cancer. Mol. Cancer. 2014;13:92. doi: 10.1186/1476-4598-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsai M.C., Manor O., Wan Y., Mosammaparast N., Wang J.K., Lan F., Shi Y., Segal E., Chang H.Y. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–693. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pandey R.R., Mondal T., Mohammad F., Enroth S., Redrup L., Komorowski J., Nagano T., Mancini-DiNardo D., Kanduri C. KCNQ1OT1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol. Cell. 2008;32:232–246. doi: 10.1016/j.molcel.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 55.Leucci E., Patella F., Waage J., Holmstrom K., Lindow M., Porse B., Kauppinen S., Lund A.H. MicroRNA-9 targets the long non-coding RNA MALAT1 for degradation in the nucleus. Sci. Rep. 2013;3:2535. doi: 10.1038/srep02535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Han Y., Liu Y., Zhang H., Wang T., Diao R., Jiang Z., Gui Y., Cai Z. Hsa-miR-125b suppresses bladder cancer development by down-regulating oncogene SIRT7 and oncogenic long non-coding RNA MALAT1. FEBS Lett. 2013;587:3875–3882. [PubMed] [Google Scholar]
- 57.Kaneko S., Bonasio R., Saldana-Meyer R., Yoshida T., Son J., Nishino K., Umezawa A., Reinberg D. Interactions between JARID2 and noncoding RNAs regulate PRC2 recruitment to chromatin. Mol. Cell. 2014;53:290–300. doi: 10.1016/j.molcel.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Budhu A., Forgues M., Ye Q.H., Jia H.L., He P., Zanetti K.A., Kammula U.S., Chen Y., Qin L.X., Tang Z.Y., et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10:99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 59.Rinn J.L., Chang H.Y. Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Denzler R., Agarwal V., Stefano J., Bartel D.P., Stoffel M. Assessing the ceRNA hypothesis with quantitative measurements of miRNA and target abundance. Mol. Cell. 2014;54:766–776. doi: 10.1016/j.molcel.2014.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Geisler S., Coller J. RNA in unexpected places: Long non-coding RNA functions in diverse cellular contexts. Nat. Rev. Mol. Cell. Biol. 2013;14:699–712. doi: 10.1038/nrm3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kutter C., Watt S., Stefflova K., Wilson M.D., Goncalves A., Ponting C.P., Odom D.T., Marques A.C. Rapid turnover of long noncoding RNAs and the evolution of gene expression. PLoS Genet. 2012;8:e1002841. doi: 10.1371/journal.pgen.1002841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xie H., Ma H.W., Zhou D.Q. Plasma HULC as a promising novel biomarker for the detection of hepatocellular carcinoma. Biomed. Res. Int. 2013;2013:136106. doi: 10.1155/2013/136106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee B.Y., Mazar J., Aftab M.N., Qi F., Shelley J., Li J.L., Govindarajan S., Valerio F., Rivera I., Thurn T., et al. Long noncoding RNAs as putative biomarkers for prostate cancer detection. J. Mol. Diagn. 2014;16:615–626. doi: 10.1016/j.jmoldx.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang Y.S., Hsieh H.Y., Shih H.M., Sytwu H.K., Wu C.C. Urinary Xist is a potential biomarker for membranous nephropathy. Biochem. Biophys. Res. Commun. 2014;452:415–421. doi: 10.1016/j.bbrc.2014.08.077. [DOI] [PubMed] [Google Scholar]
- 66.Kamimura K., Abe H., Suda T., Aoyagi Y., Liu D. Liver-directed gene therapy. JSM Gastroenterol. Hepatol. 2013;1:1005. [Google Scholar]
- 67.Jiang N., Zhang X., Zheng X., Chen D., Siu K., Wang H., Ichim T.E., Quan D., McAlister V., Chen G., et al. A novel in vivo siRNA delivery system specifically targeting liver cells for protection of ConA-induced fulminant hepatitis. PLoS One. 2012;7:e44138. doi: 10.1371/journal.pone.0044138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hattori Y., Nakamura A., Arai S., Nishigaki M., Ohkura H., Kawano K., Maitani Y., Yonemochi E. In vivo siRNA delivery system for targeting to the liver by poly-l-glutamic acid-coated lipoplex. Results Pharm. Sci. 2014;4:1–7. doi: 10.1016/j.rinphs.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hayashi Y., Yamauchi J., Khalil I.A., Kajimoto K., Akita H., Harashima H. Cell penetrating peptide-mediated systemic siRNA delivery to the liver. Int. J. Pharm. 2011;419:308–313. doi: 10.1016/j.ijpharm.2011.07.038. [DOI] [PubMed] [Google Scholar]
- 70.Kolli S., Wong S.P., Harbottle R., Johnston B., Thanou M., Miller A.D. pH-Triggered nanoparticle mediated delivery of siRNA to liver cells in vitro and in vivo. Bioconjug. Chem. 2013;24:314–332. doi: 10.1021/bc3004099. [DOI] [PubMed] [Google Scholar]