Abstract
Ruptured pea (Pisum sativum cv. Massey Gem) chloroplasts exhibited ascorbate peroxidase activity as determined by H2O2-dependent oxidation of ascorbate and ascorbate-dependent reduction of H2O2. The ratio of ascorbate peroxidase to NADP-glyceraldehyde 3-phosphate dehydrogenase activity was constant during repeated washing of isolated chloroplasts. This indicates that the ascorbate peroxidase is a chloroplast enzyme. The pH optimum of ascorbate peroxidase activity was 8.2 and the Km value for ascorbate was 0.6 millimolar. Pyrogallol, glutathione, and NAD(P)H did not substitute for ascorbate in the enzyme catalyzed reaction. The enzyme was inhibited by NaN3, KCN, and 8-hydroxyquinoline but not ZnCl2 or iodoacetate. The ascorbate peroxidase activity of sonicated chloroplasts was inhibited by light but not in the presence of substrate concentrations of ascorbate.
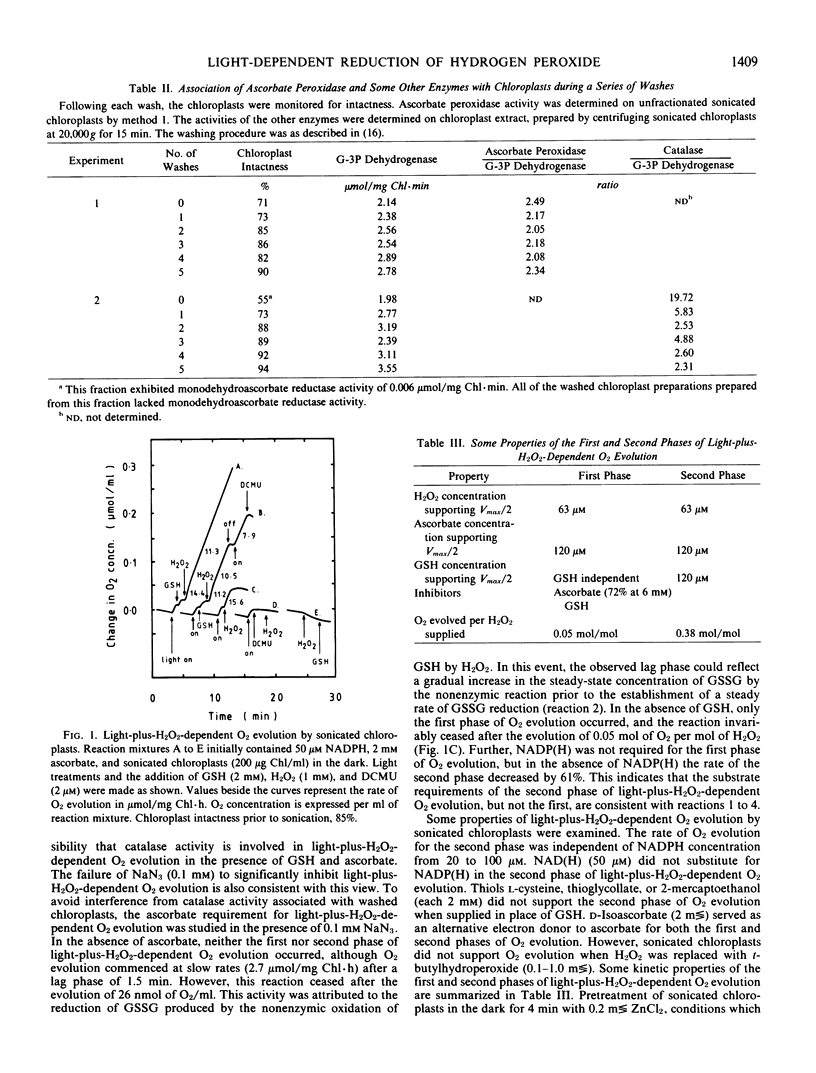
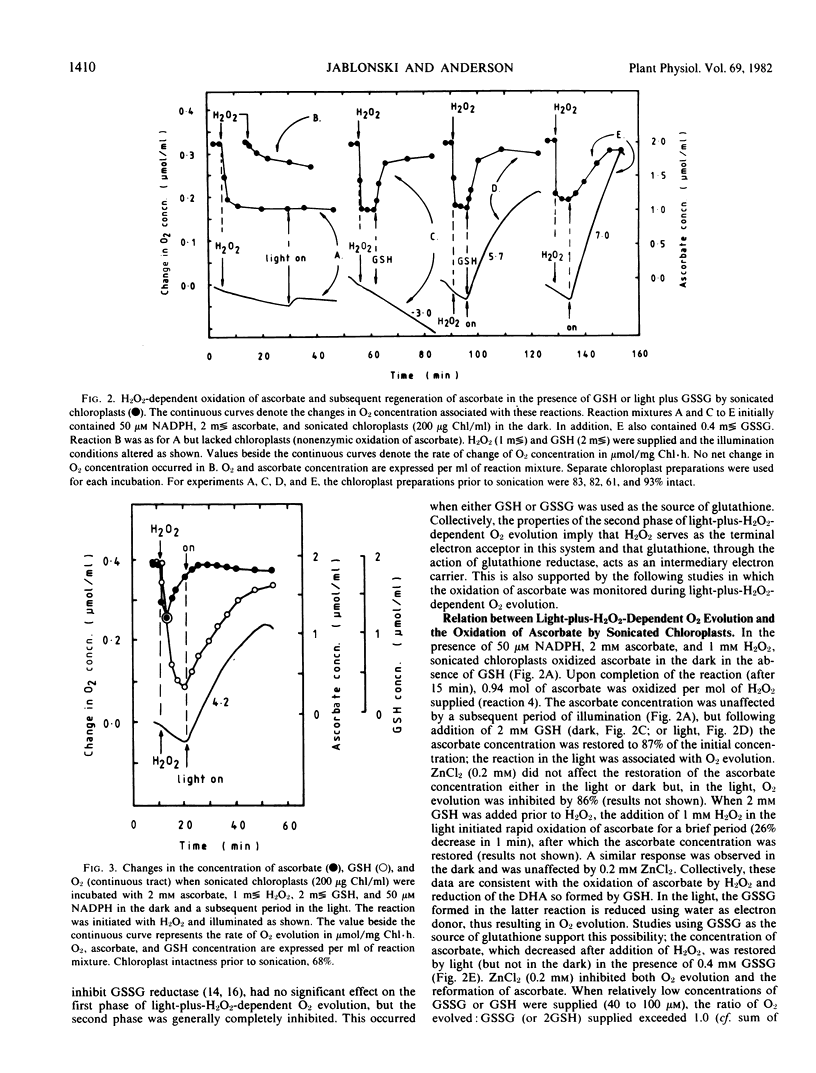
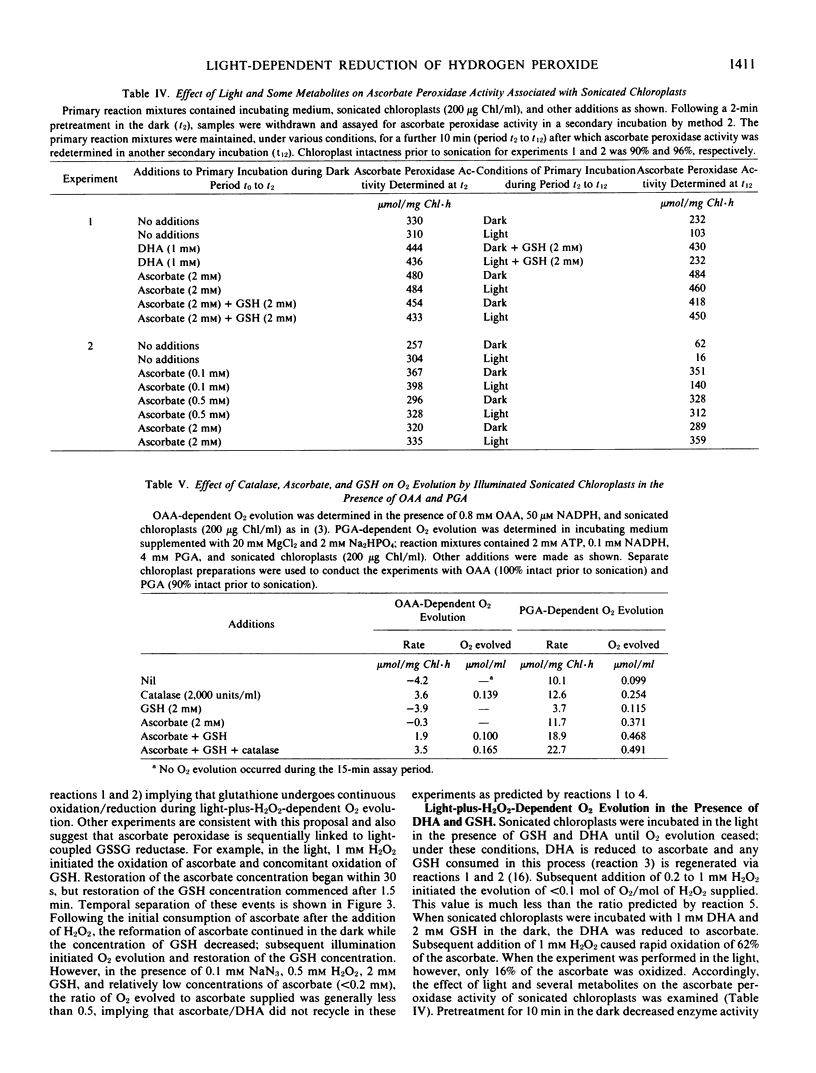
Illuminated ruptured chloroplasts, in the presence of 50 micromolar NADP(H), 2 millimolar l-ascorbate, and substrate concentrations of oxidized or reduced glutathione, catalyzed O2 evolution when H2O2 was added. Since the reaction was not inhibited by 0.1 millimolar NaN3 and did not occur in the dark, it was concluded that catalase was not involved. Light-plus-H2O2-dependent O2 evolution consisted of two distinct phases. The first phase was ascorbate-dependent and typically represented 10% of the total amount of O2 evolved. The second phase was dependent on ascorbate and glutathione. The properties of the second phase were consistent with the operation of light-coupled glutathione reductase sequentially coupled to glutathione dehydrogenase and ascorbate peroxidase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. F. Effects of washing and osmotic shock on catalase activity of intact chloroplast preparations. FEBS Lett. 1977 Dec 15;84(2):221–224. doi: 10.1016/0014-5793(77)80692-3. [DOI] [PubMed] [Google Scholar]
