Abstract
Background:
The reported risk of coronary heart disease (CHD) in patients with a history of kidney stones is conflicting.
Aims:
The objective of this meta-analysis was to assess the association between a history of kidney stones and CHD risk.
Materials and Methods:
A literature search was performed using MEDLINE, EMBASE, and Cochrane Database of Systematic Reviews from inception until April 04, 2014. Studies that reported odds ratios or hazard ratios comparing the risk of CHD in patients with a history of kidney stones versus those without a history of kidney stones were included. Pooled risk ratios (RRs) and 95% confidence interval (CI) were calculated using a random-effect, generic inverse variance method.
Results:
Seven study populations from four cohort studies and one cross-sectional study were identified and included in the data analysis. The pooled risk ratio (RR) of CHD in patients with kidney stones was 1.24 (95% CI, 1.10-1.40). This result remained significant (RR, 1.23 [95% CI, 1.08-1.41]) when the sensitivity analysis was restricted to only cohort studies. A history of kidney stones was associated with increased CHD risk in females (RR, 1.43 [95% CI, 1.12-1.82]), whereas the association was not significant in males (RR, 1.14 [95% CI, 0.94-1.38]).
Conclusions:
Our study demonstrates a statistically significant increased risk of CHD in female patients with prior kidney stones. This finding suggests that a history of kidney stones is a risk factor for CHD in females and may impact clinical management.
Keywords: Cardiovascular, gender, nephrolithiasis
Introduction
Kidney stones have recently been linked to many comorbid conditions including hypertension, metabolic syndrome, diabetes, gout, and chronic kidney disease.[1] In addition, active and prior kidney stone formers are more likely to have accumulated risk factors for coronary heart disease (CHD).[2,3]
The reported risk of CHD in patients with a history of kidney stones, however, is still conflicting. Several studies have demonstrated an association between a history of kidney stones and CHD.[4,5,6,7,8] Conversely, a few studies have shown that a history of kidney stones is not a risk factor for CHD.[9,10,11] Tang et.al. found no association of prevalent kidney stone disease with all-cause and cardiovascular mortality.[12]
There is also controversy regarding CHD risk in male and female stone former groups. Elmfeldt et.al.[13] had previously reported an increased occurrence of kidney stones among male patients with myocardial infarction. More recent studies however show that CHD risk associated with a kidney stone is more pronounced for women than men.[2,14] The objectives of this meta-analysis were (1) to evaluate the association between a history of kidney stones and CHD risk and (2) to assess this association in different gender groups.
Materials and Methods
Search strategy
Two investigators (W.C. and C.T.) independently searched published studies indexed in MEDLINE, EMBASE, and the Cochrane database from inception to April 2014 using the terms “kidney calculi”, “nephrolithiasis”, and “kidney stone” combined with the terms “coronary heart disease” and “cardiovascular disease”. A manual search for additional relevant studies using references from retrieved articles was also performed. Conference abstracts and unpublished studies were excluded.
Inclusion criteria
The inclusion criteria were as follows:
Observational studies (case-control, cross-sectional or cohort studies) published as original studies to evaluate the association between kidney stones and CHD,
Odds ratios, relative risks, hazard ratios or standardized incidence ratio with 95% confidence intervals (CIs) were provided, and
A reference group composed of participants without a history of kidney stones was used.
Study eligibility was independently determined by the two investigators noted above. Differing decisions were resolved by mutual consensus. The quality of each study was independently evaluated by each investigator using Newcastle-Ottawa quality assessment scale.[10]
Data extraction
A standardized data collection form was used to extract the following information: Last name of the first author, title of the article, study design, year of study, country of origin, year of publication, sample size, characteristics of included participants, definition of CHD,[15] method used to diagnose kidney stones and CHD, mean duration of follow-up, and adjusted effect estimates with 95% CI. The two investigators mentioned above independently performed this data extraction.
