Abstract
Background:
Breast lumps constitute a significant proportion of surgical cases in women of both developed and developing countries. The aim of this study is to look the frequency distribution of various breast lesions on fine needle aspiration (FNA).
Materials and Methods:
The 902 patients who presented with palpable breast lump, irrespective of age and sex were included in the study. Frequency distribution of various breast lesions with respect to age and sex was studied. Cytology grading in breast carcinoma was correlated in 69 cases with histology grading.
Results:
The majority (N = 871) of patients were females with maximum (N = 566) patients between second and third decade. The 773 patients had benign breast lesions and maximum (N = 341) patients were in the second decade. Fibroadenoma was the commonest benign lesion followed by fibrocystic change and mastitis. Out of 119 malignant breast lesions, 31.93% [N = 38] were between 41-50 years of age, 28.57% [N = 34] in 51-60 years and 22.68% [N = 27] in between 31-40 years of age. Out of 119 malignant breast lumps and majority were infiltrating ductal carcinoma (N = 108). Cytology grading correlated maximum with histology grade in Grade I followed by Grade II and Grade III.
Conclusion:
With experienced hands, FNA is safe, cost effective and a reliable technique for preoperative evaluation of palpable breast lumps. FNA features are more informative when combined with physical and radiology findings (Triple test). Fibroadenoma, fibrocystic change and mastitis form the major bulk of benign breast lesions. Epithelioid cells when seen in inflammatory breast FNA smears, tuberculosis must be ruled out. In India, breast carcinoma arises in younger patients as compared to western countries. Grading of breast carcinomas must be done on FNA smears for selecting neoadjuvent therapy. Clinical breast examination and mammography screening in females should be encouraged in developing countries from the third decade onwards for early detection of breast carcinoma.
Keywords: Breast, benign, cytology, FNA, grading, malignant
INTRODUCTION
Breast lumps constitute a significant proportion of surgical cases in both developed and developing countries. Vast majority of them are in women and are benign. It is needed to distinguish benign lumps from malignant preoperatively for definite treatment.[1,2] The triple test includes physical breast examination, mammography and fine-needle aspiration (FNA) and has proved a reliable tool for accurate diagnosis of palpable breast masses.
The use of FNA for preoperative assessment of breast cancer has gradually declined in United States (US), Canada and United Kingdom (UK). The reasons are high error rates due to lack of experienced cytopathologists at individual laboratories and inability to provide adequate and suitable samples for assessment of prognostic markers. It is largely replaced by image-guided core-needle biopsy. Nevertheless, FNA continues to be used worldwide, especially in developing countries and is widely accepted as a reliable technique for preoperative evaluation of palpable breast lumps.[3,4,5,6,7,8,9] Scope of FNA has now extended into identifying the subtypes of benign, malignant lesions and residual disease for the purpose of planning the therapeutic protocol and eventual follow-up.[10,11] The present study is intended to look the frequency distribution of various lesions of palpable breast lumps.
MATERIALS AND METHODS
The present study is a retrospective cohort study of 902 patients between April 2002 and May 2013 who presented with palpable breast lump. Medical records of these patients were retrieved and reviewed. Detailed clinical history, physical examination and mammography/ultrasonography (USG) findings were noted.
All the patients underwent FNA in cytology clinic after prior written consent. FNA was done with standard technique and aseptic precautions by using 10 cc disposable syringe and 22-23 gauge needles. Material was smeared on glass slides. Slides were stained with Leishman's, Hematoxylene and Eosin [H and E] and Papanicolaou stains. Ziehl Neelsen (ZN) staining was done wherever required. In case, material obtained was not satisfactory, a repeat aspiration was done. In case of more than one swelling, aspiration was done from each swelling. USG/Mammography was done in 490 patients. Surgical specimens of 525 breast lumps were received for histopathological examination. Tissues were 10% formalin fixed and paraffin processed. The 3-4 μm thick sections were stained with H and E stain. ZN staining was performed wherever required.
