Abstract
Menopause is defined as permanent irreversible cessation of menses brought by decline in ovarian follicular activity. Hormonal alteration results in various physical, psychological, and sexual changes in menopausal women. Associated dermatological problems can be classified as physiological changes, age-related changes, changes due to estrogen deficiency and due to hormone replacement therapy. Dermatosis seen due to estrogen deficiency includes Atrophic Vulvovaginitis, Vulvar Lichen Sclerosus, Dyaesthetic Vulvodynia, Hirsutism, Alopecia, Menopausal Flushing, Keratoderma Climactericum, Vulvovaginal Candidiasis. Dermatologists and gynecologists need to be familiar with the problems of menopausal women, as with increase in life expectancy, women passing through this phase is rising.
Keywords: Dermatosis, estrogen deficiency, menopause
INTRODUCTION
The life expectancy in India has been increasing, according to WHO health statistics 2011 in India, an average female life expectancy is 68 years and is projected to increase up to 73 years by 2021.[1,2] With about 25 million women passing through the menopause each year and rising, menopause and its associated symptoms have become key areas of interest.[3] Menopause is defined as the permanent, irreversible cessation of menses (not having a menstrual period for 12 consecutive months) brought about by a decline in ovarian follicular activity.[4] In most women, menopause occurs between the ages of 50-55 years, with an average age of 51.5 years, but some have their menopause before the age of 40 (premature menopause), whereas a few may menstruate until they are in their 60s. The estimated mean age of menopause is 46 years in India which is lower than Caucasians.[5]
Premature menopause can occur due to surgery, irradiation, viral infection, e. g., mumps, various enzymatic, or hormonal defects[6] and systemic disorders like Addison's disease, rheumatoid arthritis, diabetes mellitus, or myasthenia gravis. Menopause is preceded by a period, which is called menopausal transition, peri-menopause, or climacteric — a time of change and readjustment to new phase which menopause brings — ‘A Step up the ladder’.
There are many factors that influence menopausal age including[7] heredity (age of mothers menopause), smoking, parity, socioeconomic factors, exposure to various toxins, and nutrition.
Menopause syndrome due to estrogen deficiency can be classified as physical or psychological. Physical symptoms include vasomotor symptoms such as hot flushes and night sweats, urogenital symptoms, palpitations, headaches, bone and joint pain, asthenia, tiredness, disturbed sleep or insomnia, breast tenderness, and skin aging. Psychological symptoms includes depression, memory loss, irritability, poor concentration, tiredness, depressed mood, mood swings, loss of libido, anxiety, and loss of confidence.[2,8]
A multitude of factors, including lifestyle and role, body image, interpersonal relationships, and sociocultural status, can influence a woman's attitude toward the menopause and impact on her perception of symptom severity.[8]
The abundance of estrogen receptors in both dermis and epidermis and to a lesser extent progesterone receptor shows that skin is significantly affected during menopause. Dermatosis associated with menopause can classified as: [Table 1].
Table 1.
Dermatosis associated with menopause can be classed as under

PHYSIOLOGICAL CHANGES
Breast glandular tissue decreases with increased fibrous tissue. Uterus becomes small, and muscles are partly replaced by fibrous tissue. Vagina becomes narrower and shorter, vaginal and vulvar epithelium atrophies, and pH of vagina increases with increased chances of infection. External genitalia atrophies with loss of vulval subcutaneous fat. Epithelium of lower urinary tract atrophies, leading to increased tendency to prolapse and chances of urinary tract infection. Loss of elasticity in Pelvic supporting ligaments contributes to prolapse and urinary incontinence.[9,10] Pubic hair decreases, scalp hairs become depigmented with conversion of terminal hair to vellus hair.
Glands atrophies with decreased sebum and sweat production leading to dryness of skin. Melanocytes and langerhans cells decrease. Body weight increases, with fat being mainly deposited in the abdominal region with an increase in the waist-to-hip circumference ratio, thus change from the gynecoid to the android body shape.[9]
AGE-RELATED EFFECTS
Extrinsic cutaneous aging is a type of premature skin aging occurs by exposing the skin to harmful environmental factors such as poor nutrition, smoking, sun exposure, and large alcohol intake.[11] Intrinsic skin aging is believed to occur as a result of telomere shortening.[12] Telomeres also play a role in lowering oxidative damage in cells.
Atrophy of dermis occurs with decreased collagen, fibroblasts, mast cells, and blood vessels. Skin wrinkles become translucent, dry, flaky, and fragile, making it more prone to trauma, bleeding, and infection.[13]
Menopause brings changes in collagen metabolism. Postmenopausal period is marked by low amounts of soluble collagen, slow turnover, and collagen synthesis resulting in decreased skin resilience and pliability.[2] Skin loses its suppleness, with a feeling of intense tingling and formication owing to the effect on the neurovascular network in the skin collagen, which is largely influenced by sex hormones, especially estrogen, which declines after menopause.