Statistical analysis
Review Manager 5.2 software from the Cochrane Collaboration was used for data analysis. Point estimates and standard errors were extracted from individual studies and were combined by the generic inverse variance method of DerSimonian and Laird.[9] Given the high likelihood of between study variances, we used a random-effect model rather than a fixed-effect model.[16,17] Statistical heterogeneity was assessed using the Cochran's Q test. This statistic is complemented with the I2 statistic, which quantifies the proportion of the total variation across studies that is due to heterogeneity rather than chance.[18] A value of I2 of 0-25% represents insignificant heterogeneity, 26-50% low heterogeneity, 51-75% moderate heterogeneity, and >75% high heterogeneity.[19]
Results
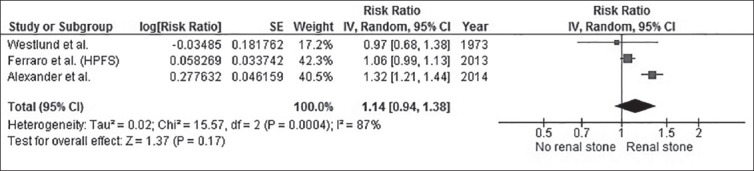
Our search strategy yielded 130 potentially relevant articles; 117 articles were excluded based on title and abstract for clearly not fulfilling inclusion criteria on a basis of the type of article, study design, population, or outcome of interest. Thirteen articles underwent full-length article review. Four articles were excluded because they reported the incidence of CHD risk factors, not incidence of CHD. Three articles were excluded because they were descriptive study without control groups. An article was excluded because it reported mortality rate from CHD, not incidence of CHD. Seven study populations from five articles (four cohort studies and one cross-sectional study) with 52,791 patients with kidney stones met our inclusion criteria and were included in the data analysis.[2,4,7,11,14] Table 1 describes the detailed characteristics and quality assessment of the included studies.
Table 1.
Main characteristics of the studies included in this meta-analysis
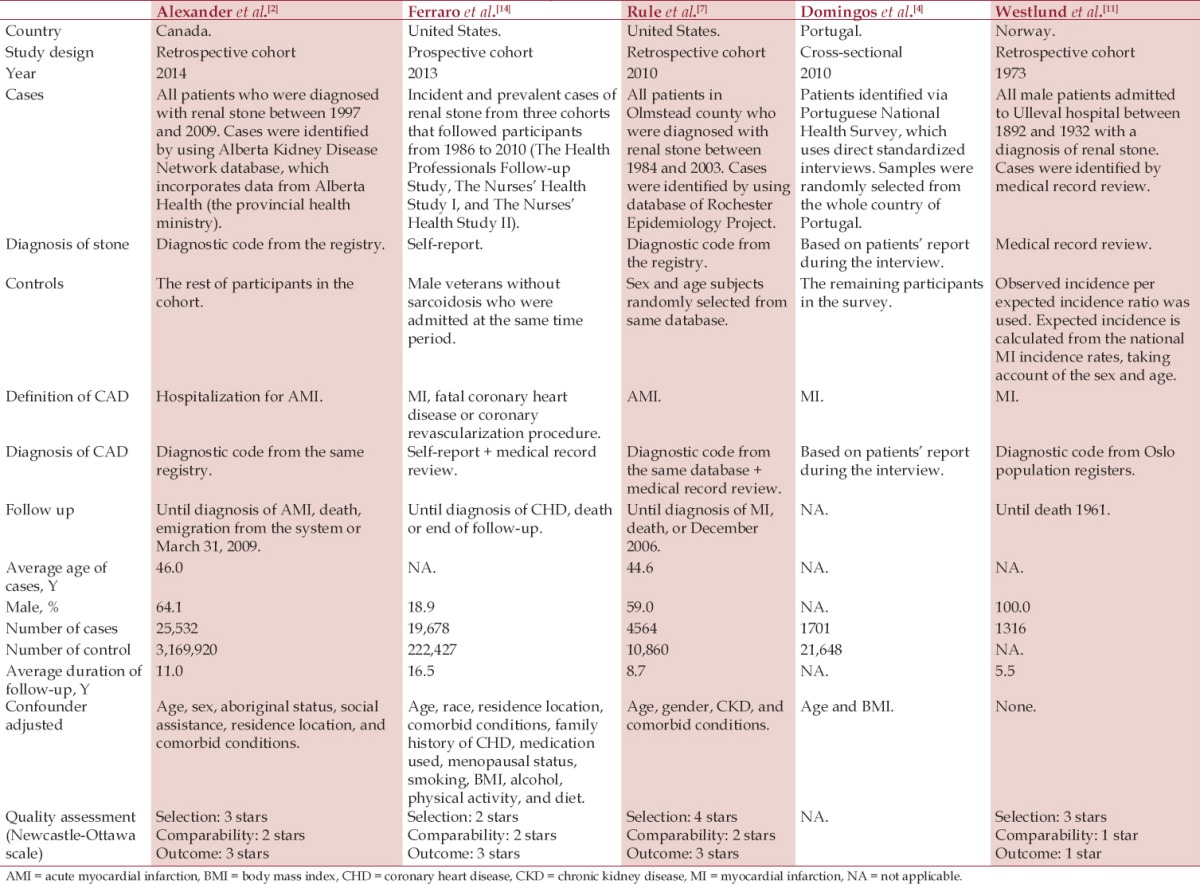
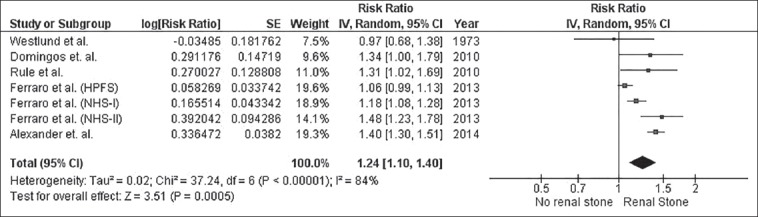
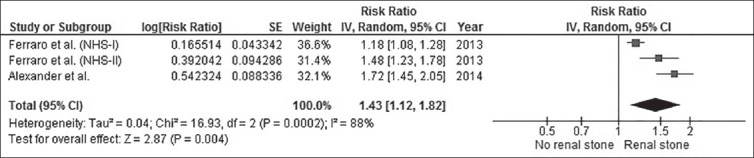
The pooled risk ratio (RR) of CHD of subjects with kidney stones versus control subjects was 1.24 (95% CI, 1.10-1.40). The statistical heterogeneity was high with an I2 of 84%. Figure 1 demonstrates the forest plot of the included studies. In addition, a history of kidney stones was associated with increased CHD risk in females (RR, 1.43 [95% CI, 1.12-1.82], I2, 88%), whereas the association was not significant in males (RR, 1.14 [95% CI, 0.94-1.38], I2, 87%). Figure 2 and Figure 3 show the forest plot of the included studies in females and males with kidney stones, respectively.
Figure 1.
Forest plot of the included studies comparing risk of CHD between patients with a history of kidney stones and those without a history of kidney stones
