Abstract
Background:
Significant toxicity in chemotherapy trials is usually defined as grade ⩾3. In clinical practice, however, multiple lower grade toxicities are often considered meaningful. The purpose of this observational cohort study was to identify which level of toxicity triggers treatment modification and early discontinuation of chemotherapy in older people.
Methods:
Patients aged 65+ were recruited in a central London hospital. A total of 108 patients were recruited at the start of new chemotherapy treatment between October 2010 and July 2012.
Results:
Mean age was 72.1±5 years, median 72 and range 65–86 years. Of the patients, 50.9% (55) were male with gastrointestinal (49), gynaecological (18), lung (15) and other cancers (26). Chemotherapy was palliative in 59.3% (64/108), curative/ neoadjuvant/adjuvant in the others. Mean number of cycles completed was 4.2±3. Treatment modifications due to toxicity occurred in 60 (55.6%) patients, 35% (21/60) of whom had no greater than grade 2 toxicity. Early treatment discontinuation because of toxicity occurred in 23 patients (21.3%), 39.1% (9/23) of whom had no greater than grade 2 toxicity.
Conclusions:
Many older patients did not complete treatment as planned. Treatment was modified/discontinued even for one or two low-grade toxicities. Further work is required to clarify whether low-grade toxicity has a greater clinical impact in older people, or whether clinicians have a lower threshold for modifying/discontinuing treatment in older people.
Keywords: geriatric oncology, low-grade toxicity, elderly, chemotherapy
Delivering chemotherapy to older people can be a challenge for clinicians. Older people are living longer with a variety of comorbidities of differing severities, functional deficits and degrees of ‘frailty'. Consequently, decision-making in respect to chemotherapy can be complex. The decision includes evaluating who is robust enough to tolerate chemotherapy, tolerate full dose treatment and/or to continue through chemotherapy treatment without modifications. A judgement needs to be made as to whether the patient will actually gain benefit from the treatment. In the absence of a unifying criteria beyond performance status (PS) to assist decision-making, a subjective decision is made by the individual clinician using the available information.
There are often concerns of increased risk of toxicity in older people (Lichtman et al, 2007; Wedding et al, 2007). Some studies indicate increased toxicity with age (Stein et al, 1995; Tsalic et al, 2003; Jantunen et al, 2006). However, these studies did not control for comorbidities that may equally affect tolerance to chemotherapy. This raises the concern that comorbidity and not age is the contributing risk factor (Wedding et al, 2007). The distinction is essential as comorbidity is potentially modifiable (e.g., renal impairment, hypertension etc.) and sometimes reversible (e.g., incontinence). Conversely, several studies (clinical trials and real life clinical practice analyses) have shown that older people have equal benefit and tolerate treatment as well as younger people (Giovanazzi-Bannon et al, 1994; Sargent et al, 2001; Garcia-Suarez et al, 2003; Goldberg et al, 2006; Mitry and Rougier, 2009; Park et al, 2012). A pooled reanalysis of large clinical trials has shown that older patients received the same benefit as younger patients without an increase in toxicity (Giovanazzi-Bannon et al, 1994. However, these pooled analyses did not report the selection criteria for the clinical trials, nor important characteristics (e.g., comorbidities) which would enable a judgement to be made regarding the relevance of these results to clinical practice. Older people included in clinical trials are likely to have a better PS and fewer comorbidities than the whole population of older people with cancer, and thus are not representative of the heterogeneous group seen in clinical practice, about which there is little published literature.
The paucity of clinical information makes it harder to make evidence-based decisions around the management of chemotherapy in older people (Lichtman et al, 2007; Wu and Goldberg, 2013). Older people are underrepresented in clinical trials (Yee et al, 2003). There are a number of factors contributing to this including the exclusion of older people from clinical trials, either because of study protocols excluding comorbidities common in older people or by upper age limits (Droz et al, 2008). In addition, even where eligible, oncologists have been observed to be less likely to include older people in trials (Benson et al, 1991). The risk/benefit decision is consequently often influenced by either observational study evidence, subgroup analyses and/or by the previous experience of the individual clinician (Wu and Goldberg, 2013). There may be less clinical confidence in assessing this risk in older people – this has been observed by oncologists in training (Kalsi et al, 2013).