Some dermatoses are seen in senile age group, which may not be specifically related with hormonal changes [Table 2]. The skin becomes rapidly thinner after menopause at a rate similar to the decrease in bone mass. The reduction in thickness can not only be attributed to age alone but also to the decrease of skin collagen. Thickness of the skin decreases by 1.13% every year in the postmenopausal period, whereas the collagen content decreases by 2.1% every postmenopausal year.[2]
Table 2.
Dermatosis aggravated with aging

Skin is a cellular repository of iron that menopause increases iron in skin, which may contribute to the manifestation of accelerated skin aging and photo aging after menopause.[14]
DERMATOSIS ASSOCIATED WITH ESTROGEN DEFICIENCY
Estrogen is essential for normal female sexual development and for the healthy functioning of the reproductive system. It is naturally produced, majority (90%) in the ovaries in premenopausal woman and smaller quantities are produced by the adrenal glands and peripheral tissues such as fat, liver, and kidneys by converting androgens into estrogens. Estrogens are also formed in the placenta during pregnancy. In a normal adult human women, three different natural estrogens predominates: Estrone (10-20%), estradiol (10-20%), and estriol (60-80%).
At menopause, the ovaries are atrophied, hence stop producing estrogen, and other sources continue to produce estrogen but in smaller quantities. Obese women may suffer less from menopause-related problems, related to estrogen depletion, as the androgens are converted to estrogen in fat cells.
Estrogen receptors are most abundant around genital areas, face, and lower limbs. So skin conditions involving these areas are more commonly affected in peri- and postmenopausal women.
Atrophic vulvovaginitis
A common intermediate complication of menopause is organ atrophy. Vaginal atrophy is due to thinning of vagina and vestibule. It often presents with vaginal dryness and burning, tenderness, pruritus, and dyspareunia. Urogenital atrophy occurs from rapid loss of collagen as a result of estrogen deficiency presenting with urgency, frequency, dysuria, painful urination, and incontinence.[15] Declining collagen in the cardinal and uterosacral ligaments, caused by low estrogen levels, may cause uterine prolapse.[16]
Vulvar lichen sclerosus
Lichen sclerosus et atrophicus (LSA) is a chronic atrophic skin disease that affects mainly anogenital areas in 85-93% cases involving both males and females.[17] Prevalence rates are difficult to establish as it is underreported due to embarrassment as well as physician fail to recognize the disorder. Females are more commonly affected with 10:1 and 6:1 ratio in different series.[18]
Patient may be completely asymptomatic or presents with intractable pruritus at night, irritation, painful intercourse, dysuria, urethral and vaginal discharge, dyspareunia, urinary and fecal incontinence, painful skin fissuring, pruritus vulvae, or sore vulvodynia.[17,18]
It presents with white atrophic polygonal, flat topped papules coalescing into plaques and extending into vulva and perianal skin in figure of eight configuration. Thickening of skin of vulva, perineum, labia majora, labia minora, fourchette, and clitoris may be seen. Sometimes clitoris disappears, labia shrink, and entrance to vagina tightens. It may present like cigarette paper with wrinkled surface and waxy thickened or fragile skin, bruises, blisters, purpura, telengectasia, erosion, or ulcers. It never affects inside vagina and cervix as it spares mucosa.
Skin around anus may be involved which aggravate the tendency of constipation as there is increased chances of genito-urinary infections.[17]
Resolution of lesions is unlikely and is associated with increased risk of vulval cancer in 5% patients in the form of premalignant changes or SCC. LSA can affect sites like inner thigh, buttocks, under breast, neck shoulders, and armpits.
It may follow or coexist with lichen simplex, genital warts, candidiasis, and erosive lichen planus. It needs to be differentiated from lichen planus, vitiligo, vulvar intraepithelial neoplasm, and extra mammillary pagets disease.
Etiology
Association with autoimmune disorders is seen in 21.5-34% patients and in 74% have autoantibodies to alopecia areata, vitiligo, thyroid disorders, and pernicious anemia. Extracellular matrix[18] protein as antibodies is detected in 75-83% of women.
Familial cases suggesting genetic tendency and significant association with HLA class II antigen DQ7 is demonstrated[19]
Bimodal peak suggests hormonal influence, but hormonal therapy does not improve disease.[20]
Infective etiology with spirohaete borrelia has been implicated.[21]
Vulvar skin has fibroblast sensitive to androgens responsible for sclerosis[22]
Oxidative stress might be one of the factor for sclerosis[23]
Histopathology — Hyperkeratosis with follicular plugging, atrophy of stratum malphigian with hydropic degeneration of basal cells, pronounced edema and homogenization of collagen, hyalinization with a band like zone of chronic inflammatory cells and absence of elastic fibers is seen in dermis.[24]
It needs to be differentiated from morphea where absence of fibrosis, presence of hydropic degeneration of basal layer and flattening of rete ridges is seen.
