Abstract
The metabolic regulation of membrane lipid composition has been examined using the cell wall-less, unicellular green alga Dunaliella salina (UTEX 1644) as a model system. Low temperature stress was employed to initiate and study the regulatory response.
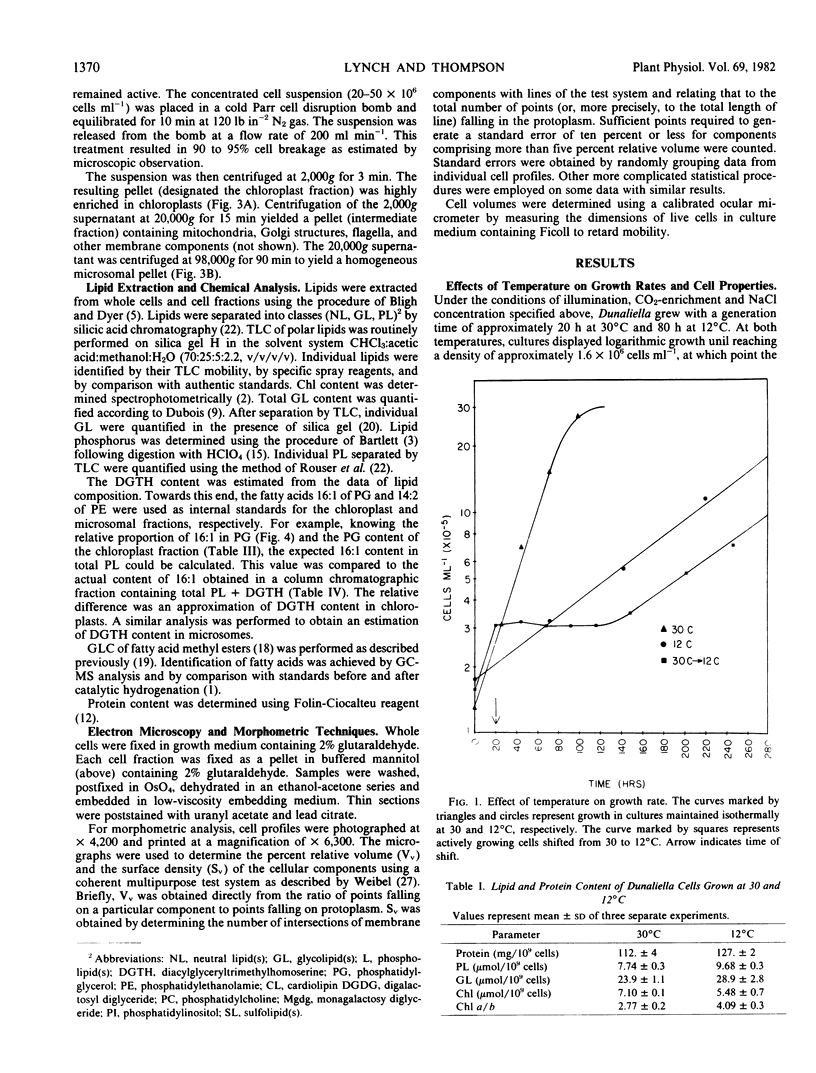
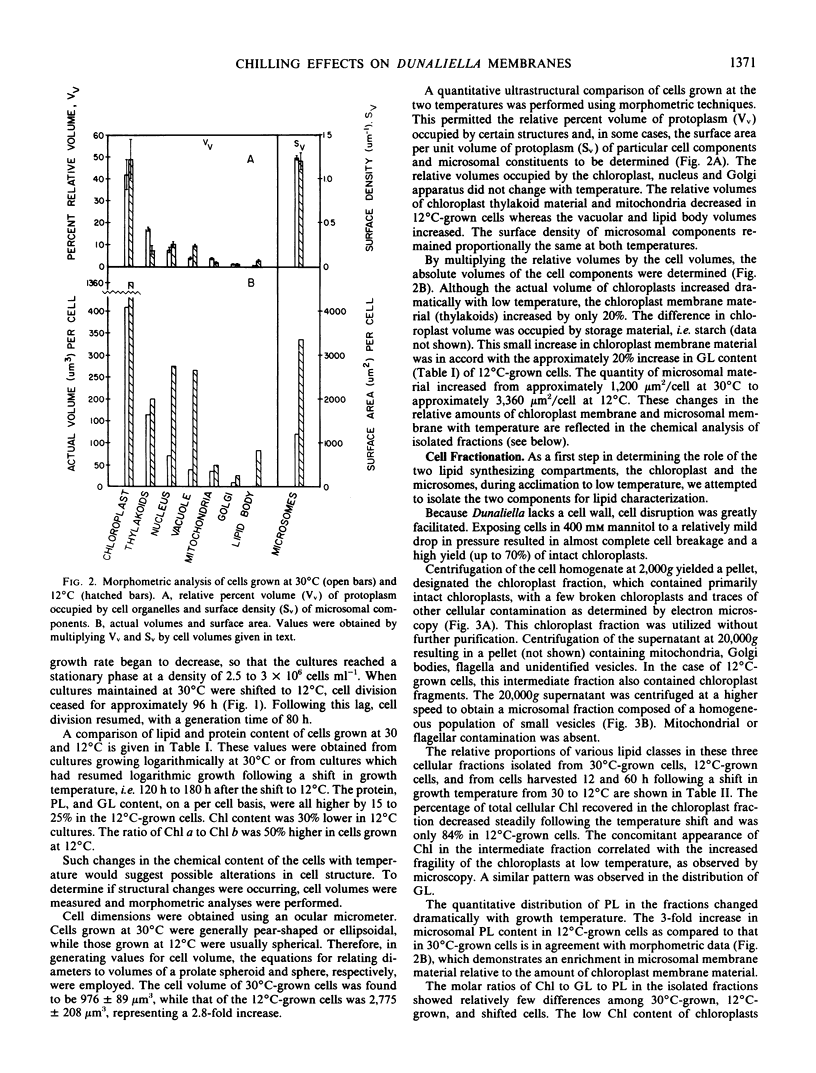
When cultures growing logarithmically at 30°C were chilled to 12°C, cell division ceased for approximately 100 hours, and then the cells resumed logarithmic growth at a slower rate. The