Figure 2.
Forest plot of the included studies comparing risk of CHD in females with a history of kidney stones and those without a history of kidney stones
Figure 3.
Forest plot of the included studies comparing risk of CHD in males with a history of kidney stones and those without a history of kidney stones
Sensitivity analysis
We performed a sensitivity analysis excluding the study by Domingos et al.[4] as this study was the only study with cross-sectional design, which could not establish a temporal relationship between kidney stones and CHD. The result remained significant (RR, 1.23 [95% CI, 1.08-1.41], I2, 86%).
Evaluation for publication bias
Funnel plot to evaluate publication bias is fairly asymmetric and, thus, providing a suggestion to the presence of publication in favor of positive studies.
Discussions
Our meta-analysis demonstrated a significant association between a history of kidney stones and coronary artery disease, with an overall 1.24-fold increased risk compared with those without a history of kidney stones. This association remained significant following sensitivity analysis restricted to cohort studies.
There are several plausible explanations for the increased risk of CHD in patients with kidney stones. First, kidney stones may be the manifestation of a systemic disorder.[20] A tendency to form stones has been associated with features of the metabolic syndrome, including obesity, hypertension and dyslipidemia.[5,8,21] Second, stone formers, especially those with uric acid stones, have demonstrated a significantly higher prevalence of diabetes and glucose intolerance.[21,22] Schwille et al.[23] found an association between postprandial insulinemia and increased urinary calcium and phosphorus excretion in patients with kidney stones.
A history of kidney stones has been demonstrated to be independently associated with CHD even after multivariable analysis for CHD risk factors.[2,4,7,14] These findings raise the hypothesis that urinary stone formation in the renal tubule has a vascular pathogenesis.[8] The underlying pathophysiology leading to calcium precipitation in the coronary arteries might also result from the same mechanism contributing to calcium precipitation in the renal tubules.[2] Khan et al.[24] recently found that the composition of vascular plaques are identical to Randall's plaque, the nidus of stone formation.[24] In addition, pyrophosphates, inhibitors of calcification are found both in blood and urine.[25] The deficiency of pyrophosphates both in blood and urine could explain the association between CHD and kidney stone formation.