Treatment
-
Topical:
- Potent topical Steroids (Clobetasol propionate, 0.05%) are given, tapered according to response, but need to be applied on regular basis (once a week) to prevent recurrences.
- Emollient cream or petrolatum several times to relieve dryness or itching.
- Calcipotriol cream
- Estrogens — is not effective, but prescribed for postmenopausal atrophy.
- Retinoids — causes irritation.
- Tacrolimus (0.03-0.1%) and Pimercolimus — causes burning
- Lignocain (5%): Post-inflammatory pain syndrome after clinical improvement of lesions, in vulvodynia, which does not respond to steroids.
-
Systemic
- Intralesional steroids (Triamcinolone acetonoid, 5-20 mg/ml once a month)
- Retinoids can be given in resistant cases
Surgery is reserved for resistant cases for vulval cancer.
Any patient with chronic genital disorders need psychosexual counseling.
Dyaesthetic vulvodynia
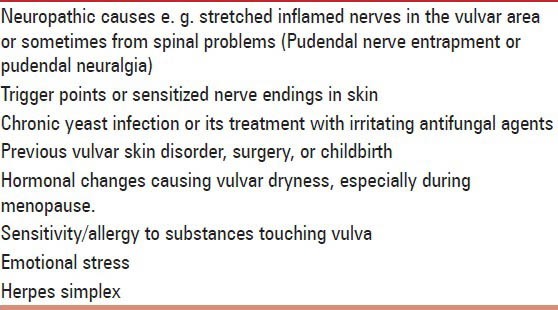
Previously called vestibulodynia,[25] now referred as generalized vulvodynia is characterized by wide-spread spontaneous pain throughout the vulvar region, labia, clitoris, vestibule, perineum, mons pubis, and inner thighs. Pain is constant or unprovoked by touch or pressure to the vulva; intercourse, bicycle riding and horse riding may worsen the symptoms.[26] Patient may also feel Poking, burning, raw feeling, irritation, throbbing, and stinging sensation in vulva. Associated stress-related and chronic pain conditions like headache, pain in face, tongue and mouth, fibromyalgia, irritable bowel syndrome fatigue, sleeping and eating disorders are also seen. Patient presents with erythema and swelling over vulvae. There are various causes of dyaesthetic vulvodynia [Table 3].
Table 3.
Various causes of dyaesthetic vulvodynia
Treatment
A combination of therapy should be used:
Physical therapy and pelvic floor exercise to relieve muscle spasms and generalized vulvar pain.
Amitriptylline — a tricyclic antidepressant, relieves pain.
Anti Convulsants — pregabalin, Carbamazepine, and Gabapentine may control pain.
Support and education to patients, their partner and families, to learn how to cope with stresses.
Biofeedback may improve if there is associated vaginismus.
Avoidance of irritants.
Regular application of local anesthetics.
Hirsutism
Hirsutism is increased hair growth in women, refers to a male pattern of hair, i. e., mustache and chin or occurring more thickly than usual on the limbs.
It is genetic in origin with increased amount of testosterone. It gradually gets more severe with age. The reduction in progesterone is known to increase the impact of androgens on the sebaceous glands and hair follicles.[27] Androgens may change the type of hair present, either lead to miniaturization of hair follicles or transform vellus to terminal hair follicles. A key trigger for excess hair growth is hyperandrogenaemia, with polycystic ovarian disease (PCOD) being the main cause of hirsutism.[27,28,29]
Post-menopausal hirsutism has also been associated with androgen therapy including testosterone therapy and androgen-estrogen hormone therapy.[30]
According to the classical or modified Ferriman-Gallwey (FG) scale, a score of ≥8 is considered to represent hirsutism.[31] However, the FG Scale may not be suitable for menopausal women due to changes in both the hair distribution pattern as well as the character of hair.
Value of detailed endocrinological workup is debatable. In addition to various serological tests like free testosterone, ultrasound of abdomen and pelvis is essential.
Treatment
The main objective is to modify any hormonal imbalance, to reduce the visible appearance of excess hair and improve the quality of life.[32]
1. Anti-androgens
Cyproterone acetate (CPA) 2 mg/day along with ethinyl estradiol (35μ gm/day, for 7 days).
Spironolactone (50-200 mg/day) — side effects includes tender breasts and irregular menstruation.
Newer anti-androgens:
• Drospirenone (3mg/day) — anti-mineralocorticoid effects.
• Flutamide and bicalutamide (250-750 mg/day).
