Abstract
Background:
The most significant problem of intra-arterial chemotherapy for advanced paranasal sinus carcinomas and residual cancers supplied by internal carotid artery (ICA) and involving the skull base is the lack of salvage therapies.
Objective:
The objective of the study was to evaluate the usefulness of intra-arterial chemotherapy including ICA infusion for treating advanced paranasal sinus carcinomas, which have invaded the skull base.
Methods:
Forty-six patients with advanced paranasal sinus carcinomas supplied by ICA were treated by intra-arterial chemotherapy using CDDP and sodium thiosulphate (STS) as a neutraliser of CDDP toxicity. After evaluating CT angiography, 150 mg m−2 of CDDP was superselectively administered weekly to each feeding artery including ICA four times.
Results:
The 10-year overall survival rate and progression-free survival rate were 70.7 and 60.2%, respectively. Compared with control group without infusing ICA, recurrences at anterior skullbase or anterior ethomoid sinus were significantly diminished. Of 32 patients in which the orbital apex had been invaded, 29 patients were treated with successful preservation of orbital contents. The CT angiography could efficiently determine all feeding arteries supplying the cancers. Consequently, chemotherapy could be administered on schedule, and side effects were minimal and acceptable.
Conclusions:
This new method has promising applications in the treatment of advanced paranasal sinus carcinomas involving the skull base.
Keywords: internal carotid artery, intra-arterial chemotherapy, skull base invasion, paranasal sinus carcinoma, organ preservation
Patients with advanced paranasal sinus carcinomas involving the skull base have a significantly poor prognosis and quality of life, especially those with unresectable, advanced carcinomas. The results of systemic chemotherapy with radiation treatment have been reported to be less than satisfactory because of the resistance to chemotherapy and radiation (Munro, 1995; El-Sayed and Nelson, 1996). Intra-arterial chemotherapy has therefore become an attractive alternative as it is a successful method for increasing the concentration of anticancer drugs in the tumour (Tomura et al, 1996; Hirai et al, 2001; Miyayama et al, 2011; Yokoyama, 2002; Hirai et al, 2004; Kaketa. et al, 2007). In 1998, we conducted CT angiography for head and neck cancer to obtain precise information in regard to the blood supply to tumours. This was the first time that this procedure had ever been carried out for head and neck cancer (Yokoyama, 2002). Our research, and subsequent research, has demonstrated that this procedure can provide accurate and detailed information about the vascular supply to head and neck cancers (Tomura et al, 1996; Miyayama et al, 2011; Yokoyama, 2002; Takayama et al, 2006; Kaketa. et al, 2007).
Materials and Methods
Forty-six patients with paranasal sinus cancer who were treated by intra-arterial (I-A) chemotherapy including internal carotid arteries concurrent to radiotherapy from January 2000 to June 2013 were included in this study. The patients' characteristics are shown in Table 1A. The number of T3 cases was 3 and the number of T4 cases was 43. The number of T4a and T4b cases were 15 and 28, respectively. There were 35 N0 cases and 11 neck metastasis cases. The mean age of the patients was 69.4 years with a range from 33 to 84. Male patients numbered 35 and female patients numbered 11. The medium follow-up time was calculated to be 37 months (range 6–160). In these patients, 150 mg m−2 of CDDP was superselectively administered weekly to each feeding artery including the internal carotid artery (ICA) four times at 5 mg min−1. During the infusion of CDDP, Sodium thiosulphate (STS) at 200-fold of CDDP was injected through a catheter placed in the brachiocephic vein introduced via the subclavian vein to neutralise the adverse effects of CDDP. The CT angiography was performed after the insertion of the catheter into a possible tumour feeding artery using conventional digital subtraction angiography (DSA). We were able to confirm the entire tumour stained by contrast medium three dimensionally using CT angiography. We used retrograde approach both via a temporal artery and a femoral artery. Intra-arterial chemotherapy was also performed via the femoral artery when the tumour invaded the contralateral side or when contralateral side lymph node metastasis occurred. CDDP was administered intra-arterially on Days 1, 8, 22, and 29. Primary tumour and nodal metastasis were irradiated to total 60 Gy in 30 fractions, 5 fractions in a week.