phospholipid, glycolipid and protein content, on a per cell basis, was, in each case, approximately 20% higher in cells grown at 12°C. The volume of the 12°C-acclimated cells was 2.8 times that of 30°C-grown cells. The quantity of chloroplast membrane, as determined by morphometric analysis, was 20% greater, whereas the content of microsomal membrane material was more elevated, being approximately 2.8 times that of 30°C-grown cells.
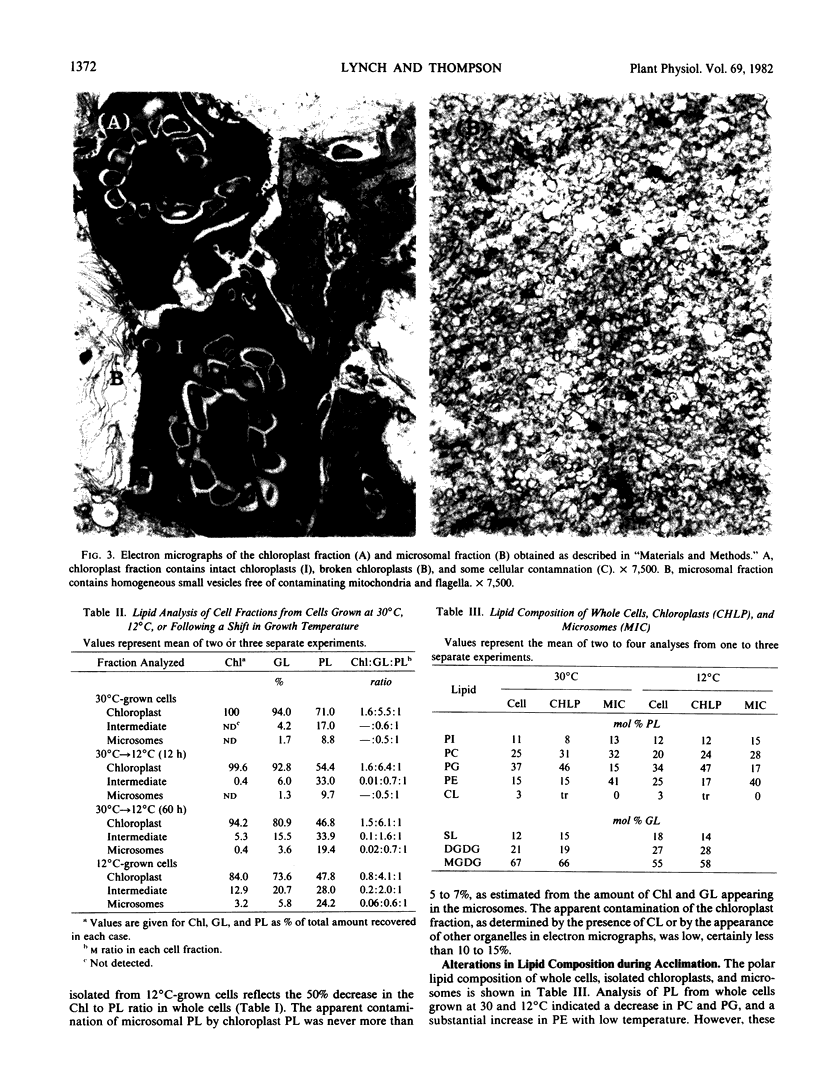
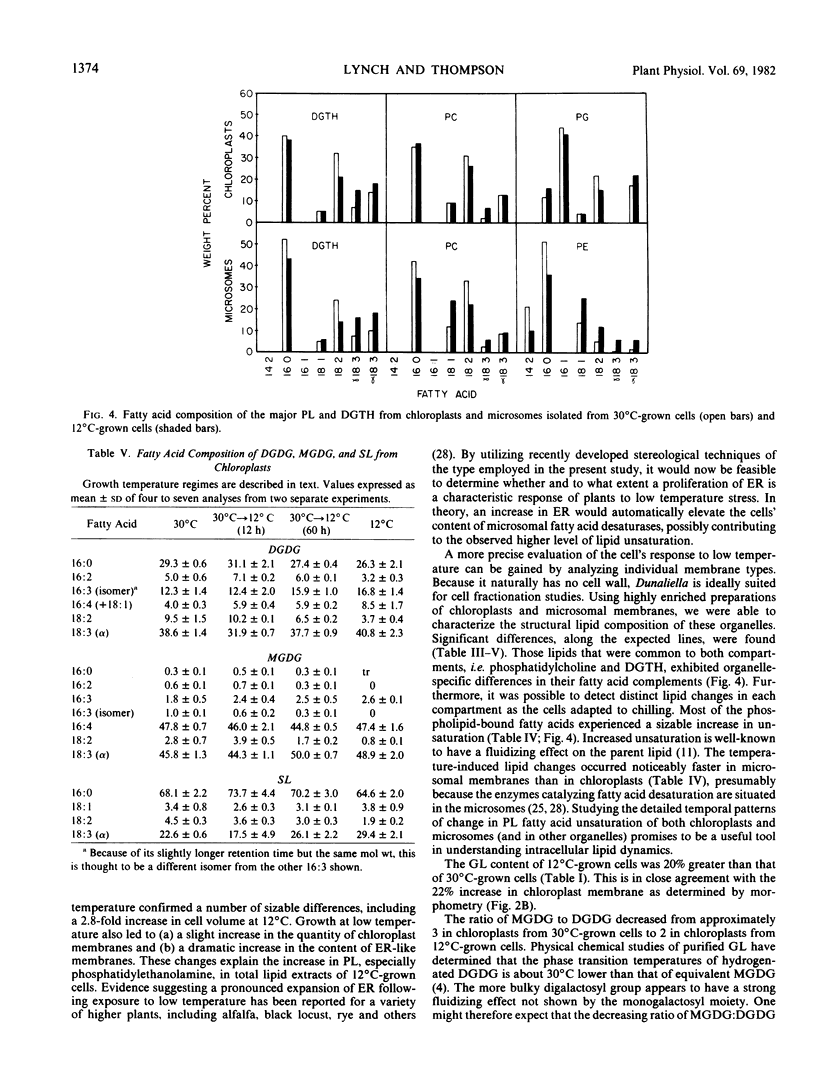
Lipid compositional analyses were carried out on purified chloroplasts and microsomes isolated from Dunaliella grown at 30 and 12°C and also from cells 12 and 60 hours following a shift from 30 to 12°C. In both chloroplast and microsomal phospholipids fatty acid unsaturation increased during acclimation to low temperature. Generally, microsomal phospholipids responded more quickly and to a greater extent than did chloroplast phospholipids. Despite these alterations, little change in the relative proportions of phospholipid classes was observed in either cell fraction.