2. Oral contraceptive pill (OCP).
3. Enzyme inhibitors.
Finasteride (2.5 mg/day)
Eflornithine (13.9% topical cream): It is approved for unwanted facial hair. It inhibits L-ornithine decarboxylase, a critical enzyme in hair growth, resulting in reduced hair density making the hair less noticeable.[33] It promotes rapid hair removal if combined with laser treatment.[34]
4. Metformin: It is insulin-sensitizing agent, it lowers testosterone levels and stimulates ovulation in PCOD patients. It reduces circulating insulin levels as well as free androgen concentration but is withdrawn due to cardiovascular concern.
5. Gonadotropic-releasing hormone (GnRH) analogues: Supress testosterone and estradiol synthesis.
Leuprolide acetate (7.5 mg IM depot monthly with 25-50 mg of transdermal estradiol.)
Nafarelin
They are expensive. If it is not combined with estrogen then severe estrogen deficiency can occur, causing menopausal symptoms such as hot flashes and osteoporosis, is not generally recommended for postmenopausal women.[32]
6. Epilation methods
Physical and chemical epilation (shaving, waxing, sugaring, threading) electrolysis or electroepilation laser or photoepilation, IPL, thermolysis.
Androgenetic Alopecia (AGA)
It is an inheritable condition caused by genetically determined sensitivity to the effects of dihydrotestosterone (DHT). It can have a severe psychological impact associated with low self-esteem, depression, introversion, and feeling of unattractiveness.
Causes
Aging, in conjunction with hormonal changes occurring during menopause, affect hair properties and give the perception of decreased hair coverage in middle-aged women. The reduction of the anagen phase and regression of scalp hair to finer, vellus hair is caused by androgens and can lead to hair loss (alopecia) during the menopause.[35]
Genes may also be involved in female pattern hair loss (FPHL). A lower incidence of FPHL in first-degree women relatives over 30 years (21%) compared with first-degree male relatives (54%) is found. Genetically, it is thought that early- and late-onset female AGA may be distinct entities.[36]
Serum ferritin concentration may be a factor in hair loss but studies shows conflicting results.[37]
Hair loss in women can be of three types: Frontal fibrosing alopecia, diffuse androgenetic alopecia and FPHL.[38]
The most common form of alopecia in elderly women is FPHL, which is progressive and often worsens during or after the menopause, particularly if it is pre-existing. It affects hair follicles from the parietal or fronto-vertical areas causing bitemporal hair thinning but leaving an intact frontal hairline.[39] Clinical evaluation of FPHL is by the Ludwig classification system, which describes three levels of severity,[40] further developed by Sinclair.[40]
Postmenopausal frontal fibrosing alopecia is a form of cicatricial alopecia, which was first described by Kossard.[41] It is a progressive condition that involves the destruction of the upper portion of the hair follicle by an inflammatory lymphocytic infiltrate, with symmetrical regression of the frontal and temporal hairline with partial or total loss of the eyebrows. It is not fully understood how the menopausal changes cause this selective targeting of the fronto-temporal scalp, and there is no proof of a hormonal basis, but some response has been reported in a few patients following androgen-dependent therapy.[42]
Diffuse androgenetic alopecia resembles male pattern baldness.
Treatment
Reassurance
Cosmetic methods improves esthetics, which includes hair styling techniques, use of camouflaging products and replacement hair pieces to cover exposed areas of scalp and provide the illusion of more volume, and even laser hair combs.[43]
Systemic anti-androgens, androgen antagonists, or enzyme inhibitors, inhibiting the synthesis of potent androgens, are used in women with hormonal dysregulation.
The combined use of CPA (50 mg) with the topical treatment is treatment of choice in treating women presenting with FPHL, in the absence of hormone dysregulation.[44]
Spironolactone inhibits ovarian androgen production.[45]
Flutamide (250 mg/day) is a potent, non-steroidal anti-androgen acts by inhibiting androgen uptake and inhibiting nuclear binding of androgens within the target tissue. It has side effects of hepatotoxicity, dryness of skin, feminization of male fetus.
4. Enzyme inhibitors:
Finasteride is a 5-α reductase inhibitor, which hinders the synthesis of the potent dihydrotestosterone at a dose of 2.5 mg/day.[46]
Dutasteride more potent than finasteride, inhibiting both type I and type II of the enzyme 5-α reductase is effective in the treatment of frontal fibrosing alopecia.[47]
5. Topical therapy
Minoxidil (2-5%) is suitable for treating postmenopausal women with or without hyperandrogenism via an androgen-independent mechanism. Other drugs used are aminexil, fluridil, anastim, saw palmetto, and synthetic and natural estrogen containing solutions.[48]
6. Hair transplantation may be considered for cases FPHL, which have not responded to medical treatments.
Menopausal flushing: (Climacteric flushing)
It occurs in 70-85% of women throughout peri-menopausal stage. Sudden feeling of intense heat, rapid heartbeat with reddening of face, neck, and upper chest often accompanied by discomfort and sweating that lasts 3-5 min, and subsides quickly, is due to dysfunction in thermoregulatory mechanism. It may be associated with throbbing pain in head and neck, waves of nausea, anxiety, and waking episodes at night. Sleep disturbance is commonly seen.[49] Hot beverages, physical exertion, and emotional upsets aggravate it.
