Abstract
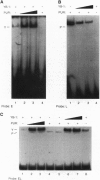
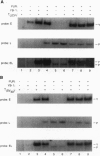
Human JC polyomavirus (JCV) is the etiologic agent of the neurodegenerative disease progressive mulifocal leukoencephalopathy. By using JCV as a model, we investigated the role of the viral early protein tumor antigen (TAg) in the binding of two cellular proteins, Pura alpha and YB-1, to JCV regulatory sequences. Results from band-shift assays with purified YB-1, Pur alpha, and TAg indicated that efficient binding of Pur alpha, a strong activator of early gene transcription, to a single-stranded target sequence corresponding to the viral lytic control element, is diminished in the presence of the late gene activator YB-1, which recognizes the opposite strand of the Pur alpha binding site. Of particular interest was the ability of Pur alpha and TAg to enhance binding of YB-1 to DNA molecules without being associated with this complex. Binding studies using a mutant peptide encompassing the N terminus of YB-1 indicated that the C terminus of YB-1 is important for its DNA binding activity. The ability of Pur alpha and TAg to increase binding of YB-1 to DNA is independent of the YB-1 C terminus. Similarly, results from band-shift assays using Pur alpha variants indicated that two distinct regions of this protein contribute either to its ability to bind DNA or to its ability to enhance YB-1 DNA binding activity. Based on the interaction of Pur alpha, YB-1, and TAg, and their binding to DNA, a model is proposed for the role of these proteins in transcription of viral early and late genes during the lytic cycle.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed S., Chowdhury M., Khalili K. Regulation of a human neurotropic virus promoter, JCVE: identification of a novel activator domain located upstream from the 98 bp enhancer promoter region. Nucleic Acids Res. 1990 Dec 25;18(24):7417–7423. doi: 10.1093/nar/18.24.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S., Rappaport J., Tada H., Kerr D., Khalili K. A nuclear protein derived from brain cells stimulates transcription of the human neurotropic virus promoter, JCVE, in vitro. J Biol Chem. 1990 Aug 15;265(23):13899–13905. [PubMed] [Google Scholar]
- Amemiya K., Traub R., Durham L., Major E. O. Interaction of a nuclear factor-1-like protein with the regulatory region of the human polyomavirus JC virus. J Biol Chem. 1989 Apr 25;264(12):7025–7032. [PubMed] [Google Scholar]
- Amirhaeri S., Wohlrab F., Major E. O., Wells R. D. Unusual DNA structure in the regulatory region of the human papovavirus JC virus. J Virol. 1988 Mar;62(3):922–931. doi: 10.1128/jvi.62.3.922-931.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergemann A. D., Johnson E. M. The HeLa Pur factor binds single-stranded DNA at a specific element conserved in gene flanking regions and origins of DNA replication. Mol Cell Biol. 1992 Mar;12(3):1257–1265. doi: 10.1128/mcb.12.3.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergemann A. D., Ma Z. W., Johnson E. M. Sequence of cDNA comprising the human pur gene and sequence-specific single-stranded-DNA-binding properties of the encoded protein. Mol Cell Biol. 1992 Dec;12(12):5673–5682. doi: 10.1128/mcb.12.12.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. F., Tada H., Khalili K. The role of a pentanucleotide repeat sequence, AGGGAAGGGA, in the regulation of JC virus DNA replication. Gene. 1994 Oct 21;148(2):309–314. doi: 10.1016/0378-1119(94)90704-8. [DOI] [PubMed] [Google Scholar]
- Chowdhury M., Kundu M., Khalili K. GA/GC-rich sequence confers Tat responsiveness to human neurotropic virus promoter, JCVL, in cells derived from central nervous system. Oncogene. 1993 Apr;8(4):887–892. [PubMed] [Google Scholar]
