Abstract
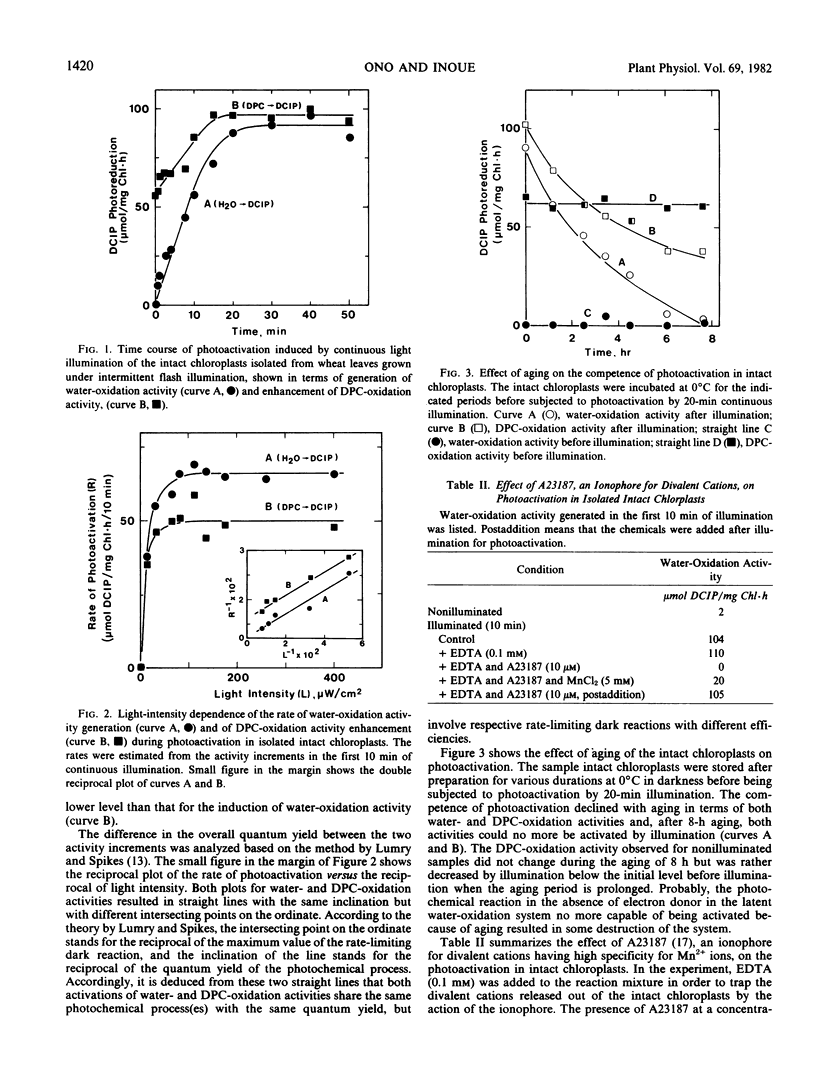
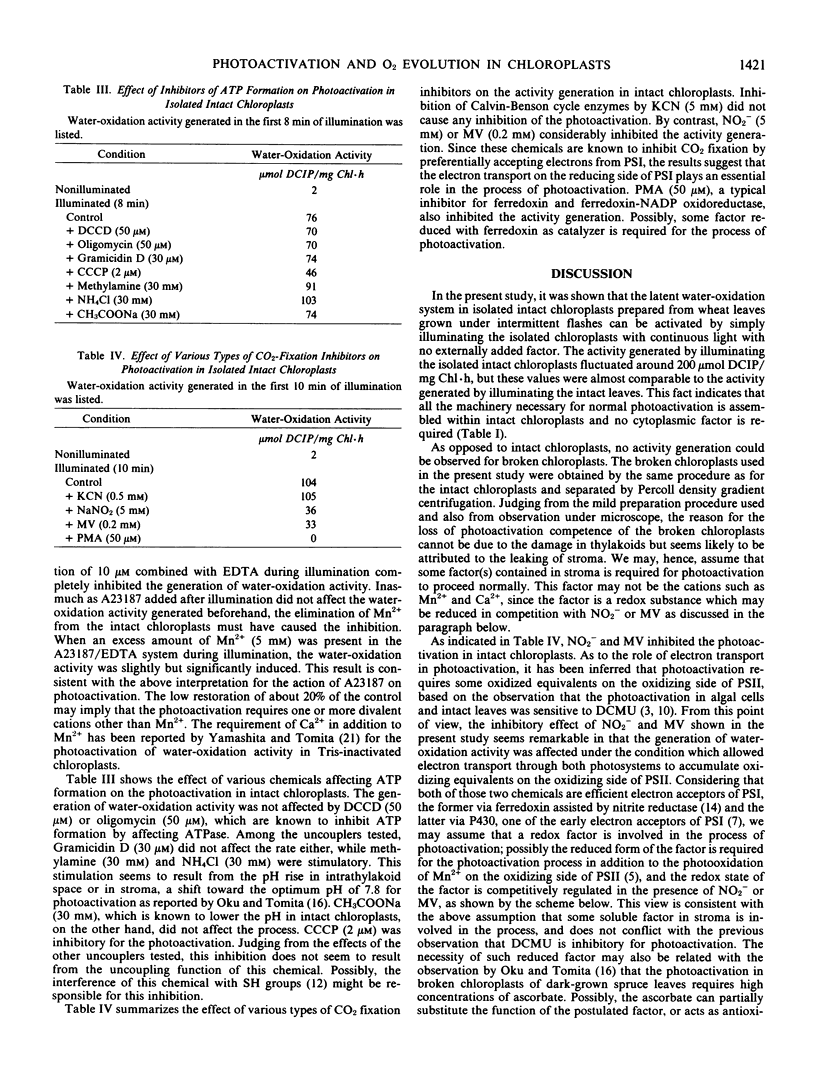
Photoactivation of the latent oxygen-evolving system in intact chloroplasts isolated from wheat (Triticum aestivum L.) leaves grown under intermittent flash illumination was investigated, and the following results were obtained: (a) The water-oxidation activity generated on illuminating the isolated intact chloroplasts was as high as that generated in intact leaves, indicating that all the machinery necessary for the activity generation is assembled within intact chloroplasts. (b) The generation of water-oxidation activity was accompanied by enhancement of the activity of diphenylcarbazide-oxidation, and both processes share the same photochemical reaction but with respective rate-limiting dark reactions of different efficiencies. (c) A23187, an ionophore for divalent cations, strongly inhibited the generation of water-oxidation activity but did not affect the activity once generated, which suggested that Mn atoms in the chloroplasts are susceptible to the ionophore before photoactivation but turn immune after photoactivation. (d) The generation of water-oxidation activity was not affected by the inhibitors of ATP formation and CO2 fixation, but was inhibited by nitrite, methylviologen and phenylmercuric acetate which suppress or inhibit the reduction of ferredoxin in intact chloroplasts. It was inferred that some factor(s) probably present in stroma to be reduced by PSI photoreaction is involved in the process of photoactivation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship R. E., Babcock G. T., Sauer K. Kinetic study of oxygen evolution parameters in Triswashed, reactivated chloroplasts. Biochim Biophys Acta. 1975 Apr 14;387(1):165–175. doi: 10.1016/0005-2728(75)90061-4. [DOI] [PubMed] [Google Scholar]
- Cheniae G. M., Martin I. F. Effects of Hydroxylamine on Photosystem II: II. Photoreversal of the NH(2)OH Destruction of O(2) Evolution. Plant Physiol. 1972 Jul;50(1):87–94. doi: 10.1104/pp.50.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheniae G. M., Martin I. F. Effects of Hydroxylamine on Photosystem II: II. Photoreversal of the NH(2)OH Destruction of O(2) Evolution. Plant Physiol. 1972 Jul;50(1):87–94. doi: 10.1104/pp.50.