In addition, the analysis and reporting of toxicity within clinical trials is more focussed on severe toxicity usually defined as National Cancer Institute Common Toxicity Criteria (NCI-CTCAE version 4.0) grade 3–4. In clinical practice, the occurrence of multiple lower grade toxicities is often considered clinically relevant and may result in changes in clinical decision-making. We hypothesise that multiple low-grade toxicities may be a common reason for treatment modification/discontinuation in older people. The purpose of this observational cohort study was to:
Identify which grade(s) of toxicity (and how many) trigger (i) treatment modification (defined as dose reductions, delays or drug omissions) and (ii) early discontinuation of treatment.
Identify the factors associated with modifications/discontinuation made because of low-grade toxicity.
Materials and Methods
Study design and setting
This prospective observational cohort study was set in a London teaching hospital between February 2010 and October 2012. A total of 516 patients aged 65+ undergoing treatment for cancer (chemotherapy, radiotherapy, surgery, watchful waiting) were recruited to the overall study with the aim of identifying comorbidity and comprehensive geriatric assessment (CGA) characteristics that are associated with poorer treatment tolerance (Ethics approval: LREC 09H71865). The primary outcomes of interest for the overall study included toxicities, surgical complications, treatment modifications/non-completion and disease control/progression. This study examines a subgroup of 108 patients who were recruited at the start of new chemotherapy treatment (recruited between October 2010 and July 2012). Patients excluded from this analysis either received a non-chemotherapy treatment modality or were not recruited at the start of chemotherapy. All participants provided consent and were asked to complete a baseline questionnaire (CGA-GOLD) (available in the online Supplementary Material). This included self-reported comorbidities and all CGA domains (e.g., falls, incontinence, delirium, psychosocial, instrumental and basic activities of daily living) (Balducci, 2003; Chen et al, 2003; Minisini et al, 2004; Terret et al, 2004; Extermann et al, 2005; Gosney, 2005; Hurria et al, 2005, 2007; Mohile et al, 2007; Rodin and Mohile, 2007; Girre et al, 2008), quality of life (EORTC QLQ-C30) (Aaronson et al, 1993) and additional questions from our user consultation.
Self-reported CGA has been feasibly conducted in cancer outpatients in the US and Europe (Ingram et al, 2002; Hurria et al, 2005; Girre et al, 2008; Marenco et al, 2008). The feasibility and utility as well as validity and reliability of CGA-GOLD has been previously reported (Kalsi et al, 2013, 2014). Oncologists were blinded to the questionnaire responses and so consented patients received unbiased care according to standard clinical management.
Subjects
Potential participants were identified from oncology clinic lists and chemotherapy day unit lists on the hospital electronic record system. Patients were mailed study information and given a minimum of 48 h to consider participation prior to consent. Questionnaires were returned by participants by post.
Patients aged 65+ with cancer recruited prior or within the first cycle of chemotherapy were included in the analysis. Patients receiving concomitant radiotherapy were also included. Patients receiving both neoadjuvant and adjuvant chemotherapy only had one of these schedules assessed for toxicity (whichever was closest to the recruitment date). The rationale was to avoid contamination of the chemotherapy toxicity outcome by post-operative side effects.