- Didier D. K., Schiffenbauer J., Woulfe S. L., Zacheis M., Schwartz B. D. Characterization of the cDNA encoding a protein binding to the major histocompatibility complex class II Y box. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7322–7326. doi: 10.1073/pnas.85.19.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisque R. J., Bream G. L., Cannella M. T. Human polyomavirus JC virus genome. J Virol. 1984 Aug;51(2):458–469. doi: 10.1128/jvi.51.2.458-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson J., Saffer J., Furneaux H. The transcription factor Sp1 binds to the JC virus promoter and is selectively expressed in glial cells in human brain. Ann Neurol. 1992 Jul;32(1):72–77. doi: 10.1002/ana.410320112. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Rosenfeld P. J., Kelly T. J., Tjian R. A cellular DNA-binding protein that activates eukaryotic transcription and DNA replication. Cell. 1987 Jan 16;48(1):79–89. doi: 10.1016/0092-8674(87)90358-8. [DOI] [PubMed] [Google Scholar]
- Khalili K., Rappaport J., Khoury G. Nuclear factors in human brain cells bind specifically to the JCV regulatory region. EMBO J. 1988 Apr;7(4):1205–1210. doi: 10.1002/j.1460-2075.1988.tb02932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch K. J., Frisque R. J. Identification of critical elements within the JC virus DNA replication origin. J Virol. 1990 Dec;64(12):5812–5822. doi: 10.1128/jvi.64.12.5812-5822.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major E. O., Amemiya K., Tornatore C. S., Houff S. A., Berger J. R. Pathogenesis and molecular biology of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev. 1992 Jan;5(1):49–73. doi: 10.1128/cmr.5.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakshatri H., Pater A., Pater M. M. Activity and enhancer binding factors for JC virus regulatory elements in differentiating embryonal carcinoma cells. Virology. 1990 Aug;177(2):784–789. doi: 10.1016/0042-6822(90)90550-b. [DOI] [PubMed] [Google Scholar]
- Ranganathan P. N., Khalili K. The transcriptional enhancer element, kappa B, regulates promoter activity of the human neurotropic virus, JCV, in cells derived from the CNS. Nucleic Acids Res. 1993 Apr 25;21(8):1959–1964. doi: 10.1093/nar/21.8.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S., Wells R., Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986 Oct 17;234(4774):364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Tada H., Khalili K. A novel sequence-specific DNA-binding protein, LCP-1, interacts with single-stranded DNA and differentially regulates early gene expression of the human neurotropic JC virus. J Virol. 1992 Dec;66(12):6885–6892. doi: 10.1128/jvi.66.12.6885-6892.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada H., Lashgari M. S., Khalili K. Regulation of JCVL promoter function: evidence that a pentanucleotide "silencer" repeat sequence AGGGAAGGGA down-regulates transcription of the JC virus late promoter. Virology. 1991 Jan;180(1):327–338. doi: 10.1016/0042-6822(91)90037-c. [DOI] [PubMed] [Google Scholar]
- Tada H., Lashgari M., Rappaport J., Khalili K. Cell type-specific expression of JC virus early promoter is determined by positive and negative regulation. J Virol. 1989 Jan;63(1):463–466. doi: 10.1128/jvi.63.1.463-466.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Sumita K., Hirose S., Mikoshiba K. Core promoter of the mouse myelin basic protein gene governs brain-specific transcription in vitro. EMBO J. 1990 Oct;9(10):3101–3108. doi: 10.1002/j.1460-2075.1990.tb07507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner M., Drolet D. W., Rosenfeld M. G. Regulation of JC virus by the POU-domain transcription factor Tst-1: implications for progressive multifocal leukoencephalopathy. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4743–4747. doi: 10.1073/pnas.90.10.4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffe A. P., Tafuri S., Ranjan M., Familari M. The Y-box factors: a family of nucleic acid binding proteins conserved from Escherichia coli to man. New Biol. 1992 Apr;4(4):290–298. [PubMed] [Google Scholar]