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G. E., Robinson S. P., Tyler N. J., Walker D. A. Photosynthesis by isolated protoplasts, protoplast extracts, and chloroplasts of wheat: influence of orthophosphate, pyrophosphate, and adenylates. Plant Physiol. 1978 Aug;62(2):313–319. doi: 10.1104/pp.62.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama T., Ke B. A new photosynthetic pigment, "P430": its possible role as the priary electron acceptor of photosystem I. Proc Natl Acad Sci U S A. 1971 May;68(5):1010–1013. doi: 10.1073/pnas.68.5.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt R. C., Krogmann D. W. Inhibition of chloroplast reactions with phenylmercuric acetate. Plant Physiol. 1972 Mar;49(3):376–380. doi: 10.1104/pp.49.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y., Furuta S., Oku T., Shibata K. Light-dependent development of thermoluminescence, delayed emission and fluorescence variation in dark-grown spruce leaves. Biochim Biophys Acta. 1976 Dec 6;449(3):357–367. doi: 10.1016/0005-2728(76)90147-x. [DOI] [PubMed] [Google Scholar]
- Kaback H. R., Reeves J. P., Short S. A., Lombardi F. J. Mechanisms of active transport in isolated bacterial membrane vesicles. 18. The mechanism of action of carbonylcyanide m-chlorophenylhydrazone. Arch Biochem Biophys. 1974 Jan;160(1):215–222. doi: 10.1016/s0003-9861(74)80028-7. [DOI] [PubMed] [Google Scholar]
- Neyra C. A., Hageman R. H. Dependence of nitrite reduction on electron transport chloroplasts. Plant Physiol. 1974 Oct;54(4):480–483. doi: 10.1104/pp.54.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed P. W., Lardy H. A. A23187: a divalent cation ionophore. J Biol Chem. 1972 Nov 10;247(21):6970–6977. [PubMed] [Google Scholar]
- Rieske J. S., Lumry R., Spikes J. D. The Mechanism of the Photochemical Activity of Isolated Chloroplasts. III. Dependence of Velocity on Light Intensity. Plant Physiol. 1959 May;34(3):293–300. doi: 10.1104/pp.34.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosiuk R. A., Buchanan B. B. Activation of Chloroplast NADP-linked Glyceraldehyde-3-Phosphate Dehydrogenase by the Ferredoxin/Thioredoxin System. Plant Physiol. 1978 Apr;61(4):669–671. doi: 10.1104/pp.61.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]



