Abstract
Objective:
Croton tiglium seeds, known as Jamālgoṭa in Hindi, Marathi, and Urdu is well-known for its toxicity (severe purgative action). In Ayurvedic texts, the plant is known as Kumbhinī and is used for the treatment of constipation after Śodhana (detoxification process) of the seeds with Godugdha (cow milk).
Material and Methods:
In the present study, C. tiglium seeds were purified with cow milk as reported in Ayurvedic classics. Phorbol esters equivalent to phorbol-12-myristate-13-acetate (PMA) and crotonic acid contents were quantified by high-performance liquid chromatography method in the seeds of C. tiglium before and after the purification process.
Results:
The content of the phorbol ester equivalent to PMA in unpurified and purified sample was found to be 5.2 mg/100 g and 1.8 mg/100 g of dried seeds of C. tiglium, respectively. The quantity of crotonic acid in unpurified seeds of C. tiglium was found to be 0.102 mg/100 g of dried seeds while it was absent in the purified seed extract of C. tiglium.
Conclusion:
The toxicity of C. tiglium seeds may be due to the presence of phorbol esters and crotonic acid along with other constituents. These constituents are oil soluble and may be removed by cow milk during the process of Śodhana. Reduction in the level of these constituents after the purification decreases the toxicity of C. tiglium seeds. Reduction in the oily content from the seeds of C. tiglium during the purification process is also supported by the results obtained from the physiochemical parameters.
KEY WORDS: Croton tiglium, crotonic acid, phorbol ester, purification
INTRODUCTION
The family Euphorbiaceae includes some 280 genera and 8000 species which occur in tropical and temperate regions all over the world.[1] Croton tiglium L. is an important medicinal plant of the family Euphorbiaceae which is used for the treatment of constipation, dyspepsia, dysenteriae, gastrointestinal disorders, intestinal inflammation, rheumatism, peptic ulcer, visceral pain, and headache.[2,3,4,5] C. tiglium seeds oil is reported to contain phorbol esters and crotonic acid along with the fatty acids.[6,7] C. tiglium, commonly known as Kumbhinī is well-known for its severe purgative action.[6,7]
Ayurveda is being practiced throughout the India including tribal and remote areas where other systems of medicine are not readily available. Though it does not have the scientific evidence regarding the mechanism of action, it plays a major role in meeting the health-care needs of a large section of India.[8] In Ayurveda, Śodhana is a unique process of detoxification which is employed. In Ayurveda, Śodhana is a unique process of detoxification which is employed partly to purify/detoxify and partly to potentiate the effect of various kinds of drugs used in Ayurvedic medicine with a view to reduce their toxic contents/effects as well as to enhance their therapeutic properties. Commonly used ingredients for Śodhana are cow's milk, cow's urine, ghee, and juice of few plants.[9,10] Milk is considered as the best among all the media (Śodhanīya dravya) used in the Śodhana process. In Ayurvedic text, it is reported that C. tiglium seeds are used for the treatment of constipation after Śodhana with Godugdha (cow milk).[11,12]
In the present study, an attempt was made to identify the toxic principles responsible for the severe purgative action of C. tiglium seeds. High-performance liquid chromatography (HPLC) of extract of C. tiglium seeds before and after the purification process were performed for the identification and quantification of the toxic principles along with other physiochemical parameters.
MATERIALS AND METHODS
Chemicals and instrumentation
Croton tiglium seeds were purchased from Goladina Nath market, Varanasi. Standard crotonic acid and phorbol-12-myristate-13-acetate (PMA) were purchased from Tocris Bioscience (Bristol, UK). All other chemicals used were of analytical grade. HPLC (Detoxification of Croton tiglium) used for the quantitative estimation of PMA and crotonic acid.
Śodhana of Kumbhinī
Croton tiglium seeds (250 g) were soaked in water overnight, outer cover (testa), and cotyledon of the seeds was removed with the help of a knife. The endosperm obtained was converted into a coarse powder. The coarse powder was wrapped in a cotton cloth to form a poṭṭali. Poṭṭali was hung in an iron pot containing cow's milk in such a manner that the poṭṭali was completely immersed in milk but did not touch bottom of the vessel. Heating (120°C) provided to the vessel by hot plate continuously for 3 h (1 prahara) to aid the svedana process. During the svedana process, a sufficient amount of fresh milk was added at regular intervals to maintain the level of milk in the vessel. After 3 h, the poṭṭali was removed from the milk and washed with hot water thrice and dried by spreading on the outer surface of an earthen pot. The whole process was repeated for thrice.[11]
Preparation of crude extract
About 12 g each of purified and unpurified powdered plant materials were macerated with methanol (100 ml) for 24 h. In this, they were shaken frequently for 6 h and allowed to stand for 18 h. The macerate was filtered, and filtrate obtained was concentrated in a rotary evaporator. The residue obtained (1.4 g) was suspended in 20 ml of water and partitioned with hexane and diethyl ether, thrice with each.[13] Diethyl ether fraction was pooled and used for the quantitative estimation of PMA. Hexane soluble fraction was used for quantitative estimation of crotonic acid.