Data collection
Data were collected using the hospital electronic patient records. Patient characteristics (age, comorbidities, PS) were identified from patient records. Clinical outcomes were followed up for 6 months from the first dose of chemotherapy. Toxicity data and treatment changes were identified by review of prospectively recorded oncology electronic notes and clinical letters. Reasons for treatment changes (i.e., if toxicity related) were also identified in the same way. Patients were followed up for toxicity data to the end of the treatment course, or if chemotherapy was given for longer than 6 months, toxicity follow-up ceased at 6 months to avoid skewing of data as a validated approach (Extermann et al, 2011). Toxicity during the treatment course was recorded as per the grades documented in the clinical notes entry. Where clinicians had not graded toxicity but had adequately described the toxicity, grading was assigned retrospectively using NCI-CTCAE version 4.0. Laboratory results during chemotherapy were reviewed to identify haematological toxicity and graded using NCI-CTCAE version 4.0.
Patients who were identified as having treatment modified or discontinued because of toxicity were then further examined to identify the grades of toxicities during their treatment course. Those with modifications/discontinuation who only suffered low-grade toxicity were further examined for the number of low-grade toxicities occurring in their treatment course.
Statistical analysis
Toxicity data were dichotomised to low-grade (grade 1–2) and high-grade (grade 3+) toxicity. Participants were described as suffering low or high toxicity based on the highest recorded grade of toxicity for the individual. Predictors were also prospectively dichotomised (age: <75 vs 75+, comorbidities: <4 vs 4+, PS: 0–1 vs 2–3 and treatment intent: curative/adjuvant/neoadjuvant vs palliative). Associations between predictors and treatment modification and discontinuation because of low-grade toxicity were investigated with univariate analysis using χ2 or Fisher's exact test if the expected frequency was less than 5. SPSS version 19 statistical software package (SPSS, Inc., Chicago, IL, USA) was used for data analysis.
Results
Fifty-three participants were recruited prior to the first cycle and 55 within the first cycle of chemotherapy. All 108 completed follow-up as planned. Ninety-one completed the 6-month follow-up, 17 (15.7%) to the point prior to 6 months at which they died (Figure 1).
Figure 1.
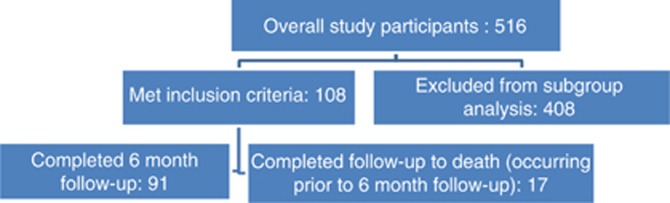
Results – participants.
The mean age of participants was 72.1±5 years, median 72, range 65–86. Of them, 50.9% (n=55) were male and 89.5% (n=94) white; 82.5% were PS 0–1, 14.6% PS 2 and 2.9% PS 3. Participants had gastrointestinal (49), gynaecological (18), lung (15) and other cancers (26). More than three active comorbidities were present in 50% of the cohort. The most common comorbidities were hypertension (39.6%), cardiac disease (23.6%), musculoskeletal disease (22.6%), hypercholesteraemia (21.7%), respiratory disease (17.0%), thrombo-embolism (17.9%) and diabetes (14.2%).
Chemotherapy was palliative in 59.3% (64/108) and curative/adjuvant/neoadjuvant in 40.7% (44/108). Forty-seven different chemotherapy regimens were administered, 16.7% with concomitant radiotherapy; 76.9% (83) had full dose at the outset. Median chemotherapy cycles completed were 4, mean 4.2±3, range 1–12.
Table 1 describes toxicity incidence and Table 2 describes the incidence of the most common individual toxicities by grade. Toxicity (all grades) occurred in 93.5% of participants, 50.9% with grade 3+ and 42.6% with low grade. The most common low-grade toxicities included fatigue, nausea, diarrhoea, constipation and haematological toxicity. The number of missing toxicity data was small (0.46%).