Diagnosis of each lump was based on physical examination, FNA and/or mammography/USG features and/or histological features. Cytology grading of breast carcinomas done by using Robinson's grading system based on six cytomorphology features viz: Cell dissociation, cell size, cell uniformity, nucleoli nuclear margins, and chromatin pattern. Carcinomas were graded into Grade I, II and III. Out of 115 breast carcinomas, surgical specimens were received in 69 cases. Histology grading was done by Elston and Ellis's modification of Bloom-Richardson method based on histological features viz: Proportion of tubule formation, nuclear pleomorphism and mitotic count/10 hpf. Cytology grading was correlated with histology grading in 69 cases.
RESULTS
Out of 902 patients, 3.43% (N = 31) were male and 96.56% (N = 871) were female. Age of the patients ranged from 17-72 years with 62.74% (N = 566) were in the age group of 21N40 years. The 85.69% (N = 773) had benign breast lumps and 13.19% (N = 119) had malignant breast lumps. In 10 (1.10%) patients, FNA was inconclusive. Table 1 show FNA diagnosis of 902 breast lumps in various age-groups Out of 773 benign breast lesions, 44.11% (N = 341) were in the age-group of 21-30 years and 24.57% (N = 190) were in the age-group of 31-40 years. Out of 119 malignant breast lesions, 31.93% (N = 38) were between 41-50 years of age, 28.57% (N = 34) in 51-60 years and 22.68% (N = 27) in between 31-40 years of age.
Table 1.
Frequency of FNA diagnosis of 902 breast lesions in various age groups
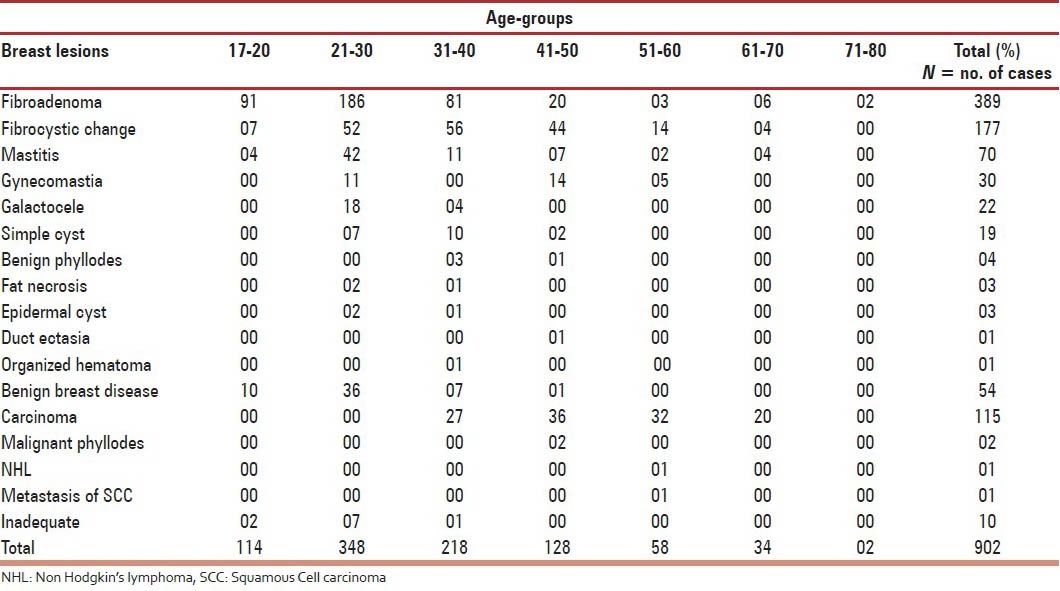
Out of 70 mastitis lesions, 75.71% (N = 53) were acute mastitis and tuberculous mastitis was seen in 20% (N = 14) cases. Table 2 shows type of mastitis in various age groups. Table 3 shows FNA diagnosis of 119 malignant lesions and cyto-histological correlation in 73 cases. Table 4 shows correlation of cytology grading with histology grading in 69 cases of breast carcinoma.
