Abstract
Background:
The underlying role of intracellular glycogen in atrial fibrillation is unknown. Experimental models developed in the goat have shown an increase of intracellular glycogen concentration in atrial myocytes resulting from prolonged pacing induced atrial fibrillation (AF). These observed glycogen molecules are as a result of structural remodeling and are known to replace the intracellular myofibrils causing myolysis in studies done in different animal models. The accumulation of glycogen is progressively and directly related to the duration of pacing-induced AF. Similar responses have been seen in clinically derived atrial tissues.
Aims:
We intend to present an endocrine hypothesis supported by published evidence that stress acting through the hypothalamic-pituitary-adrenal axis (HPA) is a contributing metabolic factor responsible for the increase of glucose levels via the hormone cortisol. This excess glucose is then metabolized by the myocytes during each heart beat and stored as glycogen. A literature search was done, and published evidence supporting stress was shown to be the main factor for the formation of glucose leading to glycogen deposition to in the cardiac myocytes.
Results:
Stress on the HPA axis stimulates the adrenal glands to release the hormone cortisol in the blood stream; this in turn increases the cardiac tissue glycogen concentration. It is also known that during each beat, excess glucose is removed by the myocytes and stored as glycogen. As aforementioned, in the cardiac myocytes, dense glycogen content with/without loss of myofibrils has been detected in both human and animal models of AF.
Conclusions:
We hypothesize that the increase of the intrinsic glycogen concentration and distribution is a result of a metabolic disruption caused by stress through the HPA Axis. For example, in atrial myocytes, the glycogen molecules impede the normal intercellular communications leading to areas of slow conduction favoring reentrant-based AF.
Keywords: Adrenal Glands, Atrial fibrillation and AF, Cortisol, Endocrine imbalance, Glycogen and AF, Hypothalamic-pituitary-adrenal axis, Stress
Introduction
It is known that stress on the brain triggers the Hypothallamus-Pituitary-Adrenal Axis (HPA) to release cortisol in the blood stream; this in turn causes an increase in the glucose level in the atrial myocytes of the cardiac tissue. Responding to this glucose excess the atrial myocytes (with each beat) convert glucose to glycogen. Presumably, by the action of cell depolarization and consequent intracellular flow, the glycogen then concentrates against the gap junctions pores [Figure 1], giving the histological visual appearance of an intracellular log jam effect. We hypothesize that this glycogen concentration impedes the normal function of the intercellular communication leading to areas of slow conduction. Not only the increased glycogen concentration but also its location within the myocytes provides a non-uniform wavefront of activation, favoring reentry and ultimately atrial fibrillation (AF).
Figure 1.
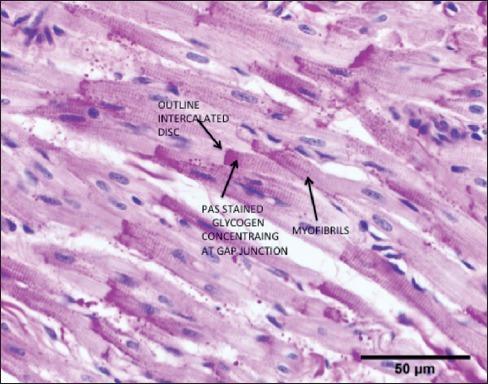
Heart Goat- Control Normal Sinus Rhythm. Right Atrial appendage (RAA) showing stained glycogen by the PAS/Schiff Diastase Method. Notice the heterogeneity and concentration of the glycogen distribution in the atrial myocytes. Arrows highlight one of many myocytes where the glycogen concentrates against the gap junctions. Cell-to-Cell flow could be impeded by the large molecular size of the glycogen molecules
Brief global historical perspective
Atrial fibrillation is considered one of the most prevalent cardiac arrhythmias seen clinically. Its presence has a profound negative impact on the quality of life and possible complications such as a stroke that could be life threatening. A systematic review of publication-based studies from the 21 Global Burden of Disease (GBD) regions, estimated that the number of individuals with AF in 2010 was 33.5 million.[1] “Mortality associated with AF was higher in females, and increased by 2-fold in females and 1.9 in males between 1990 and 2010”. The article concludes, “These findings provide evidence of progressive increases in overall burden, incidence, prevalence and AF-associated mortality between 1990-2010.” This preamble summarizes the concern in the world community to find a cause and cure for this disease. We introduce a hypothesis correlating the glycogen increase as a result of stress-related dysfunction as a major mechanism causing AF. This manuscript presents a new concept in which we postulate that the response to stress via the HPA is identified as the initiator of a metabolic dysfunction in heart tissues starting a cascade of effects leading to AF.