Table 1. Incidence of toxicity.
| All grades % (N) | Grade 1–2 % (N) | Grade 3+ % (N) | |
|---|---|---|---|
| Non haematological toxicities | 92.6 (100/108) | 60.2 (65/108) | 32.4 (35/108) |
| Haematological toxicity | 66.7 (72/108) | 37.0 (40/108) | 29.6 (32/108) |
| Treatment modification due to toxicity | 55.6 (60/108) | 19.4 (21/108) | 36.1 (39/108) |
| Treatment discontinued due to toxicity | 21.3 (23/108) | 8.3 (9/108) | 13.0 (14/108) |
Table 2. Incidence of most common toxicities by grade.
| Toxicity | Grade 1–2 % (N) | Grade 3+ % (N) |
|---|---|---|
| Fatigue | 50.9 (55/108) | 10.2 (11/108) |
| Nausea | 37.0 (40/108) | 2.8 (3/108) |
| Lymphocytopenia | 35.2 (38/108) | 11.1 (12/108) |
| Diarrhoea | 34.2 (37/108) | 4.6 (5/108) |
| Thrombocytopenia | 25.0 (27/108) | 3.7 (4/108) |
| Constipation | 25.0 (27/108) | 0 (0/108) |
| Peripheral neuropathy | 23.1 (25/108) | 3.7 (4/108) |
| Anaemia | 19.4 (21/108) | 11.1 (12/108) |
Treatment changes due to toxicity
Treatment modification due to toxicity occurred in 60 patients, 35% (21/60) of whom had no greater than grade 2 toxicity. Of these 21, the mean number of low-grade toxicities resulting in treatment modification was 2.19±1.33, 7 patients had only one grade 2 toxicity. Most common low-grade toxicity types resulting in treatment modification were fatigue (8), haematological (8), gastrointestinal (6) and infections (5).
Non-completion of treatment because of toxicity occurred in 23 patients, 39.1% (9/23) of whom had no greater than grade 2 toxicity. Of these nine, the mean number of low-grade toxicities resulting in treatment discontinuation was 1.78±1.20, three had only one grade 2 toxicity, one patient only grade 1 toxicity. Most common low-grade toxicities resulting in treatment discontinuation were fatigue (5) and haematological toxicity (4). A further 24 patients did not complete treatment because of disease progression.
Factors associated with toxicity-related treatment modifications/discontinuation
Table 3 demonstrates univariate associations of age, comorbidity, PS and treatment intent with low-grade toxicity (vs high) resulting in treatment modifications and discontinuation. Low-grade toxicity triggering treatment modification was associated with a higher comorbid burden (4+ comorbidities), P=0.01. There was no association between low-grade toxicity and age, PS or treatment intent triggering treatment modification or discontinuation.
Table 3. Associations with low-grade toxicity resulting in treatment modification and treatment discontinuation.
|
Treatment modification (N=60) |
Treatment discontinuation (N=23) |
|||||
|---|---|---|---|---|---|---|
| Low-grade toxicity (N=21) | High-grade toxicity (N=39) | Low-grade toxicity (N=9) | High-grade toxicity (N=14) | |||
| % (N) | % (N) | P-value | % (N) | % (N) | P-value | |
|
Age | ||||||
| <75 years | 31.6 (12) | 68.4 (26) | 0.47 | 30.8 (4) | 69.2 (9) | 0.42 |
| 75+ years | 40.9 (9) | 59.1 (13) | 50.0 (5) | 50.0 (5) | ||
|
Comorbidity | ||||||
| <4 comorbidities | 24.4 (10) | 75.6 (31) | 0.01 | 33.3 (5) | 66.7 (10) | 0.66 |
| 4+ comorbidities | 57.9 (11) | 42.1 (8) | 50.0 (4) | 50.0 (4) | ||
|
Performance status (one missing data) | ||||||
| PS 0–1 | 37.5 (18) | 62.5 (30) | 0.73 | 40.0 (6) | 60.0 (9) | 1.00 |
| PS 2–3 | 27.3 (3) | 72.7 (8) | 42.9 (3) | 57.1 (4) | ||
|
Treatment intent | ||||||
| Curative/adjuvant/neoadjuvant | 34.8 (8) | 65.2 (15) | 0.98 | 50.0 (4) | 50.0 (4) | 0.66 |
| Palliative | 35.1 (13) | 64.9 (24) | 33.3 (5) | 66.7 (10) | ||
Discussion
Low-grade toxicity appears to have clinical significance in older people undergoing chemotherapy. Many older patients did not complete treatment as planned. Low-grade toxicity resulted in treatment modification for 19.4% (21/108) and treatment discontinuation for 8.3% (9/108) of the whole cohort. Treatment was modified/discontinued even for one or two low-grade toxicities. The accumulation of these low-grade toxicities appear important in determining future dosing and ongoing treatment. Fatigue and haematological toxicity were the most common low-grade toxicities impacting on treatment change.