Table 1. Patients' characteristics (TNM classification).
|
TNM classification | ||||
|---|---|---|---|---|
| T/N | N0 | N1 | N2b | |
|
(A) Patients' characteristics with this new procedure | ||||
| T3 | 3 | 0 | 0 | 3 |
| T4a | 12 | 0 | 3 | 15 |
| T4b | 20 | 3 | 5 | 28 |
| Total |
35 |
3 |
8 |
46 |
|
(B) Patients' characteristics of the historical control cases | ||||
| T3 | 1 | 1 | 2 | |
| T4a | 3 | 1 | 4 | |
| T4b | 4 | 1 | 5 | |
| Total | 8 | 2 | 1 | 11 |
Abbreviation: TNM=tumour, nodes and metastases.
We also compared this new method with the historical control that did not employ infusion of the internal carotid artery and evaluated the usefulness of this new method in regard to the overall survival rate, progression-free survival rate, and recurrent sites. The historical control group included 11 patients with paranasal sinus cancer who were treated by I-A chemotherapy without infusing ICA concurrent to radiotherapy from April 1996 to April 2002. The control patients' characteristics are shown in Table 1B. The numbers of T3, T4a, and T4b cases were two, four, and five, respectively. N0 cases numbered eight and neck metastasis numbered three. The mean age of the patients was 66.5 years with a range from 45 to 84 years. Male patients numbered six and female patients numbered five. The medium follow-up time was calculated to be 150 months (range 76–186). Patients characteristics recorded between the two groups displayed no differences in regard to TNM classification, age, and male to female ratio.
All toxicities encountered during therapy were evaluated according to the Common Terminology Criteria for Adverse Events (version 3.0).
Informed consent was obtained from each patient before treatment, and the study was approved by the Human Ethics Review Committee of Juntendo University.
Statistics
Larynx preservation rates and overall survival rates were calculated by using the Kaplan–Meier method. Larynx preservation rates were compared between the two groups using the log-rank test.
An illustrative case
The following represents an example of our procedure in treating a case of advanced ethomoid cancer associated with blindness. The patient was a 60-year-old man experiencing loss of vision, headaches, and temporary consciousness disorder. The patient had lost vision 2 months before his initial visit to our hospital.
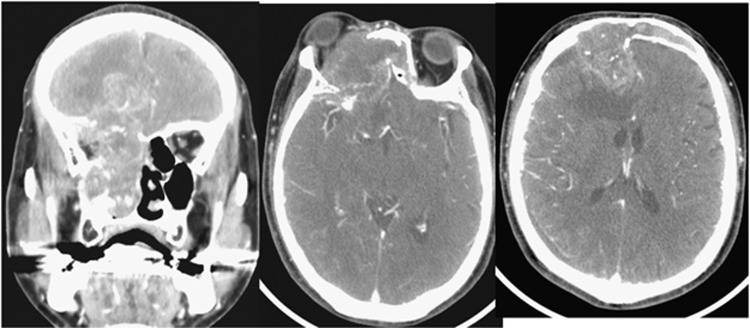
As in Figure 1 CTs showed that advanced cancer had extensively invaded the frontal lobe, and this case was diagnosed as being unresectable.
Figure 1.
Pretreatment CT for advanced ethomoid cancer associated with blindness. A 60-year-old man with ethomoid cancer had already lost vision 2 months before.