The site of dysfunction lies in central catecholaminergic system. Temperature, pulse, and respiratory rate are increased[50] due to pulsatile release of LH. Patient develops blotchy erythema.
Causes
Neurokinin B (NKB) gene expression is elevated in hypothalamus in postmenopausal women. NKB is involved in GnRH release from median eminence, which control LH release from pituitary causing flushing.[51]
Alteration of hypothalamic catecholamine levels and a failure of normal central thermoregulatory centers through GnRH-dependent mechanism, there is change in body core temperature.[52]
Low estrogen level causes night sweats.
Due to opiate-dependent mechanism.[53]
Treatment
Selective serotonin reuptake inhibitors
Antidepressants most commonly used in the treatment of depression and some personality disorders. Paroxetine became the first and only non-hormonal therapy for menopausal hot flashes approved by FDA.[54]
Isoflavones
Commonly found in legumes such as soy and red clover.[55] The two soy isoflavones, genistein, and daidzein are also known as phytestrogens. Red clover (Trifoliumpratense)[56] contains isoflavones similar to soy, have different mechanism of action at relatively low concentrations. Flaxseed is the richest source of lignans,[57] which is one of three major classes of phytestrogen. Phytotestrogens reduce hot flashes and improve mood and quality of life in postmenopausal women. Lifestyle changes may help alleviate hot flashes. These include avoiding caffeine, hot drinks, chocolate, spicy or hot foods, and alcohol.
Hormonal therapy
Estrogen therapy is most effective for symptomatic hot flushes.[49] Estrogen replacement decreases both the no. of NKB mRNA — expressing neurons and the level of expression in the individual cell.
NonHormonal Therapy: Clonidine hydrochloride (0.05 mg HS).
Miscellaneous drugs: Progestins, venlafaxine, gabapentin, and blackcohosh.[54]
Keratoderma climactericum
(Haxthausen's disease,[58] acquired plantar keratoderma)
Originally reported as specific association with menopause,[59] It is characterized by hyperkeratosis of palms and soles, especially heels beginning at menopause. Erythema and hyperkeratosis with fissuring make walking painful. Seen commonly in obese and hypertensive menopausal women.
Topical estradiol 0.05% and 25-40% urea are used as topical preparation. Therapeutic response with systemic retinoids is also reported.[60]
Vulvovaginal candidiasis
It is fungal infection around vaginal region with over growth of candida albicans. Use of OCP, hormonal therapy, diabetes mellitus, steroid therapy, and oral antibiotics aggravates the condition.
It is common in younger women but can be seen in menopausal women.
It presents with heavy white curd-like vaginal discharge and burning sensation in vagina and vulva associated with pruritus. White plaques on vaginal wall with underlying erythema and surrounding edema seen extending to labia and perineal area.
It is seen associated with lichen planus, psoriasis, and LSA.
Oral antifungal medications and vaginal suppositories are mainly given as treatment.
Hormone replacement therapy
Menopausal women on various hormonal therapy to prevent osteoporosis and cardiovascular disease are prone to cancer of breast and genital tract. Estrogen therapy in these women can cause aggravation of various dermatosis [Table 4][49,61]
Table 4.
Dermatosis aggravated by estrogen therapy

CONCLUSION
In menopausal women, hormone alterations often result in unpleasant physical, psychological, and sexual changes, which can have a negative impact on their quality of life. As the average life expectancy increases, dermatologists need to be familiar with skin diseases, seen in menopausal women. Attention needs to be directed toward identifying and referring the patients of menopausal age group presenting with dermatosis to dermatologists, which in turn will help in extending optimal care to the aging women.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Meeta, Digumarti L, Agarwal N, Vaze N, Shah R, Malik S. Clinical practice guidelines on menopause: An executive summary and recommendations. J Midlife Health. 