Modifications due to low-grade toxicity was not associated with age. A number of older people were able to complete a reasonable amount of chemotherapy (median 4 cycles). Treatment modification for low-grade toxicity occurred more often in those with multiple comorbidities. This study would thus support that treatment decision-making should not be driven by chronological age and that comorbid burden appears far more relevant.
To the authors' knowledge, this is the first study specifically investigating the impact of low-grade toxicity in older people undergoing chemotherapy. Other studies in older people have been performed investigating the impact of toxicity in general on chemotherapy completion. In a study of 171 patients with small cell lung cancer, 117 of whom who received chemotherapy, 40 patients had a dose reduction and 56 patients did not complete treatment as planned (Fisher et al, 2012). Toxicity (grades not specified) was the most common reason for treatment change. Of those with dose reductions, 75% were due to haematological toxicity and 25% due to frailty/PS. Of those with early discontinuation, 56% were discontinued because of haematological toxicity, 34% non-haematological toxicity, 44% related to frailty/PS and 25% medical reasons. Another study of 108 elderly patients with a variety of cancer types also identified haematological toxicity to be associated with relative dose intensity in regression analyses (Luciani et al, 2006). A study of 532 patients (all ages) with a variety of tumour groups also looked at the impact of adverse drug reactions on chemotherapy completion (Llopis-Salvia et al, 2010). Of the 3553 chemotherapy cycles, 12.9% were not delivered as planned because of adverse drug reactions. Adverse drugs reactions caused treatment delays ⩾7 days for 307 cases, dose reductions for 91, dose omissions in 29 and treatment discontinuation in 112 cases.
This study investigates a very large but understudied population. We examined impact according to age, PS, comorbidity and treatment intent. A variety of tumour and chemotherapy types have been included in the analysis improving generalisability.
This study has limitations. The cohort is small and our sub-optimal sample size may contribute to some non-significant results. We did not study patients under the age of 65. We cannot exclude that a similar study conducted in younger patients may reveal similar findings. A high proportion of participants had PS 0–1. Future work could compare younger matched controls and/or specifically target older people with PS 2+. Toxicity was also identified from that documented in routine clinical practice because of the limitations in size of the research team. This may risk underreporting of toxicity when compared with a clinical trial setting. These results need internal and external validation in a larger sample size to allow for appropriate multivariate analysis to more thoroughly investigate for associations.
This study has potentially significant implications. It highlights that the measure and reporting of lower grade toxicity and its impact should be considered in the design of future clinical trials, especially low-grade fatigue and haematological toxicity. This would better reflect real life clinical decision-making and would assist clinicians in making evidence-based decisions regarding the risks of a particular chemotherapy.