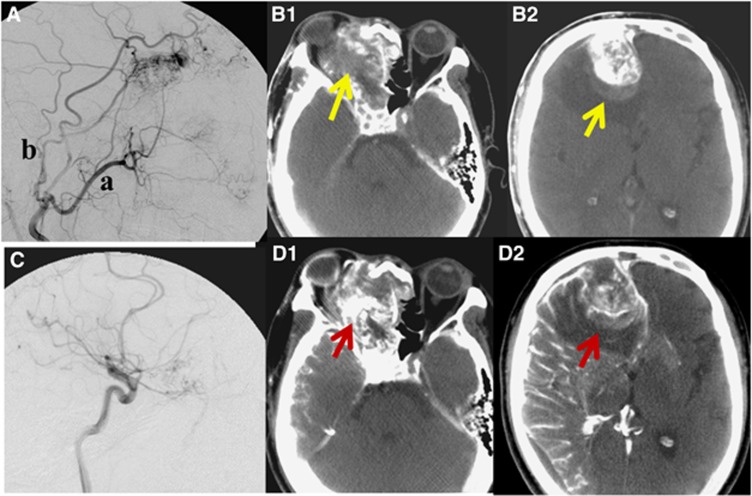
Intra-arterial chemotherapy with concurrent radiotherapy was conducted four times. Figure 2A illustrates a lateral view of the external carotid angiography. A tumour feeding artery (a) and (b) in Figure 2A shows the maxillary artery and superficial temporary artery, respectively. After DSA, CT angiography of the external carotid artery was conducted to visually identify the accurate range of the external carotid artery supplying the cancer three dimensionally (Figure 2B). Almost all of the cancer area was enhanced by contrast medium in CT angiography of the external carotid artery. However, the arrows in Figure 2 indicate areas of filling defect of the contrast medium. These areas of the cancer were not supplied with anticancer drugs by external carotid artery. In such cases, we conducted internal carotid angiography to supply blood to the filling defect of the external carotid artery. Figure 2C shows a lateral view of the internal carotid angiography.
Figure 2.
Angiography and CT angiography. The upper row; external carotid angiography and CT angiography of the external carotid artery. (A) Lateral view of the external carotid angiography. a: maxillary artery; b: superficial temporary artery. (B1, B2) CT angiography of external carotid artery. These arrows indicate filling defects of the contrast medium supplied by the external carotid artery. The lower row; the internal carotid angiography and CT angiography of internal carotid artery. (C) The lateral view of internal carotid angiography. (D1, D2) CT angiography of internal carotid artery. CT angiography of internal carotid artery could identify the cancer areas that were not stained by the external carotid artery were clearly supplied by the internal carotid artery.
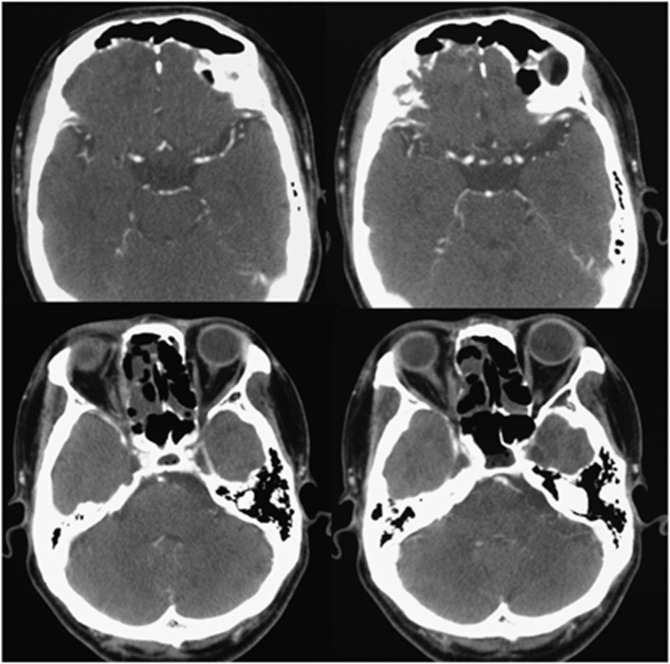
We could identify accurately the range of ICA supplying blood to the cancer using CT angiography (Figure 2D). Furthermore, CT angiography enabled us to identify the cancer areas that were not supplied by the external carotid artery, but clearly supplied by the ICA (Figure 2D). Post treatment CT taken after 2 years revealed that there was no recurrence of tumour after treatment (Figure 3).
Figure 3.
Post treatment CT. Two years post treatment CT demonstrates no tumour.
