Abstract
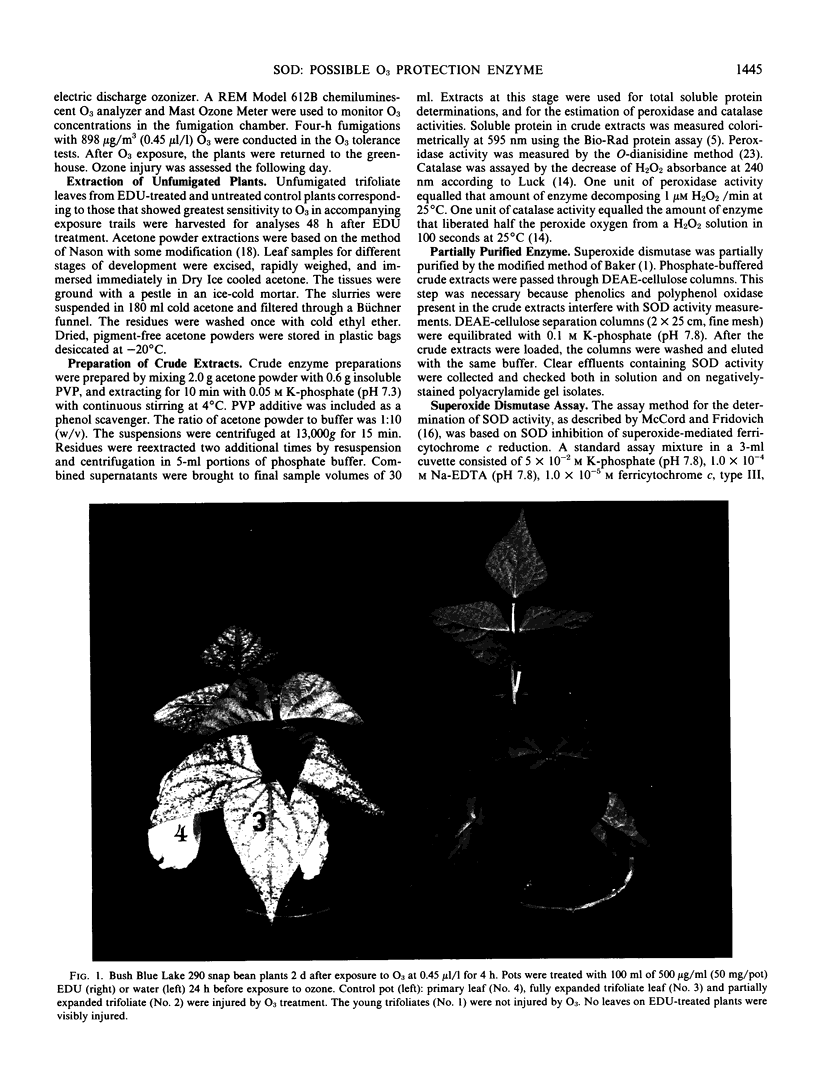
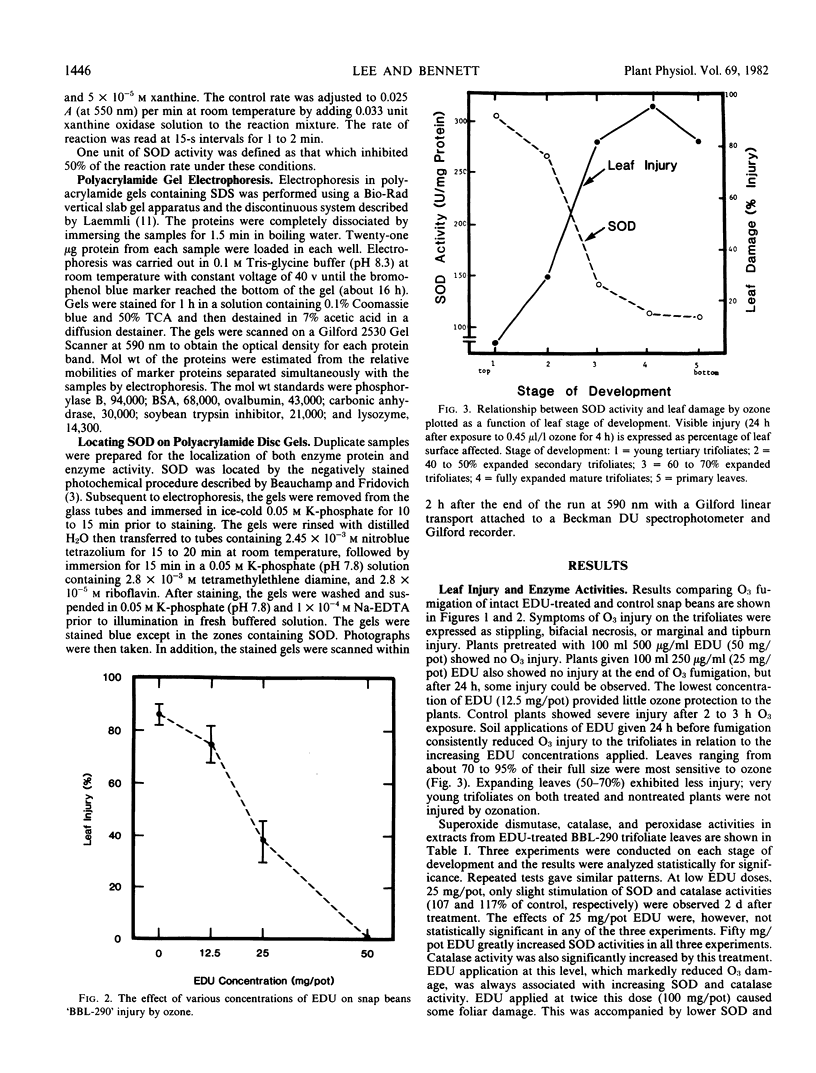
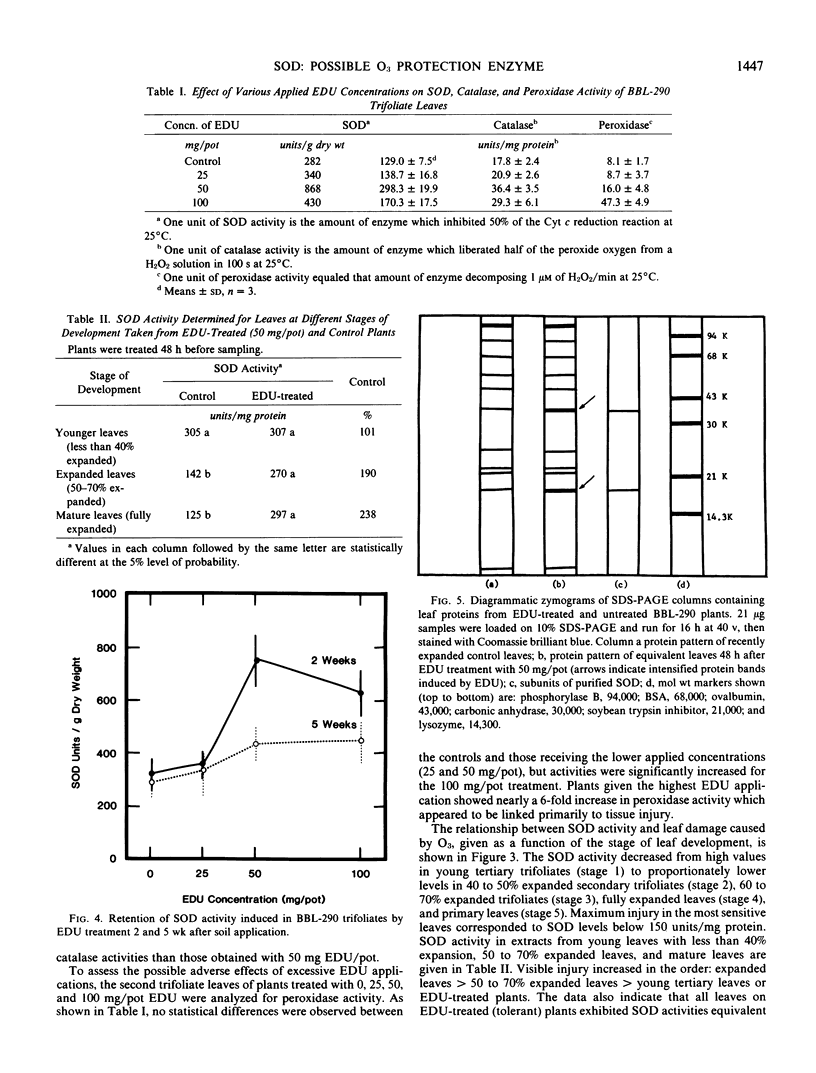
An experimental chemical N-[2-(2-oxo-1-imidazolidinyl)ethyl]-N′-phenylurea (EDU), is an effective protectant against acute and chronic foliar injury due to ozone (03) when sprayed on intact leaves or supplied to the plants through soil application. An 03-sensitive snap bean cultivar (Phaseolus vulgaris L. `Bush Blue Lake 290') was systemically treated with EDU (0, 25, 50, and 100 milligrams per 15-centimeter diameter pot) to determine if EDU-induced or activated protective oxyradical and peroxyl scavenging enzymes. EDU-enhanced tolerance to O3 injury always correlated with increases in superoxide dismutase (SOD) and catalase activities in the leaves. Peroxidase levels correlated more closely with foliar injury. Greater SOD levels in young leves compared to older leaves were associated with lower ozone sensitivities in these tissues.