Animal models for the study of AF
Numerous animal models were designed to study cardiac metabolism, as well as the disorganized and chaotic rhythm in the atrium known as AF. For example, Invertebrates organisms have been found to be a suitable model for mammalians neuroendocrine investigations.[2] When mussels are cerebrolectomized, the glycogen content in all body tissues is statistically and significantly reduced when compared to controls.[3] That report links the brain with changes in body tissue glycogen concentration. Recently,[4] we reported an intrinsic control glycogen presence in atria myocytes in the goat. Glycogen in the atrial tissue has been reported to progressively increase in concentration as AF proceeds from the paroxysmal to the persistent forms. The development of AF by pacing in goats has been shown to be representative of patient populations with AF.[5] In 1995, Wijffels et al.[6] published a seminal report providing an understanding of the mechanisms underlying AF. They coined the phrase “Atrial Fibrillation Begets Arial Fibrillation”. After several weeks of AF in instrumented goats, it was found that the episodes were occurring without pacing until AF became continuous. The progression was associated with not only electrophysiological changes favoring the reentrant form of AF, but also there were structural changes among which was the progressive accumulation of glycogen within atrial myocytes. This latter finding fits with our hypothesis that intracellular glycogen increase in AF is time dependent and may be a contributing factor in the genesis of this arrhythmia.
Materials and Methods
A cascade effect addressing the effect of stress on the brain is presented to explain the genesis and perpetuation of AF. Relevant published evidence in the medical literature, as well as our own work in progress, are in support and are essential elements to this hypothesis (see Appendix).
Results
Preliminary data supporting the hypothesis
How and where the excess glucose is deposited in the body and converted into glycogen is a key to our endocrine dysfunction and AF hypothesis. Examples of data supporting our hypothesis are: A recent paper by Embi et al.[4] correlates “glycogen deposition in the left atrial appendage concentrating against the intercalated discs and side-to-side connections is an impediment of cell-to-cell conduction that could result in a non-uniform wave of activation, with areas of slow conduction, predisposing the left atrium to reentrant-based atrial fibrillation”. A seminal study done in instrumented goats, Wijffels MC et al.[6] described the progression of AF from paroxysmal (intermittent) to persistent (chronic). In another unpublished work (joint cooperative study from the Oklahoma State University and other Universities in China by Ling Zhang et al. entitled “Structural Changes in the Progression of AF: Potential role of Glycogen and Fibrosis as Perpetuating Factors” is presently under review with the conclusion, “evidence is presented linking glycogen accumulation and fibrosis in the long standing persistent form of AF”. Engel and WC.[7] found that the “sensitization of endocrine organs to anterior pituitary hormone by the autonomic nervous system” is strong evidence “for the capability of autonomic neural activity to alter the functional sensitivity of endocrine glands”. Furthermore, in 2010, Lei Zaghn et al.[8] documented that rapid atrial stimulation induces left atrial mechanical remodeling in the dog. They found “Marked changes seen in cellular substructures, such as loss of myofibrils, accumulation of glycogen, and changes in mitochondrial shape and size”. The electron microscopic pictures also showed disrupted intercalated discs.