Key questions are also raised. Does low-grade toxicity truly have a greater clinical impact on older people? Or is there a lower threshold for modifying/discontinuing treatment in older people? If so, why? Or is treatment modified for low-grade toxicity similarly in younger people? And finally, as highlighted by the 2012 Department of Health report ‘Cancer Services coming of Age' (Department of Health, 2012), it may be beneficial to medically ‘optimise' older people for chemotherapy. Comorbidities and functional deficits should be thoroughly assessed and modified where possible prior to starting chemotherapy. Strategies should be sought aimed at ameliorating/optimising significant grade 2 toxicities such as fatigue and haematological toxicity (e.g., increasing the use of growth factor support, pre-treatment optimisation of anaemia).
Cancer services should consider reconfiguration of the multidisciplinary team to include geriatricians to support the wider needs of older people. Medical and social optimisation may improve the impact of low-grade toxicity on the ability of patients to receive and complete chemotherapy as planned.
Acknowledgments
TK and DH are supported by grants from the Macmillan Cancer Support and the UK Department of Health and from Guys and St Thomas' Charity. YW is supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at Guy's & St Thomas' NHS Foundation Trust and Kings College London.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JCJM, Kaasa S, Klee M, Osoba D, Razavi D, Rofe PB, Schraub S, Sneeuw K, Sullivan M, Takeda F (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85(5): 365–376. [DOI] [PubMed] [Google Scholar]
- Balducci L (2003) New paradigms for treating elderly patients with cancer: the comprehensive geriatric assessment and guidelines for supportive care. J Support Oncol 1(4 Suppl 2): 30–37. [PubMed] [Google Scholar]
- Benson AB, Pregler JP, Bean JA, Rademaker AW, Eshler B, Anderson K (1991) Oncologists' reluctance to accrue patients onto clinical trials: an Illinois Cancer Center study. J Clin Oncol 9(11): 2067–2075. [DOI] [PubMed] [Google Scholar]
- Chen H, Cantor A, Meyer J, Corcoran MB, Grendys E, Cavanaugh D, Antonek S, Camarata A, Haley W, Balducci L, Extermann M (2003) Can older cancer patients tolerate chemotherapy? A prospective pilot study. Cancer 97(4): 1107–1114. [DOI] [PubMed] [Google Scholar]
- Department of Health (2012) https://www.gov.uk/government/publications/ improving-older-peoples-access-to-cancer-treatment-services.
- Droz JP, Aapro M, Balducci L (2008) Overcoming challenges associated with chemotherapy treatment in the senior adult population. Crit Rev Oncol Hematol 68(1): 26. [DOI] [PubMed] [Google Scholar]
- Extermann M, Aapro M, Bernabei R, Cohen HJ, Droz JP, Lichtman S, Mor V, Monfardini S, Repetto L, Sorbye L, Topinkova E (2005) Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit Rev Oncol Hematol 55(3): 241–252. [DOI] [PubMed] [Google Scholar]
- Extermann M, Boler I, Reich RR, Lyman GH, Brown RH, DeFelice J, Levine RM, Lubiner ET, Reyes P, Schreiber FJ, Balducci L (2011) Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer 118(13): 3377–3386. [DOI] [PubMed] [Google Scholar]