Quantitative estimation of phorbol ester
Phorbol ester was quantified by HPLC method developed by Hass and Mittelbach[14] and modified by Saetae and Suntornsuk.[15] The analytical column used was a Zorbax, C18 (4 μm particle size and 150 mm × 3.9 mm internal diameter) column. The column was thermally controlled at 25°C. A mixture of acetonitrile and deionized water (80:20, v: v) was used as the mobile phase at a flow rate of 1 ml/min. The diode array detector wavelength was set at 254 nm. The PMA was used as an external standard [Figures 1 and 2].
Figure 1.
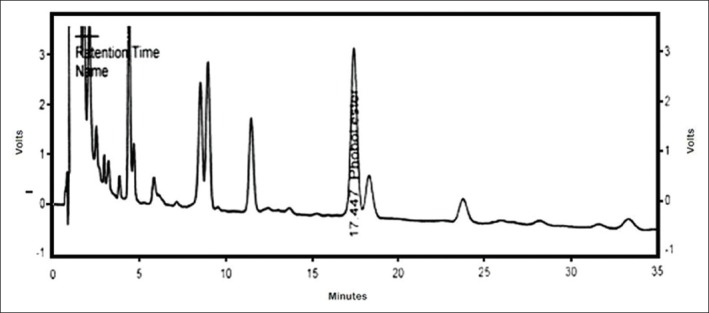
High-performance liquid chromatography of unpurified seed extract of Croton tiglium for phorbol-12-myristate-13-acetate
Figure 2.
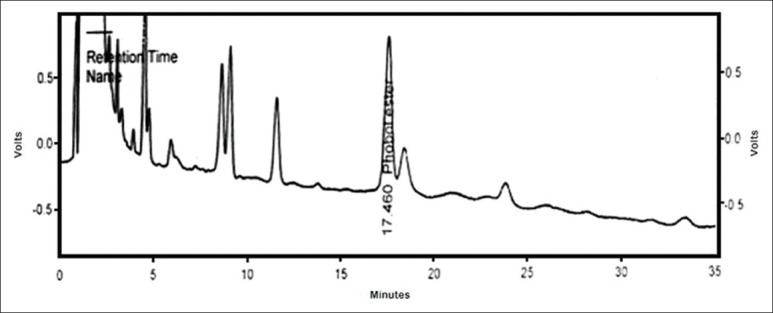
High-performance liquid chromatography of purified seed extract of Croton tiglium for phorbol-12-myristate-13-acetate
Quantitative estimation of crotonic acid
Crotonic acid was quantified by HPLC method developed by Kobayashi et al.[16] with few modifications. The analytical column used was a Zorbax, C18 (4 μm particle size and 150 × 3.9 mm internal diameter) column. The column was thermally controlled at 25°C. A mixture of 5 mM KH2PO4 (pH 2.9) and acetonitrile (3:1) was used as mobile phase at a flow rate of 1 ml/min. The diode array detector wavelength was set at 240 nm. Crotonic acid was used as an external standard [Figures 3 and 4].
Figure 3.
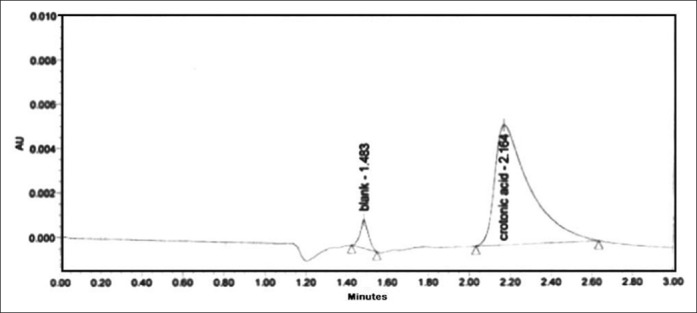
High-performance liquid chromatography of unpurified seed extract of Croton tiglium for crotonic acid content
Figure 4.