In 2014, in a research correspondence to the Journal of the American College of Cardiology,[9] dark chocolate consumption is described as “substantially lowering cardiovascular mortality due to the high content of polyphenolic flavonoids, but underlying mechanisms remain unclear (italics ours)”. The letter continues, “Animal studies suggest that flavonoid administration may protect from adverse effects by reducing stress responses including HPA axis activation (italics ours)”. We believe our hypothesis helps to explain the aforementioned underlying mechanism that is, that glycogen accumulation in the atrial myocytes is one more factor to be added to the mechanisms providing the genesis and perpetuation of AF.
Brain response to stress
The HPA axis is seen by the neurologist as one system that responds to stress by initiating a cascade effect that starts with the hypothalamus releasing the hormone corticotropin to the pituitary gland.[10,11] The pituitary gland, in turn, releases the adrenocorticotropic hormone (ACTH) into the blood stream affecting the adrenal glands. The adrenal cortex produces three hormones one of them being a corticosteroid, cortisol. Cortisol (colloquially known as the stress hormone) has been reported to increase the glucose level in the blood stream.[12] providing the basis for its action on the heart tissues (see Appendix). As aforementioned, cortisol has been reported to increase the glucose level in the heart. Cortisol also decreases the body immune response. The drug dexamethasone (DEX) a synthetic corticosteroid, has similar functions when compared to the natural hormone cortisol.[13] Drug dexamethasone also acts on occasion as a cortisol inhibitor; this paradox of an exogenous hormone inhibiting an endogenous counterpart's action will be explained below. The use of the synthetic glucocorticoid, DEX, has been also found to increase cardiac tissue glucose and glycogen levels.[14]
Excess glucose increases glycogen in atrial tissue
The metabolic response of the cardiac tissue to the excess glucose is proposed as a mechanism leading to increased glycogen in atrial myocytes. For example, in a basic research paper done in rats in vitro.[15] one of the conclusions was: “ When young rat heart cells in culture are actively beating (italics ours), they oxidize free fatty acids at a rate parallel with cellular ATP production. Both fatty acid oxidation and ATP production remain constant while the cells continue to beat. Furthermore, glucose is removed from the growth medium by the cells and stored as glycogen (italics ours)”. Supporting the latter, in other animal models studying the effect of exogenous fuel on glycogen utilization in cardiac myocytes in vitro, Milligan reported.[16] “in the presence of exogenous fuel (5 mmmoll-1 glucose) glycogen was spared and noradrenaline stimulated glycogenolysis (breakdown of glycogen) was apparently inhibited (italics ours)”. In the same article, reporting of hearts (in vivo) in stressful situations such as exercise it was concluded that “lactate utilization would exceed glucose utilization. Lactate oxidation is limited by lactate oxidase. However, CO2 production from glucose utilization continued to increase”. Milligan's and Anatasia's[15,16] findings are key for our conceptualization as to how glycogen levels progressively increase in the atrial myocytes during AF.
Proposed Hypothesis of glycogen as factor in AFWe propose that in paroxysmal and persistent AF, the arrhythmia is triggered by a metabolic effect on the heart-by hormone exchanges that increases the blood glucose levels, and the aforementioned mechanisms result in the marked intracellular glycogen increase. When rapid atrial pacing AF is induced experimentally and sustained for at least one or more hours, the rapid electrical impulses causes stress in the atrial tissue, which in turn stresses the HPA axis, causing the adrenal glands to release cortisol that in turn, increases gluconeogenesis (formation of glucose). This increase in glucose reaches the atrial tissue via the blood stream. Due to the prolonged rapid atrial pacing there is a depletion of the normal lactate supply and glycogen is deposited in the intercellular space as glucose utilization continues.
In cardiac cells, it can be said “permeability of the nexus (between adjacent cells) falls with increasing molecular weight of the compound”. A seminal experiment done in vitro.[17] by Muir [Figure 2] who first reported that, when subjected to centrifugation, the glycogen molecules were unable to permeate through the Purkinje fibers gap junctions in the sheep. Notably, the Purkinje cells have a greater number of gap junction pores than atrial myocytes, but both types of junction become impermeable to ionic passage and therefore electrical conduction is blocked. Recently, in vivo studies demonstrated that the atrial gap junctions are impermeable to larger molecules, such as glycogen.[18]
Figure 2.