- Fisher S, Al-Fayea TM, Winget M, Gao H, Butts C (2012) Uptake and tolerance of chemotherapy in elderly patients with small cell lung cancer and impact on survival. J Cancer Epidemiol 2012(708936): 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Suarez J, Krsnik I, Reyes E, De Miguel D, Hernanz N, Barr-Ali M, Burgaleta C (2003) Elderly haematological patients with chemotherapy-induced febrile neutropenia have similar rates of infection and outcome to younger adults: a prospective study of risk-adapted therapy. Br J Haematol 120(2): 209–216. [DOI] [PubMed] [Google Scholar]
- Giovanazzi-Bannon S, Rademaker A, Lai G, Benson AB (1994) Treatment tolerance of elderly cancer patients entered onto phase II clinical trials: an Illinois Cancer Center study. J Clin Oncol 12(11): 2447–2452. [DOI] [PubMed] [Google Scholar]
- Girre V, Falcou MC, Gisslebrecht M, Gridel G, Mosseri V, Bouleuc C, Poinsot R, Vedrine L, Ollivier L, Garabige V, Piera JY, Dieras V, Mignot L (2008) Does a geriatric oncology consultation modify the cancer treatment plan for elderly patients? J Gerontol A Biol Sci Med Sci 63(7): 724–730. [DOI] [PubMed] [Google Scholar]
- Goldberg RM, Tabah-Fisch I, Bleiberg H, De Gramont A, Tournigand C, Andre T, Rothenberg ML, Green E, Sargent DJ (2006) Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol 24(25): 4085–4091. [DOI] [PubMed] [Google Scholar]
- Gosney MA (2005) Clinical assessment of elderly people with cancer. Lancet Oncol 6(10): 790–797. [DOI] [PubMed] [Google Scholar]
- Hurria A, Gupta S, Zauderer M, Zuckerman EL, Cohen HJ, Muss H, Rodin M, Panageas KS, Holland JC, Saltz L, Kris MG, Noy A, Gomez J, Jakubwski A, Hudis C, Kornblith AB (2005) Developing a cancer-specific geriatric assessment: a feasibility study. Cancer 104(9): 1998–2005. [DOI] [PubMed] [Google Scholar]
- Hurria A, Lichtman SM, Gardes J, Li D, Limaye S, Patil S, Zuckerman E, Tew W, Hamlin P, Abou-Alfa K, Lachs M, Kelly E (2007) Identifying vulnerable older adults with cancer: integrating geriatric assessment into oncology practice. J Am Geriatr Soc 55(10): 1604–1608. [DOI] [PubMed] [Google Scholar]
- Ingram SS, Seo PH, Martell RE, Clipp EC, Doyle ME, Montana GS, Cohen HJ (2002) Comprehensive assessment of the elderly cancer patient: the feasibility of self-report methodology. J Clin Oncol 20(3): 770–775. [DOI] [PubMed] [Google Scholar]
- Jantunen E, Kuittinen T, Penttila K, Lehtonen P, Mahlamaki E, Nousiainen T (2006) High-dose melphalan (200 mg/m2) supported by autologous stem cell transplantation is safe and effective in elderly (>or=65 years) myeloma patients: comparison with younger patients treated on the same protocol. Bone Marrow Transplant 37(10): 917–922. [DOI] [PubMed] [Google Scholar]
- Kalsi T, Babic-Illman G, Duraisingham SL, Ross P, Maisey N, Hughes S, Fields P, Brodie H, Wang Y, Harari D (2013) Feasibility and utility of comprehensive geriatric assessment screening via postal questionnaire (CGA-GOLD) in older people with cancer. European Geriatric Medicine 4(Suppl 1): 96. [Google Scholar]
- Kalsi T, Babic-Illman G, Hughes S, Ross P, Fields P, Maisey N, Brodie H, Wang Y, Harari D (2014) Validity & reliability of a comprehensive geriatric assessment screening questionnaire (CGA-GOLD) in older people with cancer. Age Ageing 43(Suppl 1): i30. [Google Scholar]
- Kalsi T, Payne S, Brodie H, Mansi J, Wang Y, Harari D. (2013) Are the UK oncology trainees adequately informed about the needs of older people with cancer. Br J Cancer 108(10): 1936–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman SM, Wildiers H, Chatelut E, Steer C, Budman D, Morrison VA, Tranchand B, Shapira I, Aapro M (2007) International Society of Geriatric Oncology Chemotherapy Taskforce: evaluation of chemotherapy in older patients–an analysis of the medical literature. J Clin Oncol 25(14): 1832–1843. [DOI] [PubMed] [Google Scholar]