The prevalence of kidney stones, however, is higher in males than females (10.6% vs 7.1%, respectively).[26] Coronary heart disease is also more common in males than in females (7.8% vs 4.6%).[1,27] Therefore, it is surprising that our study found that females with kidney stone were significantly more likely to develop CHD. The underlying pathophysiology remains unclear. Females with kidney stones may also be exposed to unknown factors that could increase their risk of CHD. Further studies are needed to identify the potential risk factors of CHD in females with kidney stones such as dietary, estrogen/progesterone, pregnancy, muscle to fat composition, and genetic factors. A history of kidney stones in women may be added as a possible cardiac risk factor.
Even though most of the included studies were of high quality[2,7,14] (as evaluated by Newcastle-Ottawa scale), there are some limitations. First, two studies[2,7] were conducted using medical registry-based databases; therefore, coding inaccuracies for both kidney stones and CAD may have been presented. Another two studies[4,14] used a definition of kidney stones composed of self-reporting, which reported as accurate in 97% of cases.[28] Second, there is statistical heterogeneity in this complete analysis. The difference in the definition of CHD may be one of the main sources of this heterogeneity. Two studies included only patients with acute myocardial infarction[2,7] whereas the other studies had a broader definition that included patients with old myocardial infarctions[4,11,14] and patients undergoing coronary artery interventions.[14] Third, this is a meta-analysis of observational studies with its inherent limitations. Hence, at best, it can demonstrate an association but not a causal relationship. Observational studies require multivariable adjustments that may fail to account for unanticipated or unknown confounders. Future studies are required to identify the mechanisms underlying this potential causal relationship and to assess the types of the kidney stone and their association with CHD.
Conclusion
Our study demonstrates a statistically significant increased risk of CHD in female patients with prior kidney stones. This finding suggests that a history of kidney stones is a risk factor for CHD in females and may impact clinical management. Physicians should be aware of this potential increased risk.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Goldfarb DS. Kidney stones and the risk of coronary heart disease. Am J Kidney Dis. 2013;62:1039–41. doi: 10.1053/j.ajkd.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Alexander RT, Hemmelgarn BR, Wiebe N, Bello A, Samuel S, Klarenbach SW, et al. Kidney stones and cardiovascular events: A cohort study. Clin J Am Soc Nephrol. 2014;9:506–12. doi: 10.2215/CJN.04960513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ando R, Nagaya T, Suzuki S, Takahashi H, Kawai M, Okada A, et al. Kidney stone formation is positively associated with conventional risk factors for coronary heart disease in Japanese men. J Urol. 2013;189:1340–6. doi: 10.1016/j.juro.2012.11.045. [DOI] [PubMed] [Google Scholar]
- 4.Domingos F, Serra A. Nephrolithiasis is associated with an increased prevalence of cardiovascular disease. Nephrol Dial Transplant. 2011;26:864–8. doi: 10.1093/ndt/gfq501. [DOI] [PubMed] [Google Scholar]
- 5.Hamano S, Nakatsu H, Suzuki N, Tomioka S, Tanaka M, Murakami S. Kidney stone disease and risk factors for coronary heart disease. Int J Urol. 2005;12:859–63. doi: 10.1111/j.1442-2042.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- 6.Linden V. Vitamin D and myocardial infarction. Br Med J. 1974;3:647–50. doi: 10.1136/bmj.3.5932.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rule AD, Roger VL, Melton LJ, 3rd, Bergstralh EJ, Li X, Peyser PA, et al. Kidney stones associate with increased risk for myocardial infarction. J Am Soc Nephrol. 2010;21:1641–4. doi: 10.1681/ASN.2010030253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zimmerer T, Weiss C, Hammes HP, Braun C, Hesse A, Alken P, et al. Evaluation of urolithiasis: A link between stone formation and diabetes mellitus? Urol Int. 2009;82:350–5. doi: 10.1159/000209371. [DOI] [PubMed] [Google Scholar]