Results
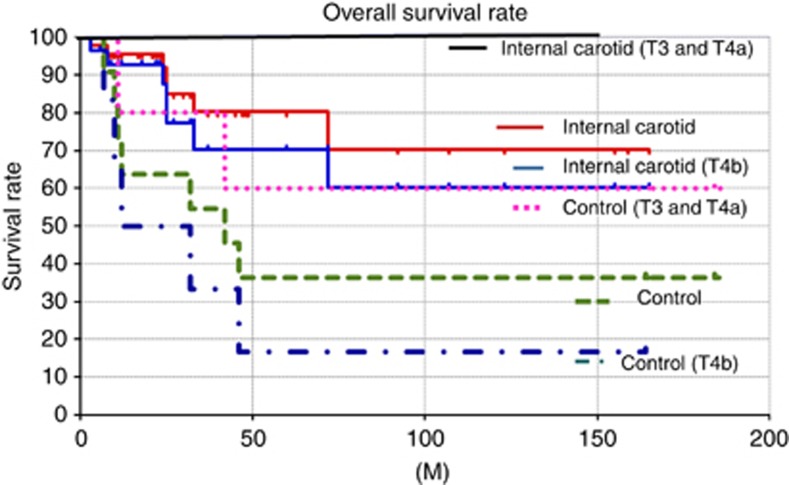
The complete and partial response rates were 37 out of 46 (81%) and 9 out of 46 (19%), respectively. The 10-year overall survival rate calculated by using the Kaplan–Meier method was 70.2 percent. The overall 10-year survival rates of T3 and T4a cases, and T4b cases were 100 and 60.2%, respectively (Figure 4). Treatment failures included two cases of local recurrences, one case of neck metastasis, and four cases of distant metastasis.
Figure 4.
The 10-year overall survival rates. The overall survival rates of each stage treated by the new method were significantly better than those of the control group.
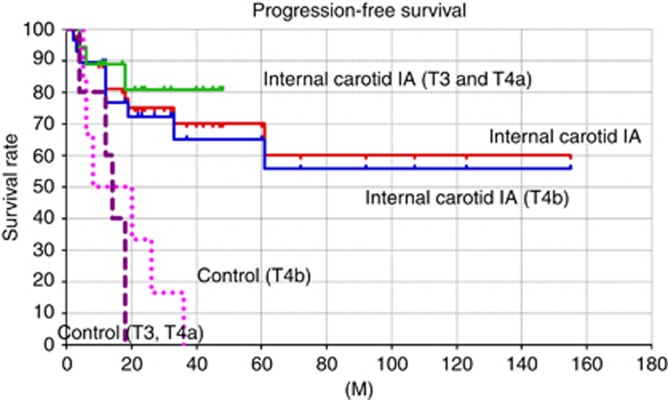
The progression-free survival rate of the new method calculated by using the Kaplan-Meier method was 60.2%. The progression-free survival rates of T3 and T4a cases, and T4b cases were 80.8 and 55.8%, respectively (Figure 5). In contrast, the 3-year progression-free survival rate of the historical control cases was only 9.1%. The 4-year progression-free survival rate was 0% due to developing recurrence at the anterior ethomoid sinus and anterior skull base (Figure 5). Of six recurrent cases of T3 and T4a, four cases were successfully salvaged; however, five recurrent cases of T4b were not able to be salvaged at all.
Figure 5.
The progression-free survival rates. The progression-free survival rates of each stage treated by the new method were significantly better than those of the control group.
Table 2 shows a list of the infused arteries. The total number of I-A chemotherapies was 212; the mean number of I-A chemotherapy cycles was 4.6 (range 3–6). The total number of superselectively infused arteries was 630. Of the 630 infused arteries, the numbers of infused maxillary arteries, facial arteries, transverse facial arteries, and internal carotid arteries were 212, 109, 98, and 161, respectively.
Table 2. Summary of infused arteries.
| Infused artery | Maxillary artery | Facial artery | Transvers facial artery | Superficial temporary artery | Occipital artery | Internal carotid artery | Posterior auricular artery | Contralaterl maxillary artery | Contralaterl internal carotid artery | Ascending pharyngeal artery |
|---|---|---|---|---|---|---|---|---|---|---|
| Total |
212 |
109 |
98 |
22 |
13 |
102 |
3 |
7 |
3 |
2 |
| Average |
4.6 |
2.4 |
2.1 |
0.48 |
0.28 |
2.2 |
0.07 |
0.15 |
0.07 |
0.04 |
| Range | 2–6 | 0–5 | 0–5 | 0–5 | 0–2 | 0–6 | 0–2 | 0–4 | 0–2 | 0–2 |
The administered dose of CDDP to the internal carotid artery was the mean CDDP dose per cycle (mg per cycle) 48.0 mg (25–100 mg). The mean total CDDP dose to the internal carotid artery was 168 mg (50–400 mg).