Table 2.
Type and frequency of mastitis on FNA in various age groups

Table 3.
Frequency of 119 malignant lesions on FNA and correlation of FNA and histology diagnosis of 73 lesions
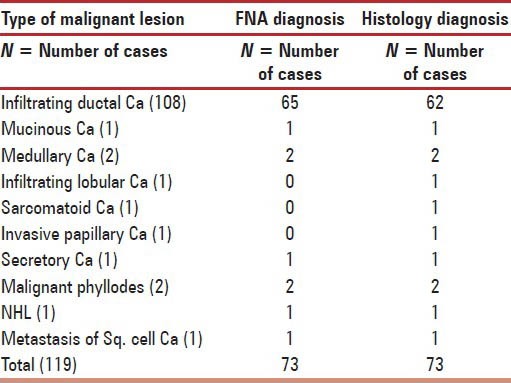
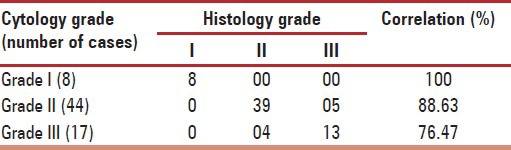
Table 4.
Correlation of cytology and histology grading of 69 breast carcinomas
DISCUSSION
FNA is widely accepted as a reliable technique in the initial evaluation of palpable breast lumps. It is simple, safe, cost-effective, minimally invasive, rapid and as sensitive as biopsy.[3,4,8] Primary goal of FNA is to separate benign lesions from malignant lesions for the purpose of planning the therapeutic protocol and uneventful follow-up.[10,11,12]
In our study, age of the patients ranged from 17-72 years with male to female ratio of 1:28. Similar age-group was observed in studies done in Asian countries.[13,14] Higher age-group in western countries was attributed to higher life expectancy.[15] Out of 902 patients, maximum patients (N = 773) had benign lesions. Malignant lesions were found in 13.19% (N = 118). It has been emphasized in the past that vast majority of the lesions in breast are benign.[1,16,17,18,19,20]
Fibroadenoma was the most frequently (N = 389) diagnosed lesion on FNA with maximum (N = 277) patients between 17-30 years. Multiple fibroadenomas were seen in 11.56% (N = 45) patients. Definitive FNA diagnosis was made in 46.27% (N = 180) patients based on diagnostic triad of cellular smears with bimodal pattern, numerous single bare bipolar nuclei and fragments of fibromyxoid stroma. Absence of any component of diagnostic triad and low cellularity are the common causes of pitfalls in correct diagnosis of fibroadenoma.[21] In remaining 209 cases, fibromyxoid stroma was not seen. Correct diagnosis in these cases was achieved by correlating FNA features with clinical and radiological features.
Another common benign breast lesion we encountered was fibrocystic change (N = 177) with maximum patients (N = 108) between 21-40 year. Though hormones play a role in its development exact pathogenesis remains obscure.[21] Fibrocystic change is not a specific cytological diagnosis. Cytology samples must be evaluated in the context of clinical and mammography findings. Some of these lesions simulate carcinoma clinically, radio logically, and microscopically.[22] More than 90% of the fibrocystic change were non-proliferative and FNA smears showed many macrophages, apocrine cells with or without scanty chronic inflammatory cells. Small clusters of ductal epithelial cells without atypia were seen. Few cases showed predominant ductal cells without atypia and were diagnosed as proliferative type. Compared to the general population, proliferative fibrocystic change with or without atypia has relative risk of developing carcinoma.[23]
Mastitis was seen in 7.76% (N = 70) patients with maximum (N = 42) patients between 21-30 years. Acute mastitis/abscesses, which is also known as puerperal or lactational mastitis, was seen in 78.57% (N = 55) with maximum (N = 40) between 21-30 years. Early diagnosis and management is of value.[23,24,25] Tuberculous mastitis is relatively rare with reported incidence varying from 3-4.5% in developing countries like India.[26] Few reports, including our dealing with cytomorphologic features have been published in the past.[27] Clinical and radiological features are not diagnostic and easily can be confused with breast cancer or pyogenic abscess. Out of 14 patients, majority (N = 12) presented with painless, firm mass and remaining two patients had hard lump. The 13 were females and one was male. FNA diagnosis was based on presence of epithelioid cells, caseous necrosis with or without acid-fast bacilli (AFB) or positive AFB culture. Out of 14 cases, 11 cases were confirmed on histology. Diagnosis of fat necrosis (N = 3) and duct ectasia was made based on FNA, clinical and radiological features.