2013;4:77–106. doi: 10.4103/0976-7800.115290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calleja-Agius J, Brincat M, Borg M. Skin connective tissue and ageing. Best Pract Res Clin Obstet Gynaecol. 2013;27:727–40. doi: 10.1016/j.bpobgyn.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Lund KJ. Menopause and the menopausal transition. Med Clin North Am. 2008;92:1253–71. doi: 10.1016/j.mcna.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Kuhle BX. An evolutionary perspective on the origin and ontogeny of menopause. Maturitas. 2007;57:329–37. doi: 10.1016/j.maturitas.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Sharma S, Tandon VR, Mahajan A. Menupausal symptoms in urban women. JK Science J Med Educ Res. 2007;13:2011. [Google Scholar]
- 6.Prakash GJ, Ravi Kanth VV, Shelling AN, Rozati R, Sujatha M. Mutational analysis of inhibin alpha gene revealed three novel variations in Indian women with premature ovarian failure. Fertil Steril. 2010;94:90–8. doi: 10.1016/j.fertnstert.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Hassa H, Tanir HM, Tekin B, Senses T, Oge T, Mutlu FS. Possible factors affecting the age at menopause among women in the central anatolian region of Turkey. Clin Exp Obstet Gynecol. 2006;33:59–60. [PubMed] [Google Scholar]
- 8.Bruce D, Rymer J. Symptoms of the menopause. Best Pract Res Clin Obstet Gynaecol. 2009;23:25–32. doi: 10.1016/j.bpobgyn.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Barbo DM. The physiology of the menopause. Med Clin North Am. 1987;71:11–22. doi: 10.1016/s0025-7125(16)30879-3. [DOI] [PubMed] [Google Scholar]
- 10.Robinson D, Cardozo LD. The role of estrogens in female lower urinary tract dysfunction. Urology. 2003;62:45–51. doi: 10.1016/s0090-4295(03)00676-9. [DOI] [PubMed] [Google Scholar]
- 11.Baumann L. Skin ageing and its treatment. J Pathol. 2007;211:241–51. doi: 10.1002/path.2098. [DOI] [PubMed] [Google Scholar]
- 12.Imbert I, Botto JM, Farra CD, Domloge N. Modulation of telomere binding proteins: A future area of research for skin protection and anti-aging target. J Cosmet Dermatol. 2012;11:162–6. doi: 10.1111/j.1473-2165.2012.00611.x. [DOI] [PubMed] [Google Scholar]
- 13.Brincat MP, Baron YM, Galea R. Estrogens and the skin. Climacteric. 2005;8:110–23. doi: 10.1080/13697130500118100. [DOI] [PubMed] [Google Scholar]
- 14.Pelle E, Jian J, Zhang Q, Muizzuddin N, Yang Q, Dai J, et al. Menopause increases the iron storage protein ferritin in skin. J Cosmet Sci. 2013;64:175–9. [PubMed] [Google Scholar]
- 15.Versi E, Cardozo L, Brincat M, Cooper D, Montgomery J, Studd J. Correlation of urethral physiology and skin collagen in postmenopausal women. Br J Obstet Gynaecol. 1988;95:147–52. doi: 10.1111/j.1471-0528.1988.tb06844.x. [DOI] [PubMed] [Google Scholar]
- 16.Calleja-Agius J, Brincat MP. Urogenital atrophy. Climacteri. 2009;12:279–85. doi: 10.1080/13697130902814751. [DOI] [PubMed] [Google Scholar]
- 17.Powell JJ, Wojnarowska F. Lichen sclerosus. Lancet. 1999;353:1777–83. doi: 10.1016/s0140-6736(98)08228-2. [DOI] [PubMed] [Google Scholar]
- 18.Tasker GL, Wojnarowska F. Lichen sclerosus. Clin Exp Dermatol. 2003;28:128–33. doi: 10.1046/j.1365-2230.2003.01211.x. [DOI] [PubMed] [Google Scholar]
- 19.Powell J, Wojnarowska F, Winsey S, Marren O, Welsh K. Lichen sclerosus premenarche: Autoimmunity and immunogenetics. Br J Dermatol. 2000;142:481–4. doi: 10.1046/j.1365-2133.2000.03360.x. [DOI] [PubMed] [Google Scholar]
- 20.Neill SM, Tatnall FM, Cox NH. British Association of Dermatologists. Guidelines for the management of lichen sclerosus. Br J Dermatol. 2002;147:640–9. doi: 10.1046/j.1365-2133.2002.05012.x. [DOI] [PubMed] [Google Scholar]
- 21.Breier F, Khanakah G, Stanek G, Kunz G, Aberer E, Schmidt B, et al. Isolation and polymerase chain reaction typing of Borrelia afzelii from a skin lesion in a seronegative patient with generalized ulcerating bullous lichen sclerosus et atrophicus. Br J Dermatol. 2001;144:387–92. doi: 10.1046/j.1365-2133.2001.04034.x. [DOI] [PubMed] [Google Scholar]