Of thirty-two patients whose orbital apex had been invaded, 29 patients were treated with preservation of the orbital contents. We established that CT angiography is an efficient method for diagnosing all feeding arteries of paranasal sinus cancers invading the orbital apex.
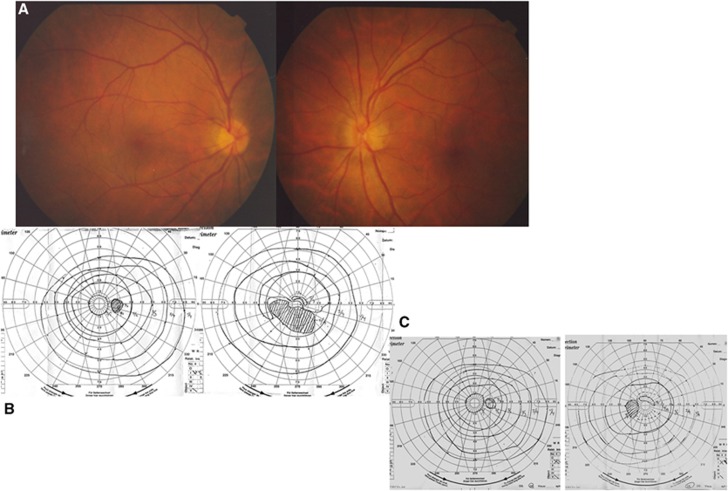
We recorded eight patients with visual loss or visual field constriction in their initial visit to our hospital. The following illustrates a case of sudden left visual sight loss caused by advanced paranasal sinus cancer. The ophthalmoscopic examination demonstrated that Marriott's blind spot was expanded, and the left optic disc was blurred and edematous (Figure 6A and B). The visual field test carried out post treatment demonstrated that the vision and visual field continued to improve 2 years later (Figure 6C).
Figure 6.
Ophthalmoscopic examination for the sudden vision loss. The ophthalmoscopic examination demonstrated that the left optic disc was blurred (A) and edematous and Marriott's blind spot was expanded (B). (C) Ophthalmoscopic examination after I-A chemotherapy. The vision and visual field had continued improving 2 years later.
Of eight patients with visual loss, all patients (with the exception of the above discussed representative case in which vision had been lost before the first visit to our hospital) regained their vision following I-A chemotherapy.
The overall 10-year survival rate of the historical control in which internal carotid artery had not been infused was 36.4%. The overall survival rates of the historical control of T3 and T4a cases, and T4b were 60 and 16.7%, respectively. Compared with the overall survival rate of the historical control, the overall survival rate of this new method was significantly better due to lower recurrence at the anterior ethomoid sinus and anterior skull base (Figure 4).
There were no complications related to the ICA administration. Chemotherapy could be administered weekly on schedule, and side effects were minimal and determined to be acceptable (Table 3).
Table 3. Summary of acute toxicity.
| Toxicity | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Ocular/Visual |
5 |
2 |
|
|
| Hearing |
1 |
2 |
|
|
| Anaemia |
18 |
20 |
2 |
|
| Leukopenia |
19 |
19 |
8 |
|
| Thrombocytopenia |
7 |
6 |
3 |
|
| Arrhythmia |
1 |
|
|
|
| Fever |
13 |
6 |
2 |
|
| Alopecia |
20 |
11 |
|
|
| Dermatitis |
7 |
17 |
2 |
|
| Nausea/vomiting |
11 |
14 |
2 |
|
| Mucositis |
10 |
24 |
10 |
2 |
| Diarrhea |
1 |
|
|
|
| Liver dysfunction |
9 |
2 |
|
|
| Neuropathy |
1 |
1 |
|
|
| Renal | 1 | 2 | 1 |
All toxicities during therapy were evaluated according to the Common Terminology Criteria for Adverse Events (version 3.0).