Gynaecomastia accounted in 3.32% (N = 30) patients with maximum (N = 19) patients between 41-60 years and 11 patients in between 20-30 years. Gynaecomastia in young age is related to hormonal pubertal changes where as in later years, it may be caused by hormonally active tumors, cirrhosis or medications.[28] Similar observations were made in our study.
Galactocele accounted for 2.43% (N = 22) patients and all the patients were lactating. We came across 19 cases of simple cyst in our study, which are easily diagnosed by ultrasonography. Confident benign diagnosis can be rendered with FNA and surgical excision can be avoided. They can be managed with follow-up imaging studies.[29,30]
Common sites for epidermal inclusion cyst (EIC) are head, neck, trunk and extremities. Published cytology literature on EIC in breast is scanty and to date less than 46 cases have been reported. They remain underreported because of their insignificant clinical presentation.[32,33,34] FNA smears showed numerous anucleate and nucleated benign squamous cells. Imaging can follow asymptomatic lesions. Potential complications such as infections and malignant changes shall be ruled out on FNA.
Cytological appearance of benign phyllodes many times overlaps with fibroadenoma. Clinical, mammography and ultrasound examination have little diagnostic value except larger size (>3 cm) and increase in size are suggestive of phyllodes.[34] We came across, four cases of benign phyllodes in our study. In three cases definitive diagnosis was given based on predominance of stromal components over epithelial, fragments of highly cellular myxoid stroma and numerous single spindle shaped bare nuclei. Nuclear atypia and mitotic figures were absent. In one case, cytomorphologic features were similar to fibroadenoma except smears were more cellular. Based on strong clinical suspicion including larger size, FNA diagnosis was suggested. Subsequently histopathology confirmed the diagnosis.
The 5.98% (N = 54) benign breast lesions could not be subcategorized into specific lesions. FNA smears in these cases had low cellular yield and showed few small cohesive sheets of ductal epithelial cells with occasional myoepithelial cell in clusters and few bare nuclei. None of these cells showed features of nuclear atypia. Inflammatory cells were not seen on the background. Repeat aspiration could be done in 32 cases and smears showed similar features. Low cellular yield can be attributed to small size, deep-seated or fibrotic/hyalinized lesions. Majority of these patients were in the younger age Hgroup. Considering the age, clinical and radiological features, these lesions were labeled as benign breast disease [Table 1]. These patients were advised follow-up. In such cases, image-guided FNA or core needle biopsy will yield more specific diagnosis.