- 22.Singh N, Thappa DM, Jaisankar TJ, Habeebullah S. A clinical study of vulval lichen sclerosus at a tertiary care hospital in South India. Indian J Sex Transm Dis. 2007;28:87–90. [Google Scholar]
- 23.Sander CS, Ali I, Dean D, Thiele JJ, Wojnarowska F. Oxidative stress is implicated in the pathogenesis of lichen sclerosus. Br J Dermatol. 2004;151:627–35. doi: 10.1111/j.1365-2133.2004.06142.x. [DOI] [PubMed] [Google Scholar]
- 24.Jaworsky C, Elder D. Livers histopathology of skin. 9th ed. Ch. 10. Connective tissue disease; pp. 293–322. [Google Scholar]
- 25.McKay M. Vulvodynia. A multifactorial problem. Arch Dermatol. 1989;125:256–62. doi: 10.1001/archderm.125.2.256. [DOI] [PubMed] [Google Scholar]
- 26.Wines N, Willsteed Menupause and the skin. Austral J Dermatol. 2001;42:149–60. doi: 10.1046/j.1440-0960.2001.00524.x. [DOI] [PubMed] [Google Scholar]
- 27.Mofid A, Seyyed Alinaghi SA, Zandieh S, Yazdani T. Hirsutism. Int J Clin Pract. 2008;62:433–43. doi: 10.1111/j.1742-1241.2007.01621.x. [DOI] [PubMed] [Google Scholar]
- 28.Rosenfield RL. Clinical practice. Hirsutism. N Engl J Med. 2005;353:2578–88. doi: 10.1056/NEJMcp033496. [DOI] [PubMed] [Google Scholar]
- 29.Shah D, Patel S. Hirsutism. Gynecol Endocrinol. 2009;25:140–8. doi: 10.1080/09513590802531567. [DOI] [PubMed] [Google Scholar]
- 30.Braunstein GD. Management of female sexual dysfunction in postmenopausal women by testosterone administration: Safety issues and controversies. J Sex Med. 2007;4:859–66. doi: 10.1111/j.1743-6109.2007.00516.x. [DOI] [PubMed] [Google Scholar]
- 31.Somani H, Harrison S, Bergfeld WF. The clinical evaluation of hirsutism. Dermatol Ther. 2008;21:376–91. doi: 10.1111/j.1529-8019.2008.00219.x. [DOI] [PubMed] [Google Scholar]
- 32.Blume-Peytavi U, Hahn S. Medical treatment of hirsutism. Dermato Ther. 2008;21:329–39. doi: 10.1111/j.1529-8019.2008.00215.x. [DOI] [PubMed] [Google Scholar]
- 33.Martin KA, Chang RJ, Ehrmann DA, Ibanez L, Lobo RA, Rosenfield RL, et al. Evaluation and treatment of hirsutism in premenopausal women: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:1105–20. doi: 10.1210/jc.2007-2437. [DOI] [PubMed] [Google Scholar]
- 34.Blume-Peytavi U, Atkin S, Gieler U, Grimalt R. Skin Academy: Hair, skin, hormones and menopause — current status/knowledge on the management of hair disorders in menopausal women. Eur J Dermatol. 2012;22:310–8. doi: 10.1684/ejd.2012.1692. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann R, Niiyama S, Huth A, Kissling S, Happle R. 17alpha-estradiol induces aromatase activity in intact human anagen hair follicles ex vivo. Exp Dermatol. 2002;11:376–80. doi: 10.1034/j.1600-0625.2002.110413.x. [DOI] [PubMed] [Google Scholar]
- 36.Blume-Peytavi U, Blumeyer A, Tosti A, Finner A, Marmol V, Trakatelli M, et al. European Consensus Group. S1 guideline for diagnostic evaluation in androgenetic alopecia in men, women and adolescents. Br J Dermatol. 2011;164:5–15. doi: 10.1111/j.1365-2133.2010.10011.x. [DOI] [PubMed] [Google Scholar]
- 37.Rushton DH. Nutritional factors and hair loss. Clin Exp Dermatol. 2002;27:396–404. doi: 10.1046/j.1365-2230.2002.01076.x. [DOI] [PubMed] [Google Scholar]
- 38.Rebora A. Pathogenesis of androgenetic alopecia. J Am Acad Dermatol. 2004;50:777–9. doi: 10.1016/j.jaad.2003.11.073. [DOI] [PubMed] [Google Scholar]
- 39.Dinh QQ, Sinclair R. Female pattern hair loss: Current treatment concepts. Clin Interv Aging. 2007;2:189–99. [PMC free article] [PubMed] [Google Scholar]
- 40.Chen W, Yang CC, Todorova A, Al Khuzaei S, Chiu HG, Worret WI, et al. Hair loss in elderly women. Eur J Dermatol. 2010;20:145–51. doi: 10.1684/ejd.2010.0828. [DOI] [PubMed] [Google Scholar]
- 41.Kossard S. Postmenopausal frontal fibrosing alopecia. Scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770–4. [PubMed] [Google Scholar]
- 42.Tosti A, Piraccini BM, Iorizzo M, Misciali C. Frontal fibrosing alopecia in postmenopausal women. J Am Acad Dermatol. 2005;52:55–60. doi: 10.1016/j.jaad.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 43.Blume-Peytavi U, Vogt A. Current standards in diagnostics and therapy of hair diseases — hair consultation. J Dtsch Dermatol Ges. 2011;9:394–410. doi: 10.1111/j.1610-0387.2011.07609.x. [DOI] [PubMed] [Google Scholar]
