Abstract
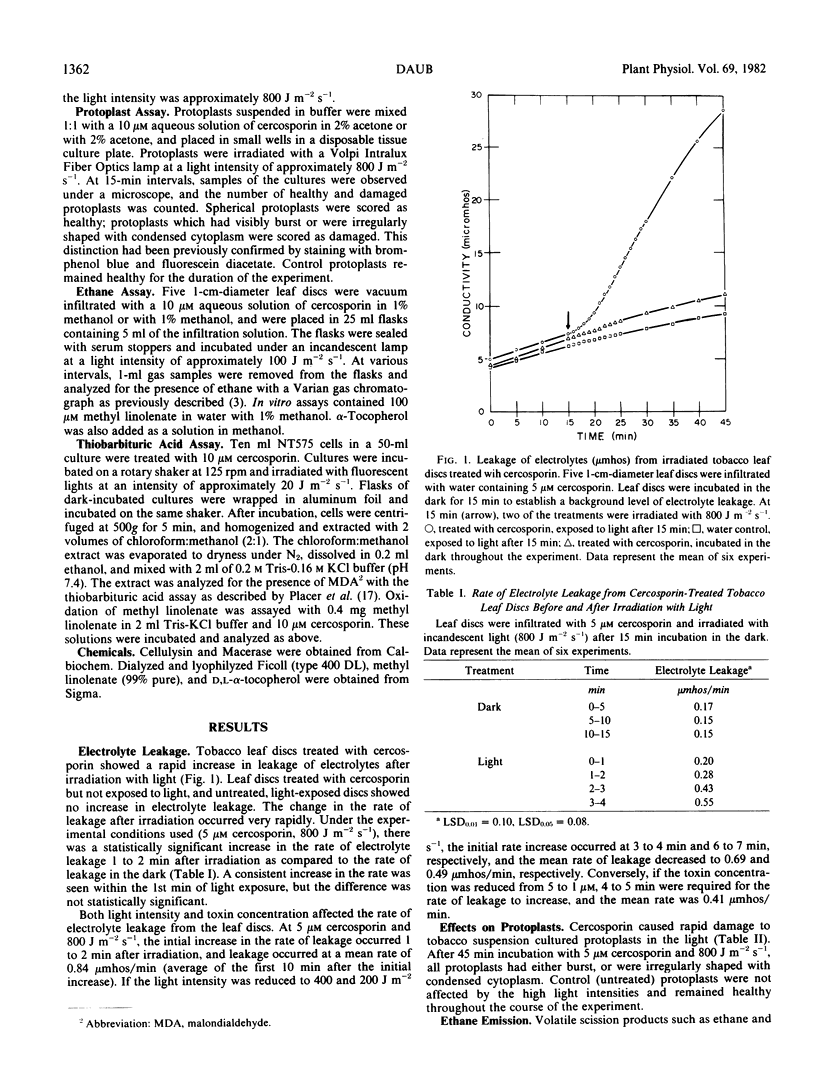
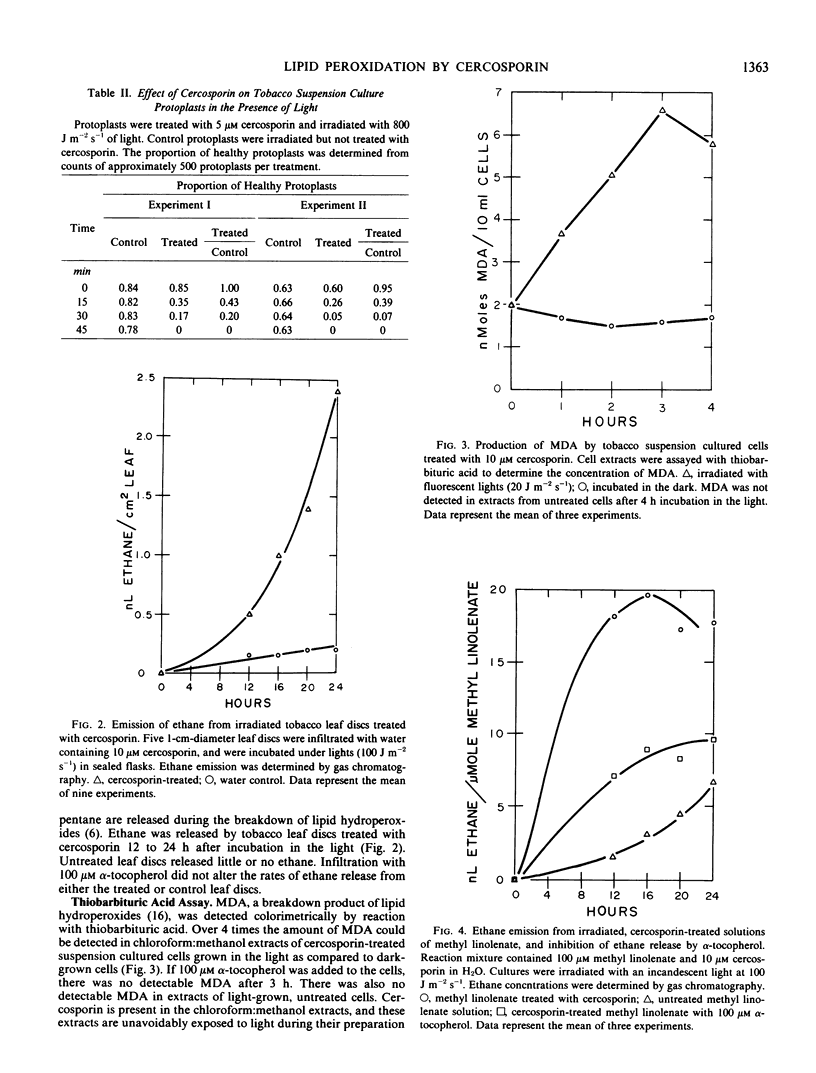
Cercosporin, a nonspecific toxin from Cercospora species, is a photosensitizing compound which rapidly kills plant cells in the light. Cell death appears to be due to a cercosporin-mediated peroxidation of membrane lipids. Tobacco leaf discs treated with cercosporin showed a large increase in electrolyte leakage 1 to 2 minutes after irradiation with light. All tobacco protoplasts exposed to cercosporin in the light were damaged within 45 minutes. Chloroform:methanol extracts of toxin-treated suspension cultures gave positive reactions for lipid hydroperoxides in the thiobarbituric acid test. Cercosporin-treated leaf discs emitted high concentrations of ethane 12 to 24 hours after incubation in the light. Cercosporin also oxidized solutions of methyl linolenate as determined by the thiobarbituric acid assay and the emission of ethane. α-Tocopherol had an inhibitory effect on the cercosporin-mediated lipid peroxidation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bressan R. A., Lecureux L., Wilson L. G., Filner P. Emission of ethylene and ethane by leaf tissue exposed to injurious concentrations of sulfur dioxide or bisulfite ion. Plant Physiol. 1979 May;63(5):924–930. doi: 10.1104/pp.63.5.