Breast cancer is the second most common cancer among Indian females next only to cervical cancer. One of the arguments for replacement of FNA by core-needle biopsy in some western countries is the high error rate due to lack of experienced cytopathologists. But in experienced hands, FNA is highly accurate diagnostic procedure with sensitivity and specificity over 95% for palpable breast lesions.[35,36] It may be more sensitive [97% vs. 90%] for core needle biopsy in the diagnosis of palpable breast cancers.[37] With advancement in the field of mammography, neo-adjuvant therapy and breast conservation surgery in breast carcinoma, cytology grading can be used for selection of neoadjuvant therapy. It allows the assessment of tumors without any surgical intervention so that morbidity associated with overtreatment of low-grade tumors can be avoided.[10,38]
Though reduction in the risk of breast carcinoma among lactated premenopausal women has been documented,[39] 27 patients were between 31-40 year; lactated and multiparous. Reports from western world state that breast cancer in women occur predominantly in fifth and sixth decades patients.[40]
Out of 119 malignant lesions, most common diagnosis was infiltrating duct carcinoma (N = 108) followed by medullary carcinoma (N = 2) and mucinous carcinoma, infiltrating lobular carcinoma, sarcomatoid carcinoma, invasive papillary carcinoma and secretory carcinoma in one case each. Remaining four lesions were malignant phyllodes (N = 2) and one case each of non-Hodgkin's lymphoma and metastasis of squamous cell carcinoma. Histopathology diagnosis was available in 73 cases.
The 66 cases in which FNA diagnosis of infiltrating duct carcinoma was given 62 cases were confirmed on histology [Table 3]. Remaining four cases turned out to be infiltrating lobular carcinoma, sarcomatoid carcinoma, invasive papillary carcinoma and secretory carcinoma one each. It is known that in few variants of breast carcinoma, histological subtype is often missed.[41] Lobular carcinoma is an important source of false negative diagnosis in breast FNA and could not be sub typed due to absence of specific features such as nuclear molding, absence of intra cytoplasmic lumina/vacuoles. Diagnosis of metaplastic carcinoma could not be done because FNA smears predominantly contain malignant ductal epithelial cells with few spindle cells. Third case which turned out to be invasive papillary carcinoma on histopathological examination showed occasional small necrotic papillary formations in FNA smears. Infracted papilla may closely mimic carcinoma. In general, a definitive cytological diagnosis of malignancy should not be made in papillary lesions and should be left on histopathological examination.[42] Secretary carcinoma is a rare form of breast carcinoma and primarily affect children. It can occur in adults.[43] Due to overlapping of cytomorphologic features between some benign and malignant lesions, rarely reports can be found in English literature.[44] In remaining malignant lesions, we did not face any difficulty in subtyping the lesion on FNA.
In 1.10% (N = 10) of the patients, FNA smears were inadequate for definite diagnosis. The rate of inadequate aspiration ranges from 0.7-25.3% and is influenced by nature of the lesion, available technology and the experience of operator. Repeat aspiration and image guidance definitely reduce the rate of inadequate aspiration.
In our study, cytology grading of malignant lesions correlated well with histology grading, which helped in selecting neo adjuvant therapy.
CONCLUSION