Discussion
In 1998, we conducted CT angiography for head and neck cancer to obtain precise information regarding the blood supply to tumours. This was the first time that this procedure had ever been carried out for head and neck cancer (Yokoyama, 2002). This procedure can provide accurate and detailed information about the vascular supply to head and neck cancers and improve prognosis (Tomura et al, 1996; Miyayama et al, 2011; Yokoyama, 2002; Takayama et al, 2006; Kaketa et al, 2007).
To minimise adverse outcomes such as visual dysfunction while at the same time improving the prognosis, CT angiography is an efficient method for diagnosing all feeding arteries into paranasal sinus cancers. In addition, we reported that the presence of dental metals can potentially interfere with the precise evaluation of paranasal sinus cancer patients when using CT angiography (Yokoyama et al, 2013). However, in cases of superior structure invasions such as skull base invasion, dental metals do not significantly interfere with the precise evaluation for paranasal sinus cancer patients when using CT angiography.
It is very important to identify the accurate range of each feeding artery three dimensionally with CT angiography to improve prognosis and to minimise adverse events. However, Rasch et al (2010) reported that I-A chemoradiation was not superior to intravenous chemoradiation for advanced head and neck cancer regarding locoregional control and survival. As they did not evaluate precisely the blood distributions of cancers using CT angiography, recurrences might develop at locoregional sites without infused CDDP. As a result, locoregional control and survival could not be improved when compared with intravenous chemoradiation.
Compared with the historical controls of advanced paranasal sinus cancers in which the ICA was not infused, this new method significantly improved the poor overall survival rate and progression-free survival rate. The control group without infusion to the ICA often developed recurrence at the anterior ethomoid sinus and anterior cranial fossa. According to Figure 2 and Table 2, 18% of anterior skull base tumours could be supplied by ICA. As the control group without infusing ICA, additionally 18% tumours should recur at the anterior skull base and anterior ethomoid sinus after the treatment. As a result, in the control group there were six cases of recurrence in the anterior skullbase, three in the anterior ethomoid sinus, one in the frontal sinus, and one in the nasal cavity. In contrast, of the recurrences in the new method group, there were five cases of distant metastasis, one case involving neck metastasis, one case of recurrence in the orbital cavity, one in the anterior skull base, one in the anterior ethomoid sinus, and one in the palate. Compared with the controls, the new method of infusing the ICA could reduce recurrences in the anterior skull base and anterior ethomoid sinus.
Concerning the surgical treatment for resectable T4 disease, the 5-year survival rate was reported to be 7–28% (Lavertu et al, 1989; Carinci et al, 1997; Myers et al, 2002). For anterior skull base invasion cases including orbital apex invasions, the orbital contents could not be preserved in almost all cases (Nibu et al, 2002). Furthermore, deep involvement of the orbit affected not only the preservation of the orbital contents, but also was a significant factor associated with poor survival for surgical treatment (Suarez et al, 2004). For our treated cases including those with >60% unresectable stage IVB, the prognosis for surgical treatment was significantly poor. Madison et al (2005) reported that superselective I-A chemotherapy concurrent radiation therapy combined with surgery for resectable advanced paranasal sinus cancers resulted in an 81% overall survival rate. However, in our new treatment strategy for cases in which the orbital apex was deeply invaded, the orbital contents could be preserved in 29 out of 32 (90.6%) and, additionally, the 5-year overall survival rate was improved to as high as 80.5%. In the total cases treated with this strategy, the orbital contents could be preserved in 42 out of 46 (91.3%) of advanced paranasal cancers supplied by the ICA.
As our mean administered per cycle dose of CDDP to the Internal carotid artery was as low as 48.0 (mg per cycle), our new strategy was also determined to be safe. We measured the tumours' CDDP concentrations on the first day and eigth day after first I-A chemotherapy and second I-A chemotherapy. The first day and eigth day CDDP concentrations in the tumours were 6.7 and 8.9 μg g−1, respectively. One cycle of additional I-A chemotherapy could increase by1.33-fold of the weekly CDDP concentration. As a result, even resistant skull base cancers were impacted by the high CDDP concentrations achieved by repeated I-A chemotherapy.