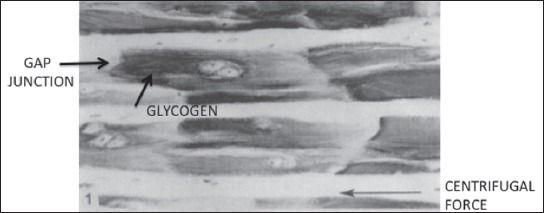
Heart Sheep: In vitro demonstration of gap junction selectivity towards the glycogen molecules. Purkinge Fibers after centrifugation. Notice the presence of the stained glycogen concentrating against the gap intercellular intercalated discs. Gap Junction. Purkinje cell are reported to have a higher density of pores that the atrial myocytes. Selectivity towards molecular size is clearly demonstrated. (Arrow denotes centrifugal force) Image printed with permissionfrom publisher: Muir AR. Further observationson the cellular structure of cardiac muscle JAnat Lond 1965;99:27-46
Gap junctions proteins alterations in atrial myocytes may lead to abnormal electrical coupling and may trigger arrhythmias.[19] This finding correlates with our hypothesis of glycogen impeding the normal syncytial electrical flow through the gap junctions. An example in humans of glycogen occupying 100% of the atrial myocyte's intracellular space in a patient with AF was suggested by Corradi et al. whose report.[20] depicted a myocytolysis myocyte with complete glycogen substitution. The glycogen appeared “trapped” inside the cell.
Renal denervation improves AF outcome
Older and recent findings are of relevant to our hypothesis: In 1968, J. Chales Daw.[21] in researching the Influences of Corticosteroids on Cardiac Glycogen Concentration in the Rat, reports that “Bilateral adrenalectomy resulted in a decrease (4.97 to 2.79 mg/g) in cardiac glycogen concentration The glucocorticoid DEX (40 μ/day) increased the cardiac glycogen concentration.”, the author continues by observing that “It is also possible that the changes in cardiac glycogen are secondary to changes in the blood glucose concentration”.
There is also further published evidence supporting our hypothesis of the adrenal glands involvement in AF through activation of sympathetic innervation. A catheter procedure for renal denervation improves the outcome in patients with AF and hypertension. “Although the exact mechanisms are still unclear, it has been suggested that ablation of afferent nerves arising from the renal arteries, or other arteries for that matter, can reduce central sympathetic outflow back to the renal nerves as well as via cardiac nerves to the heart, thereby affecting blood pressure as well as heart rate and cardiac arrhythmias’.[22,23]
Synthetic corticosteroid dexamethasone (DEX) inhibits endogenous cortisol
The paradoxical action of infusing exogenous corticosteroid reducing blood cortisol levels is a well-known negative feedback counteracting circulating corticosteroids (cortisol) released by and HPA axis. Dexamethasone exerts its inhibitory effects on the HPA axis primarily by acting at glucocorticoid receptors (GRs) in the pituitary. In experimental studies, DEX “produced a selective and significant decrease in available GR in peripheral tissues (pituitary and spleen)”.[24] Peripheral (in the context of the previous sentence) meaning outside of the blood-brain barrier. These findings support our hypothesis of the role of cortisol and cardiac glucose increase and was demonstrated in the following report: Viviano et al.,[25] proposed that “a single dose (of DEX) prior to cardiac surgery of (0.6 mg/k) can significantly diminish Post Operative Atrial Fibrillation (POAF) P = 0.027”. The authors also raise the question, “Is perioperative corticosteroid administration associated with a reduced incidence of POAF in adult cardiac surgery?” We believe the question mark is appropriate since the action of DEX regarding the HPA axis sensitivity is related to body fat distribution and dosage. The activity of the HPA axis is regulated by central GRs. “This activity can be tested by the administration of exogenous glucocorticoids, which normally inhibits cortisol secretion”. Waist hip circumference ratio (WHR) was correlated with the effect of DEX, and the conclusion was, “men with an elevated WHR, experience a decrease in the inhibition of cortisol secretion by dexamethasone…this explains their elevated sensitivity of their H-P-A Axis”.[26] In other words, DEX reduces POAF (by reducing the cortisol level in blood. Atrial fibrillation is limited by the patient WHR. The authors noted that “both lower (up to 8 mg.) and higher (236-2850 mg) dosing resulted in blunted effects”. Based on that observation, we propose that once the cortisol level is refractory to DEX, the POAF prevalence is not reduced.