Benign breast lesions constitute a majority of breast lumps in surgical cases and mainly occur in second and third decade. Fibroadenoma, fibrocystic change and mastitis forms the major bulk of benign breast lesions. Epithelioid cells when seen in FNA breast smears, tuberculosis must be ruled out. FNA is safe, cost effective and a reliable technique for preoperative evaluation of palpable breast lumps. FNA features are more informative when combined with physical and radiology features. Grading of malignant lesions on cytology smears must be done for selecting neoadjuvent therapy. Malignant lesions though are more common in fourth and fifth decade, substantial number of cases occurs in third decade. Clinical breast examination and mammography screening should be encouraged in females from the third decade onwards for early detection of breast carcinoma.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Vaidyanathan L, Barnard K, Elnicki DM. Benign breast disease: When to treat, when to reassure, when to refer. Cleve Clin J Med. 2002;69:425–32. doi: 10.3949/ccjm.69.5.425. [DOI] [PubMed] [Google Scholar]
- 2.Guray M, Sahin AA. Benign breast diseases: Classification, diagnosis, and management. Oncologist. 2006;11:435–49. doi: 10.1634/theoncologist.11-5-435. [DOI] [PubMed] [Google Scholar]
- 3.Chaiwun B, Settakorn J, Ya-In C, Wisedmongkol W, Rangdaeng S, Thorner P. Effectiveness of fine-needle aspiration cytology of breast: Analysis of 2375 cases from northern Thailand. Diagn Cytopathol. 2002;26:201–5. doi: 10.1002/dc.10067. [DOI] [PubMed] [Google Scholar]
- 4.Nguansangiam S, Jesdapatarakul S, Tangjitgamol S. Accuracy of fine needle aspiration cytology from breast masses in Thailand. Asian Pac J Cancer Prev. 2009;10:623–6. [PubMed] [Google Scholar]
- 5.Cobb CJ, Raza AS. Obituary: “Alas poor FNA of breast — We knew thee well!”. Diagn Cytopathol. 2005;32:1–4. doi: 10.1002/dc.20189. [DOI] [PubMed] [Google Scholar]
- 6.Orell SR, Miliauskas J. Fine needle biopsy cytology of breast lesion: A review of interpretative difficulties. Adv Anat Pathol. 2005;12:233–45. doi: 10.1097/01.pap.0000184175.58295.a1. [DOI] [PubMed] [Google Scholar]
- 7.Zagorianakou P, Fiaccavento S, Zagorianakou N, Makrydimas G, Stefanou D, Agnatis NJ. FNAC: Its role, limitations and perspective in the preoperative diagnosis of breast cancer. Eur J Gynaecol Oncol. 2005;26:143–9. [PubMed] [Google Scholar]
- 8.Kalhan S, Dubey S, Sharma S, Duclani S, Preeti, Divit M. Significance of nuclear morphometry in breast masses. J Cytol. 2010;27:16–21. doi: 10.4103/0970-9371.66694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muddegowda PH, Lingegowda JB, Krupad R, Konapur PG, Shivarudrappa A, Subramaniam P. The value of systematic pattern analysis in FNAC of breast lesions: 225 cases with cytohistological correlation. J Cytol. 2011;28:13–9. doi: 10.4103/0970-9371.76942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhargava V, Jain M, Agarwal K, Thomas S, Singh S. Critical appraisal of cytological nuclear grading in carcinoma of breast and its correlation with ER/PR expression. J Cytol. 2008;25:58–61. [Google Scholar]
- 11.Joshi A, Maimoon S. Limitations of fine needle aspiration cytology in subtyping breast malignancies- A report of three cases. J Cytol. 2007;24:203–6. [Google Scholar]
- 12.Kaufman Z, Shpitz B, Shapiro M, Runa R, Lew S, Dinbar A. Tripple approach in the diagnosis of dominant breast masses: Combined physical examination, mammography and fine needle aspiration. J Surg Oncol. 1994;56:254–7. doi: 10.1002/jso.2930560413. [DOI] [PubMed] [Google Scholar]