Almost all references concerning the use of the internal carotid artery infusion chemotherapy were high grade brain tumours (Madajewicz et al, 1991; Tfayli et al, 1999; Madajewicz et al, 2000). The administrated CDDP dose to the internal carotid artery was two or three times higher than that used in our cases, and the side effects were reported to be acceptable. According to our experience and these references, our new method could be safe and useful for treating advanced paranasal sinus carcinomas supplied by the ICA. In particular, this method with CT angiography could contribute to the preservation of the orbital contents and visual function.
Conclusion
This new method of chemotherapy has promising applications in the treatment of advanced paranasal sinus carcinomas involving the skull base.
Acknowledgments
This research was funded in part by a Grants-in Aid for Scientific Research from the Ministry of Education, Culture, Sports, and Technology (22591920) of Japan.
Author contributions
JY conceived of the study. JY prepared and edited the manuscript. SO and MF contributed to the acquisition of data. MF and MK performed the statistical analysis. MS, HY, and KI conducted the interpretation of data. JY and KI revised the final version of the manuscript. All authors read and approved the final manuscript.
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Carinci F, Farina A, Padula E, Calearo C. Primary malignancies of the nasal fossa and paranasal sinuses: comparison between UICC classification and a new staging system. J Craniofac Surg. 1997;8:405–412. doi: 10.1097/00001665-199708050-00014. [DOI] [PubMed] [Google Scholar]
- El-Sayed S, Nelson N. Adjuvant and adjunctive chemotherapy in the management of squamous cell carcinoma of the head and neck region. A meta-analysis of prospective and randomized trials. J Clin Oncol. 1996;14:838–847. doi: 10.1200/JCO.1996.14.3.838. [DOI] [PubMed] [Google Scholar]
- Hirai T, Korogi Y, Ono K, Maruoka K, Harada K, Aridomi S, Takahashi M. Intra-arterilal chemotherapy for locally advanced and/or recurrent hepatic tumors: evaluation of the feeding artery with an interventional CT system. Cardiovasc Intervent Radiol. 2001;24:176–179. doi: 10.1007/s002700000389. [DOI] [PubMed] [Google Scholar]
- Hirai T, Korogi Y, Ono K, Uemura S, Yamashita Y. Preoperative embolization for meningeal tumors:evaluation of vascular supply with angio-CT. AJNR Am J Neuroradiol. 2004;25:74–76. [PMC free article] [PubMed] [Google Scholar]
- Kaketa S, Korogi. Y, Miyaguni. Y, Moriya J, Ohnari N, Oda N, Nishino K, Miyamoto W. A cone-beam volume CT using a 3D angiography system with a flat panel detector of direct conversion type: usefulness for superselective intra-arterial chemotherapy for head and neck tumors. AJNR Am J Neuroradiol. 2007;28:1783–1788. doi: 10.3174/ajnr.A0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavertu P, Roberts JK, Kraus DH, Levine HL, Wood BG, Medendorp SV, Tucker HM. Squamous cell carcinoma of the paranasal sinuses: the Cleveland Clinic experience 1977-1986. Laryngoscope. 1989;99:1130–1136. doi: 10.1288/00005537-198911000-00005. [DOI] [PubMed] [Google Scholar]