- 44.Sinclair R, Wewerinke M, Jolley D. Treatment of female pattern hair loss with oral antiandrogens. Br J Dermatol. 2005;152:466–73. doi: 10.1111/j.1365-2133.2005.06218.x. [DOI] [PubMed] [Google Scholar]
- 45.Rathnayake D, Sinclair R. Innovative use of spironolactone as an antiandrogen in the treatment of female pattern hair loss. Dermatol Clin. 2010;28:611–8. doi: 10.1016/j.det.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 46.Trüeb RM. Swiss Trichology Study Group. Finasteride treatment of patterned hair loss in normoandrogenic postmenopausal women. Dermatology. 2004;209:202–7. doi: 10.1159/000079890. [DOI] [PubMed] [Google Scholar]
- 47.Georgala S, Katoulis AC, Befon A, Danopoulou I, Georgala C. Treatment of postmenopausal frontal fibrosing alopecia with oral dutasteride. J Am Acad Dermatol. 2009;61:157–8. doi: 10.1016/j.jaad.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 48.Blumeyer A, Tosti A, Messenger A, Reygagne P, Del Marmol V, Spuls PI, et al. European Dermatology Forum (EDF). Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men. J Dtsch Dermatol Ges. 2011;9:S1–57. doi: 10.1111/j.1610-0379.2011.07802.x. [DOI] [PubMed] [Google Scholar]
- 49.Utian WH. Psychosocial and socioeconomical burden of vasomotor symptoms in menupause: A comprehensive review. Health Qual Life Outcomes. 2005;3:47. doi: 10.1186/1477-7525-3-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mulley G, Mitchell JR. Menopausal flushing: Does estrogen therapy make sense? Lancet. 1976;1:1397–9. doi: 10.1016/s0140-6736(76)93040-3. [DOI] [PubMed] [Google Scholar]
- 51.Ravnikar V, Elkind-Hirsch K, Schiff I, Ryan KJ, Tulchinsky D. Vasomotor flushes and the release of peripheral immunoreactive lutenizing hormone-releasing hormone in postmenopausal women. Fertil Steril. 1984;41:881–7. doi: 10.1016/s0015-0282(16)47902-1. [DOI] [PubMed] [Google Scholar]
- 52.Mulley G, Mitchell JR, Tattersall RB. Hot flushes after hypopysectomy. Br Med J. 1977;2:1062. doi: 10.1136/bmj.2.6094.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Casper RF, Yen SS. Neuroendocrinology of menopausal flushes: An hypothesis of flush mechanism. Clin Endocrinol (Oxf) 1985;22:293–312. doi: 10.1111/j.1365-2265.1985.tb03243.x. [DOI] [PubMed] [Google Scholar]
- 54.Cheema D, Coomarasamy A, El-Toukhy T. Non-hormonal therapy of post-menopausal vasomotor symptoms: A structured evidence-based review. Arch Gynecol Obstet. 2007;276:463–9. doi: 10.1007/s00404-007-0390-9. [DOI] [PubMed] [Google Scholar]
- 55.Messina M, Hughes C. Efficacy of soyfoods and soybean isoflavone supplements for alleviating menopausal symptoms is positively related to initial hot flush frequency. J Med Food. 2003;6:1–11. doi: 10.1089/109662003765184697. [DOI] [PubMed] [Google Scholar]
- 56.Nissan HP, Lu J, Booth NL, Yamamura HI, Farnsworth NR, Wang ZJ. A red clover (Trifolium pratense) phase II clinical extract possesses opiate activity. J Ethnopharmacol. 2007;112:207–10. doi: 10.1016/j.jep.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 57.Basch E, Bent S, Collins J, Dacey C, Hammerness P, Harrison M, et al. Natural Standard Resource Collaboration. Flax and flaxseed oil (Linum usitatissimum): A review by the Natural Standard Research Collaboration. J Soc Integr Oncol. 2007;5:92–105. doi: 10.2310/7200.2007.005. [DOI] [PubMed] [Google Scholar]
- 58.Haxthausen H. Keratoderma climactericum. Br J Dermatol. 1934;46:161–7. [Google Scholar]
- 59.Deschamps P, Leroy D, Pedailles S, Mandard JC. Keratoderma climactericum (Haxthausen's disease): Clinical signs, laboratory findings and etretinate treatment in 10 patients. Dermatologica. 1986;172:258–62. doi: 10.1159/000249351. [DOI] [PubMed] [Google Scholar]
- 60.Scheinfeld NS. Obesity and dermatology. Clin Dermatol. 2004;22:303–9. doi: 10.1016/j.clindermatol.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 61.Graham-Brown R. Dermatologic problems of the menopause. Clin Dermatol. 1997;15:143–5. doi: 10.1016/s0738-081x(96)00116-2. [DOI] [PubMed] [Google Scholar]