- 13.Kumar R. A clinicopathologic study of breat lumps in Bhairahwa, Nepal. Asian Pac J Cancer Prev. 2010;11:855–8. [PubMed] [Google Scholar]
- 14.Ahmed HG, Ali AS, Almobarak AO. Utility of fine-needle aspiration as a diagnostic technique in breast lumps. Diagn Cytopathol. 2009;37:881–4. doi: 10.1002/dc.21115. [DOI] [PubMed] [Google Scholar]
- 15.Dennison G, Anand R, Makar SH, Pain JA. A perspective study of the use of fine needle aspiration cytology and core biopsy in the diagnosis of breast cancer. Breast J. 2003;9:491–3. doi: 10.1046/j.1524-4741.2003.09611.x. [DOI] [PubMed] [Google Scholar]
- 16.Caleffi M, Filho DD, Borghetti K, Graudenz M, Littrup PJ, Freeman-Gibb LA, et al. Cryoablation of benign breast tumors: Evolution of technique and technology. Breast. 2004;13:397–407. doi: 10.1016/j.breast.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Okoth C, Galukande M, Jombwe J, Wamala D. Benign proliferative breast diseases among female patients at a sub Saharan African tertiary hospital: A cross sectional study. BMC Surg. 2013;13:9. doi: 10.1186/1471-2482-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosa M, Mohammadi A, Masood S. The value of fine needle aspiration biopsy in the diagnosis and prognostic assessment of palpable breast lesion. Diagn Cytopathol. 2012;40:26–34. doi: 10.1002/dc.21497. [DOI] [PubMed] [Google Scholar]
- 19.Day C, Moatamed N, Fimbres AM, Salami N, Lim S, Apple SK. A retrospective study of the diagnostic accuracy of fine needle aspiration for breast lesions and implications for future use. Diagn Cytopathol. 2008;36:855–60. doi: 10.1002/dc.20933. [DOI] [PubMed] [Google Scholar]
- 20.Rahman MZ, Islams S. Fine needle aspiration cytology of palpable breast lump: A study of 1778 cases. Surg. 2013;S12:001. [Google Scholar]
- 21.Rosai J. 9th ed. Vol. 2. Missouri: Elsevier Publishers; 2004. Rosai and Ackerman's Surgical Pathology; p. 1779. [Google Scholar]
- 22.Marshall LM, Hunter DJ, Connolly JL, Schnitt SJ, Byrne C, London SJ, et al. Risk of breast cancer associated with atypical hyperplasia of lobular and ductal types. Cancer Epidemiol Biomarkers Prev. 1997;6:297–301. [PubMed] [Google Scholar]
- 23.Foxman B, D’Arcy H, Gillespie B, Bobo JK, Schwartz K. Lactation mastitis: Occurrence and medical management among 946 breast feeding women in the United States. Am J Epidemiol. 2002;155:103–14. doi: 10.1093/aje/155.2.103. [DOI] [PubMed] [Google Scholar]
- 24.Michie C, Lockie F, Lynn W. The challenge of mastitis. Arch Dis Child. 2003;88:818–21. doi: 10.1136/adc.88.9.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dener C, Inam A. Breast abscesses in lactating women. World J Surg. 2003;27:130–3. doi: 10.1007/s00268-002-6563-6. [DOI] [PubMed] [Google Scholar]
- 26.Shinde SR, Chandawarkar RY, Deshmukh SP. Tuberculosis of the breast masquerading as carcinoma: A study of 100 patients. World J Surg. 1995;19:379–81. doi: 10.1007/BF00299163. [DOI] [PubMed] [Google Scholar]
- 27.Chandanwale SS, Buch AC, Gore CR, Ramanpreet KC, Jadhav P. Fine needle aspiration cytology in breast tuberculosis: Diagnostic difficulties- study of eleven cases. Indian J Tuberc. 2012;59:162–7. [PubMed] [Google Scholar]
- 28.Coen P, Kulin H, Ballantine T, Zaino R, Frauenhoffer E, Boal D, et al. An aromatase-producing sex-cord tumour resulting in prepubertal gynaecomastia. N Engl J Med. 1991;324:317–22. doi: 10.1056/NEJM199101313240507. [DOI] [PubMed] [Google Scholar]
- 29.Houssami N, Irwig L, Ung O. Review of complex breast cyst: Implications for cancer detection and clinical practice. ANZ J Surg. 2005;75:1080–5. doi: 10.1111/j.1445-2197.2005.03608.x. [DOI] [PubMed] [Google Scholar]