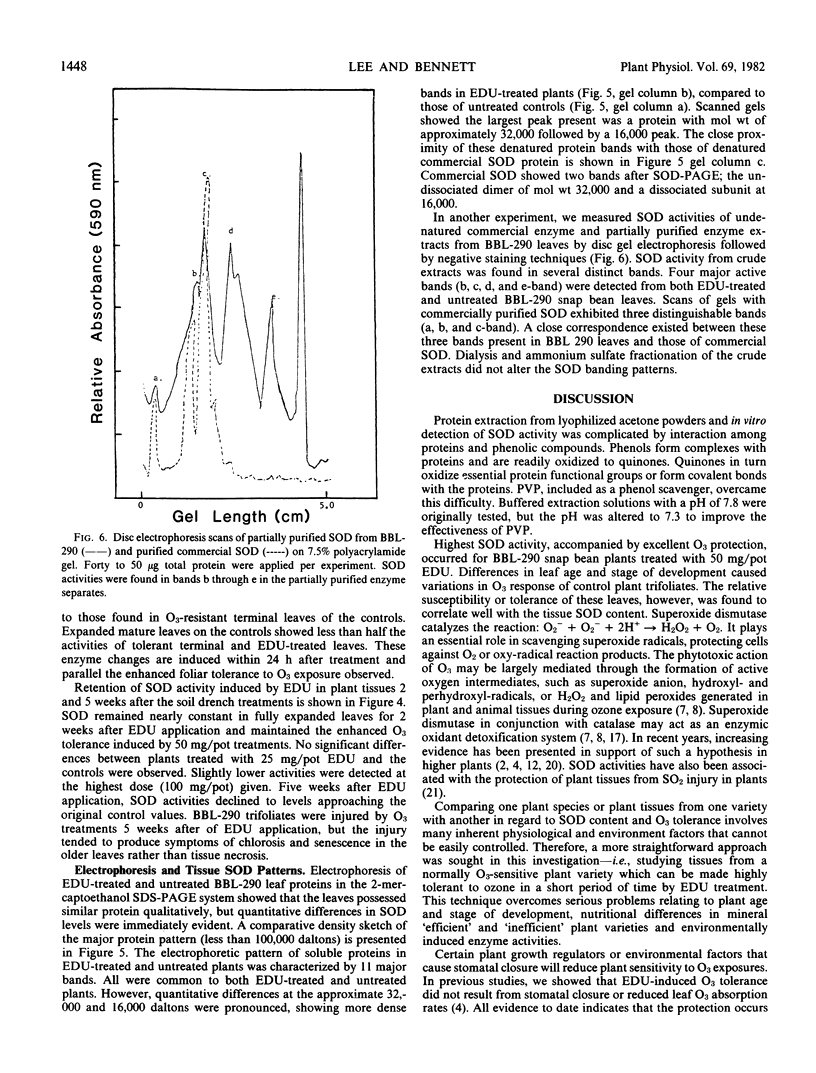
Polyacrylamide slab gel electrophoresis separations and specific determinations of SOD activity showed that EDU-treated plants possessed markedly greater SOD activity than non-treated plants. Tolerant plant tissues may have enhanced enzyme scavenging capabilities for the protection against toxic oxyradicals. Experimental confirmation for the oxyradical theory for O3 phytotoxicity and SOD involvement in the detoxification process are presented.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker J. E. Superoxide dismutase in ripening fruits. Plant Physiol. 1976 Nov;58(5):644–647. doi: 10.1104/pp.58.5.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee E. H., Bennett J. H., Heggestad H. E. Retardation of Senescence in Red Clover Leaf Discs by a New Antiozonant, N-[2-(2-Oxo-1-imidazolidinyl)ethyl]-N'-phenylurea. Plant Physiol. 1981 Feb;67(2):347–350. doi: 10.1104/pp.67.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Salin M. L., Bridges S. M. Isolation and characterization of an iron-containing superoxide dismutase from a eucaryote, Brassica campestris. Arch Biochem Biophys. 1980 May;201(2):369–374. doi: 10.1016/0003-9861(80)90524-x. [DOI] [PubMed] [Google Scholar]