Conclusions
A hypothesis has been formulated as to the mechanism explaining why glycogen accumulates in the atrial myocytes when AF is induced by rapid atrial pacing in the goat animal model. It is important to note that glycogen deposition has also been found in humans with chronic AF. We hypothesize, supported by the extensive data presented, that the normal electrophysiological balance is interrupted in the atrial syncytium by the abnormal intracellular concentration and distribution of the glycogen molecules. This, in turn, makes the atrium (specially the left) susceptible to reentrant arrhythmias, thus triggering AF.
Appendix
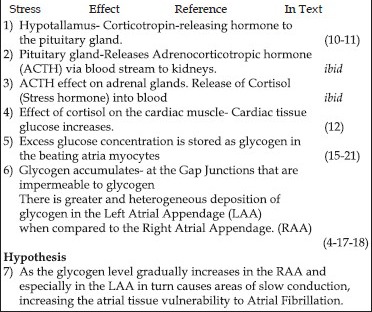
Outline of a Cascade Effect Caused by Stress on the Hypothalamic-Pituitary-Adrenal Axis in Humans and the Genesis of Atrial Fibrillation
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: A global burden of disease 2010 Study. Circulation. 2014;25(129):837–47. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ottaviani The mollusk as a suitable model for mammalian immune-neuroendocrine investigations. Invertebrate Survival Journal. 2004;1:2–4. [Google Scholar]
- 3.Dongre SB, Kure AR. Glycogen level in the different body parts of cerebrallectomized, bivalve mussel Lamillediens Corrianus. Species. 2014;7:15–29. [Google Scholar]
- 4.Embi AA, Scherlag BJ. Glycogen and the propensity for atrial fibrillation: Intrinsic anatomic differences in glycogen in the left and right atria in the goat heart. North Am J Med Sci. 2014;6:510–5. doi: 10.4103/1947-2714.143282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishida K, Michael G, Dobrev D, Nattel S. Animal models for atrial fibrillation: Clinical insights and scientific opportunities. Europace. 2010;12:160–72. doi: 10.1093/europace/eup328. [DOI] [PubMed] [Google Scholar]
- 6.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–68. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 7.Engeland WC. Sensitization of endocrine organs to anterior pituitary hormone by the autonomic nervous system. Handb Clin Neurol. 2013;117:37–44. doi: 10.1016/B978-0-444-53491-0.00004-3. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Ji XP, Wang R, Zhang W, Jiang SL, Chen WQ, et al. Short-term rapid right atrial pacing induces left atrial mechanical and anatomical remodeling in a canine model. J Geriatr Cardiol. 2010;7:30–5. [Google Scholar]
- 9.Wirtz PH, von Känel R, Meister RE, Arpagaus A, Treichler S, Kuebler U, et al. Dark chocolate intake buffers stress reactivity in humans. J Am Coll Cardiol. 2014;63:2297–9. doi: 10.1016/j.jacc.2014.02.580. [DOI] [PubMed] [Google Scholar]
- 10.Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8:383–95. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honour JW. Hypothalamic-pitituary-adrenal axis. Respir Med. 1994;88(Suppl A):9–15. doi: 10.1016/s0954-6111(05)80035-6. [DOI] [PubMed] [Google Scholar]
- 12.Khani S, Tayek JA. Cortisol increases gluconeogenesis in humans: Its role in the metabolic syndrome. Clin Sci (Lond) 2001;101:739–47. doi: 10.1042/cs1010739. [DOI] [PubMed] [Google Scholar]
- 13.MedlinePlus. National Library of Medicine NIH National Institute of Health- Update; 2010. Jan 09, Dexamethasone Oral. [Google Scholar]