- 30.Levine PH, Waisman J, Yang GC. Aspiration cytology of cystic carcinoma of the breast. Diagn Cytopathol. 2003;28:39–44. doi: 10.1002/dc.10209. [DOI] [PubMed] [Google Scholar]
- 31.Cameron DS, Hilsinger RL., Jr Squamous cell carcinoma in an epidermal inclusion cyst: Case report. Otolaryngol Head Neck Surg. 2003;129:141–3. doi: 10.1016/S0194-59980300466-2. [DOI] [PubMed] [Google Scholar]
- 32.Crystal P, Shaco-Levy R. Concentric rings within a breast mass on sonography: Lamellated keratin in an epidermal inclusion cyst. Am J Roentgenol. 2005;184:S47–8. doi: 10.2214/ajr.184.3_supplement.01840s47. [DOI] [PubMed] [Google Scholar]
- 33.Sing M, Maheshwari B, Khurana N, Jain S. Epidermal inclusion cyst in breast: Is it so rare? J Cytol. 2012;29:169–72. doi: 10.4103/0970-9371.101165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krishnamurthy S, Ashfaq R, Shin HJ, Sneige N. Distinction of phyllodes tumor from fibroadenoma. A reappraisal of an old problem. Cancer. 2000;90:342–9. [PubMed] [Google Scholar]
- 35.Chaiwn B, Thorner P. Fine needle aspiration for evaluation of breast masses. Curr Opin Obstet Gynecol. 2007;19:48–55. doi: 10.1097/GCO.0b013e328011f9ae. [DOI] [PubMed] [Google Scholar]
- 36.Ariga R, Bloom K, Reddy VB, Kluskens L, Francescatti D, Dowlat K, et al. Fine needle aspiration of clinically suspicious palpable breast masses with histopathologic correlation. Am J Surg. 2002;184:410–3. doi: 10.1016/s0002-9610(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 37.Ballo MS, Sneige N. Can core needle biopsy replace fine needle aspiration cytology in the diagnosis of palpable breast carcinoma. A comparative study of 124 women. Cancer. 1996;78:773–7. doi: 10.1002/(SICI)1097-0142(19960815)78:4<773::AID-CNCR13>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 38.Taniguchi E, Yang Q, Tang W, Nakamura Y, Shan L, Nakamura M, et al. Cytologic grading of invasive breast carcinoma. Correlation with clinicopathologic variables and predictive value of nodal metastasis. Acta Cytol. 2000;44:587–91. doi: 10.1159/000328533. [DOI] [PubMed] [Google Scholar]
- 39.Newcomb PA, Storer BE, Longnecker MP, Mittendorf R, Greenberg ER, Clapp RW, et al. Lactation and reduced risk of premenopausal breast cancer. N Engl J Med. 1994;330:81–7. doi: 10.1056/NEJM199401133300201. [DOI] [PubMed] [Google Scholar]
- 40.Anderson WF, Chatterjee N, Ersheller WB, Brawley OW. Estrogen receptor breast cancer phenotypes in the Surveillance, Epidemiology and End Results data base. Breast Cancer Res Treat. 2002;76:27–36. doi: 10.1023/a:1020299707510. [DOI] [PubMed] [Google Scholar]
- 41.Anantharamaiah H, Raja K, Suresh SP, Manikyam UK. Pitfalls in diagnosing specific subtypes of carcinoma breast on fine needle aspiration cytology: A report of two cases with review of literature. J Cancer Res Ther. 2012;8:454–6. doi: 10.4103/0973-1482.103534. [DOI] [PubMed] [Google Scholar]
- 42.Orel SR, Sterrett GF, Whitaker D. 4th ed. Elsevier: Churchill Livingstone; 2005. Fine Needle Aspiration Cytology; p. 215. [Google Scholar]
- 43.Krausz T, Jenkins D, Grontoff O, Pollock DJ, Azzopardi JG. Secretory carcinoma of the breast in adults: Emphasis is on late recurrence and metastasis. Histopathology. 1989;14:25–36. doi: 10.1111/j.1365-2559.1989.tb02111.x. [DOI] [PubMed] [Google Scholar]
- 44.Kim MK, Kwon GY, Gong GY. Fine needle aspiration cytology of cystic hypersecretory carcinoma of the breast. A case report. Acta Cytol. 1997;41:892–6. doi: 10.1159/000332724. [DOI] [PubMed] [Google Scholar]