- Allen J. F., Whatley F. R. Effects of inhibitors of catalase on photosynthesis and on catalase activity in unwashed preparations of intact chloroplasts. Plant Physiol. 1978 Jun;61(6):957–960. doi: 10.1104/pp.61.6.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. W., House C. M. Polarographic study of oxaloacetate reduction by isolated pea chloroplasts. Plant Physiol. 1979 Dec;64(6):1058–1063. doi: 10.1104/pp.64.6.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada K., Kiso K., Yoshikawa K. Univalent reduction of molecular oxygen by spinach chloroplasts on illumination. J Biol Chem. 1974 Apr 10;249(7):2175–2181. [PubMed] [Google Scholar]
- Asada K., Urano M., Takahashi M. Subcellular location of superoxide dismutase in spinach leaves and preparation and properties of crystalline spinach superoxide dismutase. Eur J Biochem. 1973 Jul 2;36(1):257–266. doi: 10.1111/j.1432-1033.1973.tb02908.x. [DOI] [PubMed] [Google Scholar]
- Egneus H., Heber U., Matthiesen U., Kirk M. Reduction of oxygen by the electron transport chain of chloroplasts during assimilation of carbon dioxide. Biochim Biophys Acta. 1975 Dec 11;408(3):252–268. doi: 10.1016/0005-2728(75)90128-0. [DOI] [PubMed] [Google Scholar]
- Groden D., Beck E. H2O2 destruction by ascorbate-dependent systems from chloroplasts. Biochim Biophys Acta. 1979 Jun 5;546(3):426–435. doi: 10.1016/0005-2728(79)90078-1. [DOI] [PubMed] [Google Scholar]