- 14.Puthanveetil P, Wang F, Kewalramani G, Kim MS, Hosseini-Beheshti E, Ng N, et al. Cardiac glycogen accumulation after dexamethasone is regulated by AMPK. Am J Physiol Heart Circ Physiol. 2008;295:1753–62. doi: 10.1152/ajpheart.518.2008. [DOI] [PubMed] [Google Scholar]
- 15.Anastasia JV, McCarl RL. Effects of cortisol on cultured rat heart cells: Lipase activity, fatty acid oxidation, glycogen metabolism, and ATP levels as related to the beating phenomenon. J Cell Biol. 1973;57:109–16. doi: 10.1083/jcb.57.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milligan LC. Adrenergic stimulation of substrate utilization by cardiac myocytes isolated from rainbow trout. J Exp Biol. 1991;159:185–202. doi: 10.1242/jeb.159.1.185. [DOI] [PubMed] [Google Scholar]
- 17.Muir AR. Further observations on the cellular structure of the cardiac muscle. J Anat. 1965;99:27–46. [PMC free article] [PubMed] [Google Scholar]
- 18.Embi AA, Scherlag BJ, Menes M, Po SS. In vivo demonstration of cytosolic calcium level shift by oxalates and cancer. J Solid Tumors. 2013;3:13–8. [Google Scholar]
- 19.Van der Velden HM, van Kempen MJ, Wijffels MC, van Zijverden M, Groenewegen WA, Allessie MA, et al. Altered pattern of connexin40 distribution in persistent atrial fibrillation in the goat. J Cardiovasc Electrophysiol. 1998;9:596–607. doi: 10.1111/j.1540-8167.1998.tb00940.x. [DOI] [PubMed] [Google Scholar]
- 20.Corradi D, Callegari S, Benussi S, Maestri R, Pastori P, Nascimbene S, et al. Myocyte changes and their left atrial distribution in patients with chronic atrial fibrillation related to mitral valve disease. Hum Pathol. 2005;36:1080–9. doi: 10.1016/j.humpath.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 21.Daw JC, Lefer AM, Berne RM. Influences of corticsteroids on cardiac glycogen concentration in the rat. Circ Res. 1968;22:639–47. doi: 10.1161/01.res.22.5.639. [DOI] [PubMed] [Google Scholar]
- 22.Scherlag MA, Scherlag BJ. Randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol. 2013;62:1129–30. doi: 10.1016/j.jacc.2013.05.068. [DOI] [PubMed] [Google Scholar]
- 23.Pokushalov E, Romanov A, Katritsis DG, Artyomenko S, Bayramova S, Losik D, et al. Renal denervation for improving outcomes of catheter ablation in patients with atrial fibrillation and hypertension: Early experience. Heart Rhythm. 2014;11:1131–8. doi: 10.1016/j.hrthm.2014.03.055. [DOI] [PubMed] [Google Scholar]
- 24.Cole MA, Kim PJ, Kalman BA, Spencer RL. Dexamethasone suppression of corticosteroid secretion: Evaluation of the site of action by receptor measures and functional studies. Psychoneuroendocrinology. 2000;25:151–67. doi: 10.1016/s0306-4530(99)00045-1. [DOI] [PubMed] [Google Scholar]
- 25.Viviano A, Kanagasabay R, Zakkar M. Is perioperative corticosterpoid administration associated with a reduced incidence of postoperative atrial fibrillation in adult cardiac surgery? Interact Cardiovasc Thorac Surg. 2014;18:225–9. doi: 10.1093/icvts/ivt486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ljung T, Anderson B, Bengtsson B, Björntorp P, Mårin P. Inhibition of cortisol secretion by dexamethasone in relation to body fat distribution: A dose response study. Obes Res. 1996;4:277–82. doi: 10.1002/j.1550-8528.1996.tb00546.x. [DOI] [PubMed] [Google Scholar]


