Abstract
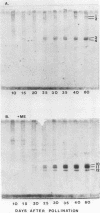
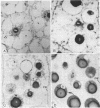
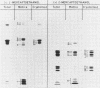
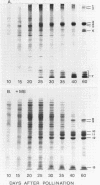
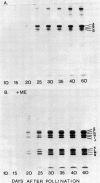
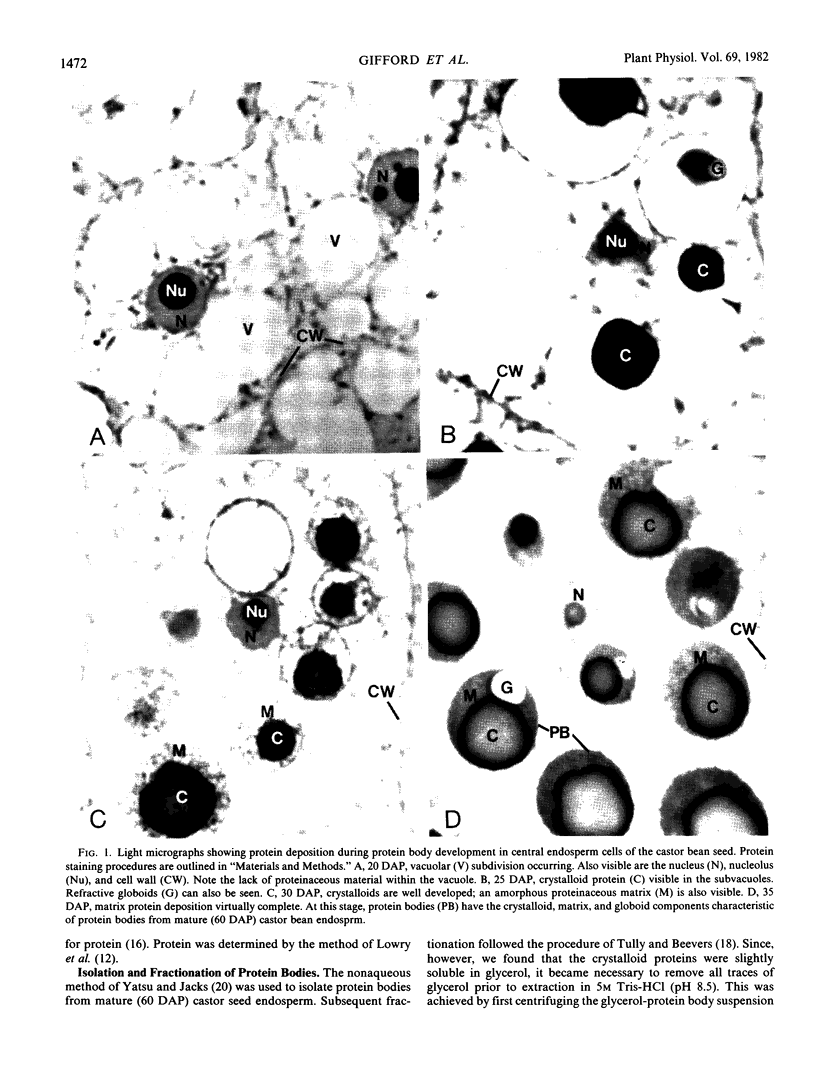
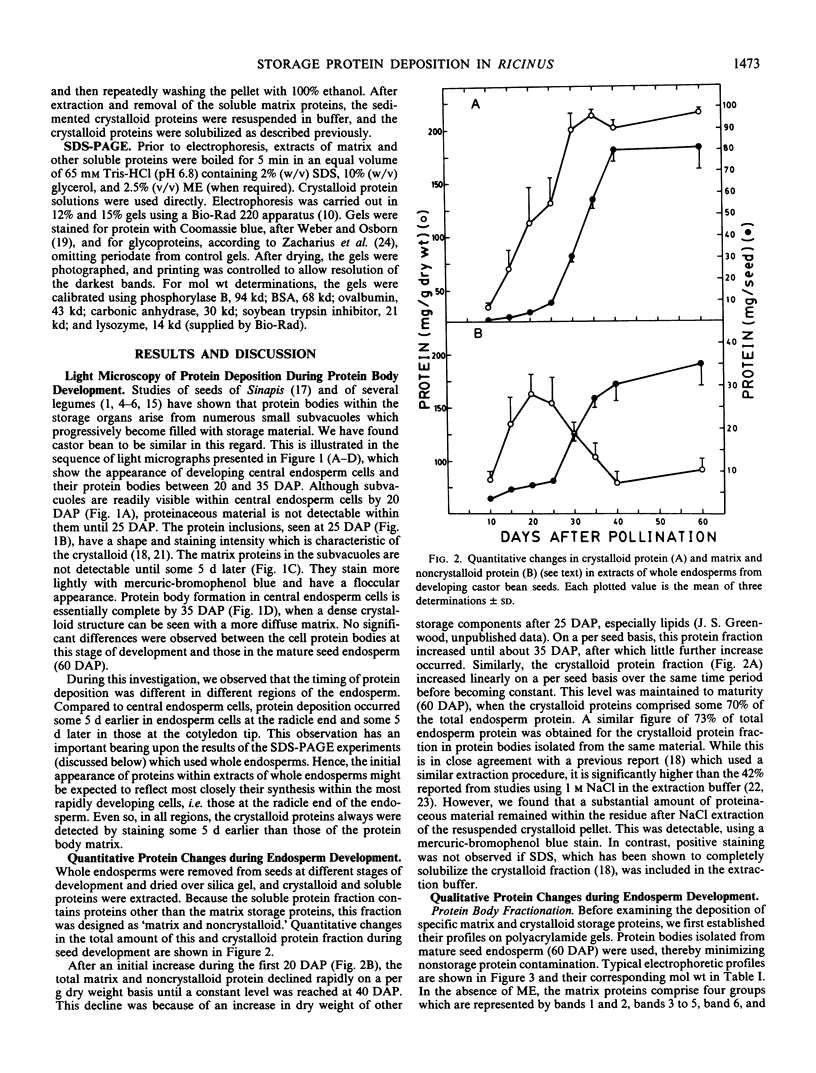
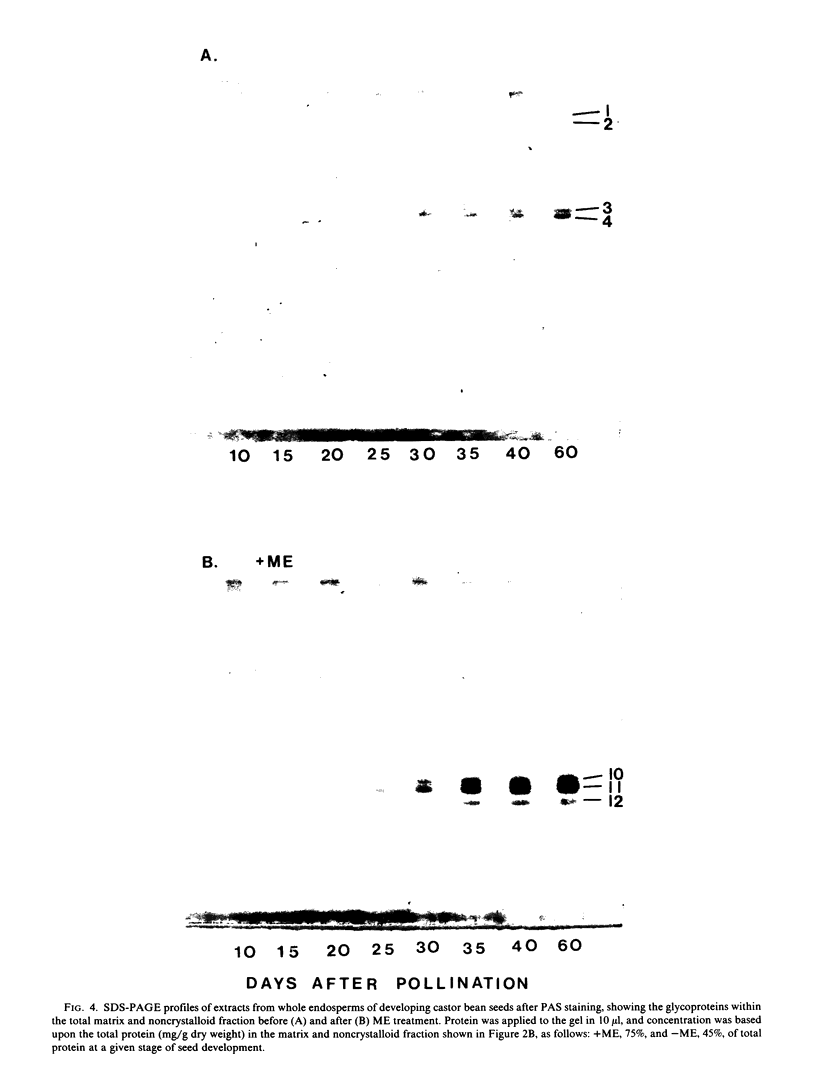
Protein bodies within the endosperm of castor bean (Ricinus communis L. cv. Hale) seeds arise from numerous small vacuoles which progressively become filled with storage protein, of which the crystalloid proteins make up approximately 70%. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) shows that the crystalloids are a family of at least four proteins which reduce to two complementary groups after 2-mercaptoethanol treatment. The matrix, which comprises the remainder, has two major components, the soluble albumins and the lectins. The lectins are the only glycoproteins within the mature protein body. Both cytochemical staining and SDS-PAGE indicate that the synthesis of the crystalloid and the majority of matrix proteins begins some 20 days after pollination. Additionally, the crystalloid proteins are synthesized concurrently, whereas there is temporal variation in the synthesis of matrix proteins.