- Halliwell B. The chloroplast at work. A review of modern developments in our understanding of chloroplast metabolism. Prog Biophys Mol Biol. 1978;33(1):1–54. doi: 10.1016/0079-6107(79)90024-5. [DOI] [PubMed] [Google Scholar]
- Jablonski P. P., Anderson J. W. Light-dependent Reduction of Oxidized Glutathione by Ruptured Chloroplasts. Plant Physiol. 1978 Feb;61(2):221–225. doi: 10.1104/pp.61.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski P. P., Anderson J. W. Light-dependent reduction of dehydroascorbate by ruptured pea chloroplasts. Plant Physiol. 1981 Jun;67(6):1239–1244. doi: 10.1104/pp.67.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C., Dench J., Moore A. L., Halliwell B., Foyer C. H., Hall D. O. Subcellular localisation and identification of superoxide dismutase in the leaves of higher plants. Eur J Biochem. 1978 Nov 15;91(2):339–344. doi: 10.1111/j.1432-1033.1978.tb12685.x. [DOI] [PubMed] [Google Scholar]
- MARRE E., ARRIGONI O. Ascorbic acid and photosynthesis. I. Monodehydroascorbic acid reductase of chloroplasts. Biochim Biophys Acta. 1958 Dec;30(3):453–457. doi: 10.1016/0006-3002(58)90089-1. [DOI] [PubMed] [Google Scholar]
- MEHLER A. H. Studies on reactions of illuminated chloroplasts. I. Mechanism of the reduction of oxygen and other Hill reagents. Arch Biochem Biophys. 1951 Aug;33(1):65–77. doi: 10.1016/0003-9861(51)90082-3. [DOI] [PubMed] [Google Scholar]
- Shigeoka S., Nakano Y., Kitaoka S. Metabolism of hydrogen peroxide in Euglena gracilis Z by L-ascorbic acid peroxidase. Biochem J. 1980 Jan 15;186(1):377–380. doi: 10.1042/bj1860377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeoka S., Nakano Y., Kitaoka S. Purification and some properties of L-ascorbic-acid-specific peroxidase in Euglena gracilis Z. Arch Biochem Biophys. 1980 Apr 15;201(1):121–127. doi: 10.1016/0003-9861(80)90495-6. [DOI] [PubMed] [Google Scholar]