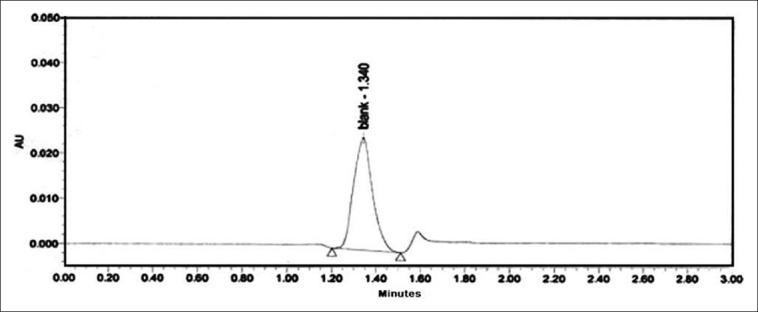
High-performance liquid chromatography of purified seed extract of Croton tiglium for crotonic acid content
Determination of physiochemical parameters
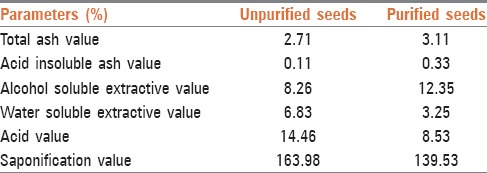
Total ash value, acid insoluble ash value, acid value, saponification value, alcohol soluble extractive value, and water soluble extractive value were determined in the C. tiglium seeds before and after the purification process as per the Ayurvedic Pharmacopoeia of India.[17]
RESULTS
The quantity of phorbol ester and crotonic acid in unpurified and purified sample was determined by HPLC. The content of the phorbol ester equivalent to PMA in unpurified and purified sample was found to be 5.2 mg/100 g and 1.8 mg/100 g of dried seeds of C. tiglium, respectively. The quantity of crotonic acid in unpurified seeds of C. tiglium was found to be 0.102 mg/100 g of dried seeds. Crotonic acid content was found to be absent in the purified seed extract of C. tiglium.
Results of physiochemical parameters in unpurified and purified seeds of C. tiglium are represented in Table 1.
Table 1.
Findings of physiochemical parameters in unpurified and purified Croton tiglium seeds
DISCUSSION
Croton tiglium seeds are well-known by the name of Kumbhinī in Ayurveda[18] for its toxicity (severe purgative action). Yet in Ayurveda, C. tiglium seeds are used in the treatment of severe constipation after its Śodhana with cow's milk. The present study was carried out to find out the phytochemical constituents responsible for the toxicity of C. tiglium seeds and changes in the level of phytochemical constituents and physiochemical parameters during the process of Śodhana. In the present study, the level of phorbol ester and crotonic acid was quantified by HPLC method. Results obtained indicate that the level of phorbol ester reduced to 1.8 mg/100 g of dried seeds of C. tiglium after the purification process from 5.2 mg/100 g in unpurified seeds of C. tiglium. The level of crotonic acid in unpurified seeds of C. tiglium was found 0.102 mg/100 g while it was absent in purified seeds extract. Milk is a natural emulsion, having both oil and water phase and has the capacity to dissolve the nonpolar, as well as polar constituents. Phorbol ester and crotonic acid are nonpolar constituents of C. tiglium seeds. During the process of Śodhana, milk removes these two constituents from the seeds. Both the constituents are reported to have irritant activity on the gastrointestinal tract[3,6,7] and subsequently responsible for severe purgative action. It may be speculated that reduction in the toxicity of C. tiglium seeds is due to the reduction of the level of these two constituents along with the other constituents. That is why C. tiglium seeds are used for the treatment of constipation in Ayurveda after its Śodhana. Results obtained from physiochemical parameters before and after purification indicate the significant change in ash value, water and alcohol soluble extractive value, acid value, and saponification value. Increase in the total ash value (from 2.71% to 3.11%), alcohol soluble extractive value (from 8.26% to 12.35%), and reduction in the acid value (from 14.46 to 8.53) and saponification value (from 163.98 to 139.53) during the process of purification indicate the loss of oil soluble constituents from the C. tiglium seeds.
