Abstract
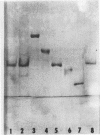
ADPglucose pyrophosphorylase from potato (Solanum tuberosum L.) tubers has been purified by hydrophobic chromatography on 3 aminopropyl-sepharose (Seph-C3-NH2). The purified preparation showed two closely associated protein-staining bands that coincided with enzyme activity stains. Only one major protein staining band was observed in sodium dodecyl sulfate polyacrylamide gel electrophoresis. The subunit molecular weight was determined to be 50,000. The molecular weight of the native enzyme was determined to be 200,000. The enzyme appeared to be a tetramer consisting of subunits of the same molecular weight. The subunit molecular weight of the enzyme is compared with previously reported subunit molecular weights of ADPglucose pyrophosphorylases from spinach leaf, maize endosperm, and various bacteria. ADPglucose synthesis from ATP and glucose 1-P is almost completely dependent on the presence of 3-P-glycerate and is inhibited by inorganic phosphate. The kinetic constants for the substrates and Mg2+ are reported. The enzyme Vmax is stimulated about 1.5- to 3-fold by 3 millimolar DTT. The significance of the activation by 3-P-glycerate and inhibition by inorganic phosphate ADPglucose synthesis catalyzed by the potato tuber enzyme is discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- Copeland L., Preiss J. Purification of Spinach Leaf ADPglucose Pyrophosphorylase. Plant Physiol. 1981 Nov;68(5):996–1001. doi: 10.1104/pp.68.5.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D. B., Preiss J. ADP glucose pyrophosphorylase from maize endosperm. Arch Biochem Biophys. 1969 Mar;130(1):119–128. doi: 10.1016/0003-9861(69)90017-4. [DOI] [PubMed] [Google Scholar]
- FRYDMAN R. B. STARCH SYNTHETASE OF POTATOES AND WAXY MAIZE. Arch Biochem Biophys. 1963 Aug;102:242–248. doi: 10.1016/0003-9861(63)90177-2. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fuchs R. L., Smith J. D. The purification and characterization of ADP-glucose pyrophosphorylase A from developing maize seeds. Biochim Biophys Acta. 1979 Jan 12;566(1):40–48. doi: 10.1016/0005-2744(79)90246-8. [DOI] [PubMed] [Google Scholar]
- Ghosh H. P., Preiss J. Adenosine diphosphate glucose pyrophosphorylase. A regulatory enzyme in the biosynthesis of starch in spinach leaf chloroplasts. J Biol Chem. 1966 Oct 10;241(19):4491–4504. [PubMed] [Google Scholar]
- Hannah L. C., Nelson O. E. Characterization of adenosine diphosphate glucose pyrophosphorylases from developing maize seeds. Plant Physiol. 1975 Feb;55(2):297–302. doi: 10.1104/pp.55.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen T. H., Ishaque A., Preiss J. Biosynthesis of bacterial glycogen. Characterization of the subunit structure of Escherichia coli B glucose-1-phosphate adenylyltransferase (EC 2.7.7.27). J Biol Chem. 1976 Dec 25;251(24):7880–7885. [PubMed] [Google Scholar]
- Heldt H. W., Rapley L. Specific transport of inorganic phosphate, 3-phosphoglycerate and dihydroxyacetonephosphate, and of dicarboxylates across the inner membrane of spinach chloroplasts. FEBS Lett. 1970 Oct 5;10(3):143–148. doi: 10.1016/0014-5793(70)80438-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu T. T., Shannon J. C. Measurement of Metabolites Associated with Nonaqueously Isolated Starch Granules from Immature Zea mays L. Endosperm. Plant Physiol. 1981 Mar;67(3):525–529. doi: 10.1104/pp.67.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Ozbun J. L., Hawker J. S., Greenberg E., Lammel C., Preiss J. Starch Synthetase, Phosphorylase, ADPglucose Pyrophosphorylase, and UDPglucose Pyrophosphorylase in Developing Maize Kernels. Plant Physiol. 1973 Jan;51(1):1–5. doi: 10.1104/pp.51.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanwal G. G., Greenberg E., Hardie J., Cameron E. C., Preiss J. Regulation of starch biosynthesis in plant leaves: activation and inhibition of ADPglucose pyrophosphorylase. Plant Physiol. 1968 Mar;43(3):417–427. doi: 10.1104/pp.43.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaltiel S., Er-El Z. Hydrophobic chromatography: use for purification of glycogen synthetase. Proc Natl Acad Sci U S A. 1973 Mar;70(3):778–781. doi: 10.1073/pnas.70.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowokinos J. R. Pyrophosphorylases in Solanum tuberosum: I. Changes in ADP-Glucose and UDP-Glucose Pyrophosphorylase Activities Associated with Starch Biosynthesis during Tuberization, Maturation, and Storage of Potatoes. Plant Physiol. 1976 Jan;57(1):63–68. doi: 10.1104/pp.57.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowokinos J. R. Pyrophosphorylases in Solanum tuberosum: II. CATALYTIC PROPERTIES AND REGULATION OF ADP-GLUCOSE AND UDP-GLUCOSE PYROPHOSPHORYLASE ACTIVITIES IN POTATOES. Plant Physiol. 1981 Oct;68(4):924–929. doi: 10.1104/pp.68.4.924. [DOI] [PMC free article] [PubMed] [Google Scholar]