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallini L., Bindoli A., Macrì F., Vianello A. Lipid peroxidation induced by cercosporin as a possible determinant of its toxicity. Chem Biol Interact. 1979 Dec;28(2-3):139–146. doi: 10.1016/0009-2797(79)90156-x. [DOI] [PubMed] [Google Scholar]
- Dumelin E. E., Tappel A. L. Hydrocarbon gases produced during in vitro peroxidation of polyunsaturated fatty acids and decomposition of preformed hydroperoxides. Lipids. 1977 Nov;12(11):894–900. doi: 10.1007/BF02533308. [DOI] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Reaction of linoleic acid hydroperoxide with thiobarbituric acid. J Lipid Res. 1978 Nov;19(8):1053–1057. [PubMed] [Google Scholar]
- Placer Z. A., Cushman L. L., Johnson B. C. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal Biochem. 1966 Aug;16(2):359–364. doi: 10.1016/0003-2697(66)90167-9. [DOI] [PubMed] [Google Scholar]
- Tappel A. L. Vitamin E and free radical peroxidation of lipids. Ann N Y Acad Sci. 1972 Dec 18;203:12–28. doi: 10.1111/j.1749-6632.1972.tb27851.x. [DOI] [PubMed] [Google Scholar]
- Tinberg H. M., Barber A. A. Studies on vitamin E action: peroxidation inhibition in structural protein-lipid micelle complexes derived from rat liver microsomal membranes. J Nutr. 1970 Apr;100(4):413–418. doi: 10.1093/jn/100.4.413. [DOI] [PubMed] [Google Scholar]