Full text
PDF

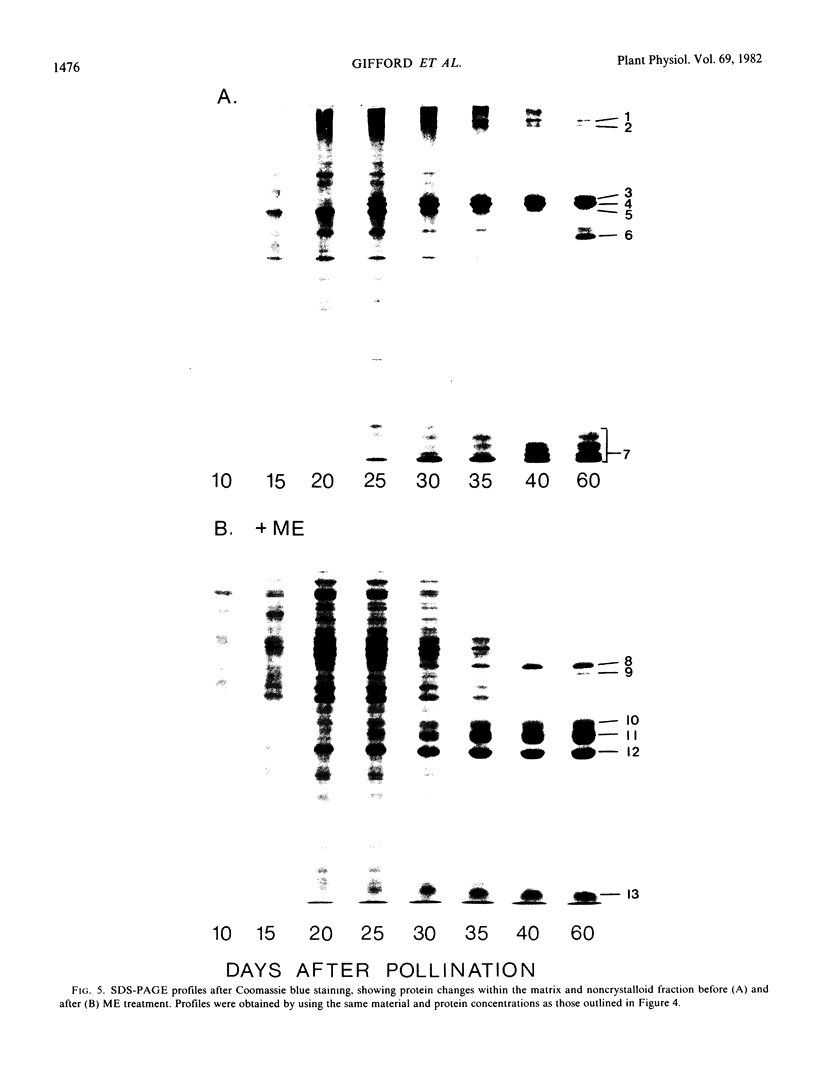
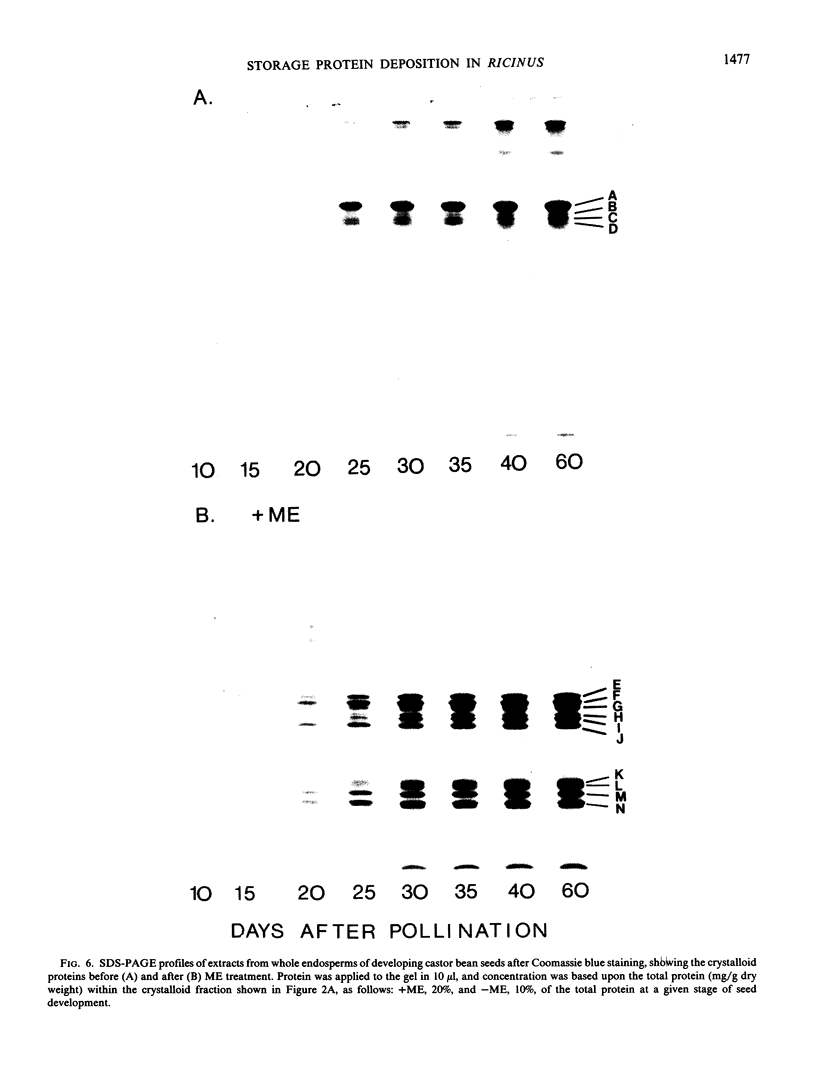

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker W. M., Leaver C. J., Weir E. M., Riezman H. Regulation of Glyoxysomal Enzymes during Germination of Cucumber: I. Developmental Changes in Cotyledonary Protein, RNA, and Enzyme Activities during Germination. Plant Physiol. 1978 Oct;62(4):542–549. doi: 10.1104/pp.62.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Blaustein J., Etzler M. E. Characterization of two plant lectins from Ricinus communis and their quantitative interaction with a murine lymphoma. Biochemistry. 1974 Jan 1;13(1):196–204. doi: 10.1021/bi00698a029. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Saltvedt E., Pihl A. Isolation and comparison of galactose-binding lectins from Abrus precatorius and Ricinus communis. J Biol Chem. 1974 Feb 10;249(3):803–810. [PubMed] [Google Scholar]
- Tully R. E., Beevers H. Protein bodies of castor bean endosperm: isolation, fractionation, and the characterization of protein components. Plant Physiol. 1976 Dec;58(6):710–716. doi: 10.1104/pp.58.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yatsu L. Y., Jacks T. J. Association of lysosomal activity with aleurone grains in plant seeds. Arch Biochem Biophys. 1968 Mar 20;124(1):466–471. doi: 10.1016/0003-9861(68)90354-8. [DOI] [PubMed] [Google Scholar]
- Youle R. J., Huang A. H. Albumin storage proteins in the protein bodies of castor bean. Plant Physiol. 1978 Jan;61(1):13–16. doi: 10.1104/pp.61.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle R. J., Huang A. H. Protein Bodies from the Endosperm of Castor Bean: Subfractionation, Protein Components, Lectins, and Changes during Germination. Plant Physiol. 1976 Dec;58(6):703–709. doi: 10.1104/pp.58.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]