In sharp contrast to the pattern of phospholipid change, chloroplast glycolipids responded to low temperature by significantly increasing the proportion of one specific class, digalactosyl diglycerides, relative to monogalactosyl diglycerides, while showing minimal change in fatty acid distribution within any given glycolipid class.
The ease and rapidity with which Dunaliella cells can be manipulated with respect to environmental stress and isolation of intact cell organelles makes it particularly well suited for research on intermembrane lipid dynamics within the plant cell.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelqvist L. A. A simple and convenient procedure for the hydrogenation of lipids on the micro- and nanomole scale. J Lipid Res. 1972 Jan;13(1):146–148. [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bishop D. G., Kenrick J. R., Bayston J. H., Macpherson A. S., Johns S. R. Monolayer properties of chloroplast lipids. Biochim Biophys Acta. 1980 Nov 4;602(2):248–259. doi: 10.1016/0005-2736(80)90308-9. [DOI] [PubMed] [Google Scholar]
- Brown A. E., Elovson J. Isolation and characterization of a novel lipid, 1(3),2-diacylglyceryl-(3)-O-4'-(N,N,N-trimethyl)homoserine, from Ochromonas danica. Biochemistry. 1974 Aug 13;13(17):3476–3482. doi: 10.1021/bi00714a009. [DOI] [PubMed] [Google Scholar]
- Douce R. Site of biosynthesis of galactolipids in spinach chloroplasts. Science. 1974 Mar 1;183(4127):852–853. doi: 10.1126/science.183.4127.852. [DOI] [PubMed] [Google Scholar]
- Israelachvili J. N., Marcelja S., Horn R. G. Physical principles of membrane organization. Q Rev Biophys. 1980 May;13(2):121–200. doi: 10.1017/s0033583500001645. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lilley R. M. Isolation of Functionally Intact Rhodoplasts from Griffithsia monilis (Ceramiaceae, Rhodophyta). Plant Physiol. 1981 Jan;67(1):5–8. doi: 10.1104/pp.67.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- Moseley K. R., Thompson G. A. Lipid Composition and Metabolism of Volvox carteri. Plant Physiol. 1980 Feb;65(2):260–265. doi: 10.1104/pp.65.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G., Batt R. D. Quantitative analysis of sulfolipid (sulfoquinovosyl diglyceride) and galactolipids (monogalactosyl and digalactosyl diglycerides) in plant tissues. Anal Biochem. 1968 Jan;22(1):74–88. doi: 10.1016/0003-2697(68)90261-3. [DOI] [PubMed] [Google Scholar]
- Roughan P. G., Holland R., Slack C. R. The role of chloroplasts and microsomal fractions in polar-lipid synthesis from [1-14C]acetate by cell-free preparations from spinach (Spinacia oleracea) leaves. Biochem J. 1980 Apr 15;188(1):17–24. doi: 10.1042/bj1880017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouser G., Fkeischer S., Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970 May;5(5):494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- Yamada T. A., Nozawa Y. An unusual lipid in the human pathogenic fungus Epidermophyton floccosum. Biochim Biophys Acta. 1979 Sep 28;574(3):433–439. doi: 10.1016/0005-2760(79)90239-x. [DOI] [PubMed] [Google Scholar]