- 9.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 10.Stang A. Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 11.Westlund K. Urolithiasis and coronary heart disease: A note on association. Am J Epidemiol. 1973;97:167–72. doi: 10.1093/oxfordjournals.aje.a121497. [DOI] [PubMed] [Google Scholar]
- 12.Tang J, Mettler P, McFann K, Chonchol M. The association of prevalent kidney stone disease with mortality in us adults: The national health and nutrition examination survey III, 1988-1994. Am J Nephrol. 2013;37:501–6. doi: 10.1159/000350691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elmfeldt D, Vedin A, Wilhelmsson C, Tibblin G, Wilhelmsen L. Morbidity in representative male survivors of myocardial infarction compared to representative population samples. J Chronic Dis. 1976;29:221–31. doi: 10.1016/0021-9681(76)90076-x. [DOI] [PubMed] [Google Scholar]
- 14.Ferraro PM, Taylor EN, Eisner BH, Gambaro G, Rimm EB, Mukamal KJ, et al. History of kidney stones and the risk of coronary heart disease. JAMA. 2013;310:408–15. doi: 10.1001/jama.2013.8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ungprasert P, Wannarong T, Panichsillapakit T, Cheungpasitporn W, Thongprayoon C, Ahmed S, et al. Cardiac involvement in mixed connective tissue disease: A systematic review. Int J Cardiol. 2014;171:326–30. doi: 10.1016/j.ijcard.2013.12.079. [DOI] [PubMed] [Google Scholar]
- 16.Ungprasert P, Charoenpong P, Ratanasrimetha P, Thongprayoon C, Cheungpasitporn W, Suksaranjit P. Risk of coronary artery disease in patients with systemic sclerosis: A systematic review and meta-analysis. Clin Rheumatol. 2014;33:1099–104. doi: 10.1007/s10067-014-2681-4. [DOI] [PubMed] [Google Scholar]
- 17.Ungprasert P, Srivali N, Wijarnpreecha K, Thongprayoon C, Cheungpasitporn W, Knight EL. Is the incidence of malignancy increased in patients with sarcoidosis? A systematic review and meta-analysis. Respirology. 2014;19:993–8. doi: 10.1111/resp.12369. [DOI] [PubMed] [Google Scholar]
- 18.Cheungpasitporn W, Thongprayoon C, O’Corragain OA, Edmonds PJ, Ungprasert P, Kittanamongkolchai W, et al. The risk of kidney cancer in patients with kidney stones: A systematic review and meta-analysis. QJM. 2014 doi: 10.1093/qjmed/hcu195. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakhaee K. Nephrolithiasis as a systemic disorder. Curr Opin Nephrol Hypertens. 2008;17:304–9. doi: 10.1097/MNH.0b013e3282f8b34d. [DOI] [PubMed] [Google Scholar]
- 21.Taylor EN, Stampfer MJ, Curhan GC. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int. 2005;68:1230–5. doi: 10.1111/j.1523-1755.2005.00516.x. [DOI] [PubMed] [Google Scholar]
- 22.Cameron MA, Maalouf NM, Adams-Huet B, Moe OW, Sakhaee K. Urine composition in type 2 diabetes: Predisposition to uric acid nephrolithiasis. J Am Soc Nephrol. 2006;17:1422–8. doi: 10.1681/ASN.2005121246. [DOI] [PubMed] [Google Scholar]
- 23.Schwille PO, Schmiedl A, Herrmann U, Wipplinger J. Postprandial hyperinsulinaemia, insulin resistance and inappropriately high phosphaturia are features of younger males with idiopathic calcium urolithiasis: Attenuation by ascorbic acid supplementation of a test meal. Urol Res. 1997;25:49–58. doi: 10.1007/BF00941906. [DOI] [PubMed] [Google Scholar]
- 24.Khan SR, Rodriguez DE, Gower LB, Monga M. Association of Randall plaque with collagen fibers and membrane vesicles. J Urol. 2012;187:1094–100. doi: 10.1016/j.juro.2011.10.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlieper G, Westenfeld R, Brandenburg V, Ketteler M. Inhibitors of calcification in blood and urine. Semin Dial. 2007;20:113–21. doi: 10.1111/j.1525-139X.2007.00257.x. [DOI] [PubMed] [Google Scholar]
- 26.Scales CD, Jr, Smith AC, Hanley JM, Saigal CS. Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160–5. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention (CDC). Prevalence of coronary heart disease--United States, 2006-2010. MMWR Morb Mortal Wkly Rep. 2011;60:1377–81. [PubMed] [Google Scholar]
- 28.Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med. 1997;126:497–504. doi: 10.7326/0003-4819-126-7-199704010-00001. [DOI] [PubMed] [Google Scholar]