- Llopis-Salvia P, Sarrio-Montes G, Garcia-Llopis P, Bargues-Ruiz A (2010) Chemotherapy dose intensity reductions due to adverse drug reactions in an oncology outpatient setting. J Oncol Pharm Pract 16(4): 256–261. [DOI] [PubMed] [Google Scholar]
- Luciani A, Marussi D, Ascione G, Caldiera S, Ferrari D, Oldani S, Uziel L, Zonato S, Foa P (2006) Do elderly cancer patients achieve an adequate dose intensity in common clinical practice? Oncology 71(5-6): 382–387. [DOI] [PubMed] [Google Scholar]
- Marenco D, Marinello R, Berruti A, Gaspari F, Stasi MF, Rosato R, Bertetto O, Molaschi M, Ciccone G (2008) Multidimensional geriatric assessment in treatment decision in elderly cancer patients: 6-year experience in an outpatient geriatric oncology service. Crit Rev Oncol Hematol 68(2): 157–164. [DOI] [PubMed] [Google Scholar]
- Minisini A, Atalay G, Bottomley A, Puglisi F, Piccart M, Biganzoli L (2004) What is the effect of systemic anticancer treatment on cognitive function? Lancet Oncol 5(5): 273–282. [DOI] [PubMed] [Google Scholar]
- Mitry E, Rougier P (2009) Review article: benefits and risks of chemotherapy in elderly patients with metastatic colorectal cancer. Aliment Pharmacol Ther 29(2): 161–171. [DOI] [PubMed] [Google Scholar]
- Mohile SG, Bylow K, Dale W, Dignam J, Martin K, Petrylak DP, Sadler WM, Rodin M (2007) A pilot study of the vulnerable elders survey-13 compared with the comprehensive geriatric assessment for identifying disability in older patients with prostate cancer who receive androgen ablation. Cancer 109(4): 802–810. [DOI] [PubMed] [Google Scholar]
- Park SC, Whan LJ, Sik RJ (2012) Docetaxel-based systemic chemotherapy in elderly Korean men with castration-resistant prostate cancer. Actas Urol Esp 36(7): 425–430. [DOI] [PubMed] [Google Scholar]
- Rodin MB, Mohile SG (2007) A practical approach to geriatric assessment in oncology. J Clin Oncol 25(14): 1936–1944. [DOI] [PubMed] [Google Scholar]
- Sargent DJ, Goldberg RM, Jacobson SD, Macdonald JS, Labianca R, Haller DG, Shepherd LE, Seitz JF, Francini G (2001) A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med 345(15): 1091–1097. [DOI] [PubMed] [Google Scholar]
- Stein BN, Petrelli NJ, Douglass HO, Driscoll DL, Arcangeli G, Meropol NJ (1995) Age and sex are independent predictors of 5-fluorouracil toxicity. Analysis of a large scale phase III trial. Cancer 75(1): 11–17. [DOI] [PubMed] [Google Scholar]
- Terret C, Albrand G, Droz JP (2004) Geriatric assessment in elderly patients with prostate cancer. Clin Prostate Cancer 2(4): 236–240. [DOI] [PubMed] [Google Scholar]
- Tsalic M, Bar-Sela G, Beny A, Visel B, Haim N (2003) Severe toxicity related to the 5-fluorouracil/leucovorin combination (the Mayo Clinic regimen): a prospective study in colorectal cancer patients. Am J Clin Oncol 26(1): 103–106. [DOI] [PubMed] [Google Scholar]
- Wedding U, Honecker F, Bokemeyer C, Pientka L, Hoffken K (2007) Tolerance to chemotherapy in elderly patients with cancer. Cancer Control 14(1): 44–56. [DOI] [PubMed] [Google Scholar]
- Wu C, Goldberg RM (2013) Managing choices for older patients with colon cancer. Am Soc Clin Oncol Educ Book 2013: 190–193. [DOI] [PubMed] [Google Scholar]
- Yee KW, Pater JL, Pho L, Zee B, Siu LL (2003) Enrollment of older patients in cancer treatment trials in Canada: why is age a barrier? J Clin Oncol 21(8): 1618–1623. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