The toxicity of C. tiglium seeds is due to the presence of phorbol esters and crotonic acid along with other constituents. These constituents are oil soluble that may have been removed by cow's milk during the process of Śodhana. Reduction in the level of these constituents after the purification reduced the toxicity of C. tiglium seeds. Reduction in the toxicity of C. tiglium seeds helps aid the therapeutic activity of the seeds after the purification process.
ACKNOWLEDGMENT
Authors are thankful to Mr. D. D. Chauhan, Elder Pharmaceuticals Ltd., Dehradun, for providing the facility for HPLC.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Hecker E. Cocarcinogenic principles from the seed oil of Croton tiglium and from other Euphorbiaceae. Cancer Res. 1968;28:2338–49. [PubMed] [Google Scholar]
- 2.Qiu HX. Beijing: Science Press; 1996. Flora of China; p. 133. [Google Scholar]
- 3.Wangx, Lan M, Wu HP, Shi YQ, Lu J, Ding J, et al. Direct effect of croton oil on intestinal epithelial cells and colonic smooth muscle cells. World J Gastroenterol. 2002;8:103–7. doi: 10.3748/wjg.v8.i1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsai JC, Tsai S, Chang WC. Effect of ethanol extracts of three Chinese medicinal plants with laxative properties on ion transport of the rat intestinal epithelia. Biol Pharm Bull. 2004;27:162–5. doi: 10.1248/bpb.27.162. [DOI] [PubMed] [Google Scholar]
- 5.Morimura K. The role of special group article in ancient Chinese medical prescription. Hist Sci (Tokyo) 2003;13:1–12. [PubMed] [Google Scholar]
- 6.Hu J, Gao WY, Gao Y, Ling NS, Huang LQ, Liu CX. M3 muscarinic receptor- and Ca2+ influx-mediated muscle contractions induced by croton oil in isolated rabbit jejunum. J Ethnopharmacol. 2010;129:377–80. doi: 10.1016/j.jep.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 7.Jasicka-Misiak I, Wieczorek PP, Kafarski P. Crotonic acid as a bioactive factor in carrot seeds (Daucus carota L.) Phytochemistry. 2005;66:1485–91. doi: 10.1016/j.phytochem.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Mishra LC. 1st ed. United States of America: CRC Press; 2004. Scientific Basis for Ayurvedic Therapies; p. 4. [Google Scholar]
- 9.Joshi D, Nagaraju V. Study on the concept on Sodhana with special reference to visopavisas. Anc Sci Life. 1988;7:195–200. [PMC free article] [PubMed] [Google Scholar]
- 10.Belge RS, Belge AR. Ayurvedic Śodhana treatments and their applied aspect with special reference to loha. J Pharm Biol Sci. 2012;2:45–9. [Google Scholar]
- 11.Sastri L. Varanasi: Chaukhambha Prakashan; 2010. Yogaratnakara; p. 168. [Google Scholar]
- 12.Sharadini AD, Urmila MT. Bombay: Popular Prakashan Private Ltd; 1989. Ayurveda Revisited; p. 91. [Google Scholar]
- 13.El-Mekkawy S, Meselhy MR, Nakamura N, Hattori M, Kawahata T, Otake T. Anti-HIV-1 phorbol esters from the seeds of Croton tiglium. Phytochemistry. 2000;53:457–64. doi: 10.1016/s0031-9422(99)00556-7. [DOI] [PubMed] [Google Scholar]
- 14.Hass W, Mittelbach M. Detoxification experiments with the seed oil from Jatropha curcas L. Ind Crops Prod. 2000;12:111–8. [Google Scholar]
- 15.Saetae D, Suntornsuk W. Antifungal activities of ethanolic extract from Jatropha curcas seed cake. J Microbiol Biotechnol. 2010;20:319–24. [PubMed] [Google Scholar]
- 16.Kobayashi M, Yanaka N, Nagasawa T, Yamada H. Purification and characterization of a novel nitrilase of Rhodococcus rhodochrous K22 that acts on aliphatic nitriles. J Bacteriol. 1990;172:4807–15. doi: 10.1128/jb.172.9.4807-4815.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anonymous. 1st ed. Part – II. I. New Delhi: Ministry of Health and Family Welfare, Govt of India; 2007. The Ayurvedic Pharmacopoeia of India; pp. 140–201. [Google Scholar]
- 18.Anonymous. 1st ed. Part – I. II. New Delhi: Ministry of Health and Family Welfare, Govt of India; 2007. The Ayurvedic Pharmacopoeia of India; p. 61. [Google Scholar]