- Madajewicz S, Chowhan N, Iliya A, Roque C, Beaton R, Davis R, Fertman S, Meek A, Alvarez O, Pampati M. Intracarotid chemotherapy with etoposide and cisplatin for malignant brain tumors. Cancer. 1991;67:2844–2849. doi: 10.1002/1097-0142(19910601)67:11<2844::aid-cncr2820671123>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Madajewicz S, Chowhan N, Tfayli A, Roque C, Meek A, Davis R, Wolf W, Cabahug C, Roche P, Manzione J, Iliya A, Shady M, Hentschel P, Atkins H, Braun A. Therapy for patients with high grade astrocytoma using intraarterial chemotherapy and radiation therapy. Cancer. 2000;88:2350–2356. doi: 10.1002/(sici)1097-0142(20000515)88:10<2350::aid-cncr20>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Madison MichaelL, II, Sorenson JeffreyM, Samant Sandeep, Jon HRobertson. The treatment of advanced sinonasal malignancies with pre-operative intra-arterial cisplatin and concurrent radiation. J Neuro-Oncol. 2005;72:67–75. doi: 10.1007/s11060-004-2712-0. [DOI] [PubMed] [Google Scholar]
- Miyayama S, Yamashiro M, Hattori Y, Orito N, Matsui K, Tsuji K, Yoshida M, Yoshida M, Kikuchi Y, Tanaka T, Tsuda G, Matsui O. Usefulness of C-arm CT during superselective infusion chemotherapy for advanced head and neck carcinoma. J Med Imaging Radiat Oncol. 2011;55:368–372. doi: 10.1111/j.1754-9485.2011.02290.x. [DOI] [PubMed] [Google Scholar]
- Munro AJ. An overview of randomized controlled trials of adjuvant chemotherapy in head and neck cancer. Br J Cancer. 1995;71:83–91. doi: 10.1038/bjc.1995.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers LL, Nussenbaum B, Bradford CR, Teknos TN, Esclamado RM, Wolf GT. Paranasal sinus malignancies: an 18-year single institution experience. Laryngoscope. 2002;112:1964–1969. doi: 10.1097/00005537-200211000-00010. [DOI] [PubMed] [Google Scholar]
- Nibu K, Sugasawa M, Asai M, Ichimura K, Mochiki M, Terahara A, Kawahara N, Asato H. Results of multimodality therapy for squamous cell carcinoma of maxillary sinus. Cancer. 2002;94:1476–1482. doi: 10.1002/cncr.10253. [DOI] [PubMed] [Google Scholar]
- Rasch CR, Hauptmann M, Schornagel J, Wijers O, Buter J, Gregor T, Wiggenraad R, de Boer JP, Ackerstaff AH, Kroger R, Hoebers FJ, Balm AJ, Hilgers FJ. Intra-arterial versus intravenous chemoradiation for advanced head and neck cancer: Results of a randomized phase 3 trial. Cancer. 2010;116:2159–2165. doi: 10.1002/cncr.24916. [DOI] [PubMed] [Google Scholar]
- Suarez C, Llorente JL, Fernandez De Leon R, Maseda E, Lopez A. Prognostic factors in sinonasal tumors involving the anterior skull base. Head Neck. 2004;26:136–144. doi: 10.1002/hed.10358. [DOI] [PubMed] [Google Scholar]
- Takayama O, Yokoyama J, Ito S. Treatment of recurrent myoepithelial carcinoma by Superselective Intra-arterial infused high dose CDDP Chemotherapy. ANL. 2006;33:235–238. doi: 10.1016/j.anl.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Tfayli A, Hentschel P, Madajewicz S, Manzione J, Chowhan N, Davis R, Roche P, Iliya A, Roque C, Meek A, Shady M. Toxicities related to intraarterial infusion of cisplatin and etoposide in patients with brain tumors. J Neurooncol. 1999;42:73–77. doi: 10.1023/a:1006116523041. [DOI] [PubMed] [Google Scholar]
- Tomura N, Hashimoto M, Sashi R, Hirano H, Kobayashi M, Hirano Y, Satoh K, Watarai J, Kowada M. Superselective Angio-CT of brain tumors. AJNR Am J Neuroradiol. 1996;17:1073–1080. [PMC free article] [PubMed] [Google Scholar]
- Yokoyama J. Usefulness of CT-angiography for superselective intra-arterial chemotherapy for advanced head and neck cancers. Gan To Kagaku Ryoho. 2002;29:2302–2306. [PubMed] [Google Scholar]
- Yokoyama J, Ohba S, Fujimaki M, Kojima M, Suzuki M, Ikeda K. Significant improvement in superselective intra-arterial chemotherapy for advanced paranasal sinus cancer by using Indocyanine green fluorescence. Eur Arch Otorhinolaryngol. 2013;271:2795–2801. doi: 10.1007/s00405-013-2846-9. [DOI] [PMC free article] [PubMed] [Google Scholar]