Abstract
Background:
Recent data suggest the possible benefits of α-tocopherol and β-carotene supplementation on liver cancer and chronic liver disease (CLD), but the long-term trial data are limited.
Methods:
We evaluated the efficacy of supplemental 50 mg day−1 α-tocopherol and 20 mg day−1 β-carotene on incident liver cancer and CLD mortality in a randomised trial of 29 105 Finnish male smokers, who received supplementation for 5–8 years and were followed for 16 additional years for outcomes.
Results:
Supplemental α-tocopherol, β-carotene, or both, relative to placebo, did not reduce the risk of liver cancer or CLD, either overall, during the intervention or during the post-intervention period.
Conclusions:
Long-term supplemental α-tocopherol or β-carotene had no effect on liver cancer or CLD mortality over 24 years of follow-up.
Keywords: randomised trial, vitamin supplement, liver cancer, chronic liver disease
As inflammation and oxidative stress are thought to be important causes of liver cancer and liver disease (Poli, 2000; Luedde and Schwabe, 2011), antioxidants might have a beneficial effect. Several previous observational studies have indicated that higher serum levels of serum α-tocopherol, β-carotene, and retinol are associated with a reduced risk of liver cancer or disease (Pan et al, 1993; Yu et al, 1995; Yamamoto et al, 1998; Yu et al, 1999; Clemente et al, 2002; Ito et al, 2006; Yuan et al, 2006). Other studies, including trials, have reported beneficial effects of vitamin E supplementation on these outcomes (Sanyal et al, 2004; Yakaryilmaz et al, 2007; Bjelakovic et al, 2011; Zhang et al, 2012) including recent data on non-diabetic persons with NASH (non-alcoholic steatohepatitis) in the PIVENS (Pioglitazone versus Vitamin E versus Placebo for the Treatment of Nondiabetic Patients with Nonalcoholic Steatohepatitis) trial (Sanyal et al, 2010) that has served as the basis of recent AASLD clinical recommendations (Chalasani et al, 2012). Concordant results were recently found for the use of vitamin E supplements and liver cancer in an observational cohort in Shanghai, China (Zhang et al, 2012). However, the long-term effect of vitamin E supplementation on liver disease is unclear (Lirussi et al, 2007; Musso et al, 2010; Bjelakovic et al, 2011).
Materials and methods
We evaluated the effects of α-tocopherol and β-carotene supplementation on liver cancer incidence and chronic liver disease (CLD) mortality in the Alpha-Tocopherol Beta-Carotene Cancer Prevention (ATBC) study, a large randomised placebo-controlled trial of 29 105 Finnish male smokers, aged 50–69 at baseline, without self-reported cirrhosis who received 50 mg day-1 DL-α-tocopheryl acetate, 20 mg day-1 β-carotene, both, or placebo from enrolment in 1985–1988 until April 30, 1993 (The ATBC Cancer Prevention Study Group, 1994). Follow-up was through cancer diagnosis, death, or until 31 December 2009. Although designed to examine lung cancer, ATBC provided a unique opportunity to evaluate the long-term effects of α-tocopherol and β-carotene supplementation on liver cancer incidence and CLD mortality.
Incident liver cancer cases (n=208; ICD-9=155, and ICD-10=C22) and mortality from CLD (n=237; ICD-9=571, and ICD-10=K70, K73, or K74) were identified through the Finnish Cancer Registry (Korhonen et al, 2002) and Finnish Register of Causes of Death, respectively. All statistical analyses were based on intention-to-treat. Kaplan–Meier survival curves were plotted for the four intervention groups on the scale of time since randomisation. The effect of intervention was estimated by Cox proportional hazards regression with age as the time scale and allowing for left truncation.
We examined the results in the pre-specified baseline subgroups, defined by age, alcohol use, BMI, cigarettes per day, baseline serum α-tocopherol, serum β-carotene, or serum total cholesterol. These analyses were designed primarily to assess consistency of the overall results, rather than discover subsets where treatment effects might be present. We also assessed associations among a subset of participants with measured hepatitis B (HBV) and C (HCV) viral status, and fasting glucose and insulin. Diabetes status was defined as: without diabetes (blood glucose <100 mg dl−1 and no history of diabetes), prediabetes (blood glucose between 100 and <126 mg dl−1 and no history of diabetes), and diabetes (blood glucose ⩾126 mg dl−1 or self-reported history of diabetes). This subset analysis was motivated by findings in the literature suggesting a benefit from α-tocopherol in the absence of diabetes. We used a P-value of 0.05 for statistical significance and all the tests were two-sided. Additional details on the methods can be found in the supplement.
Results
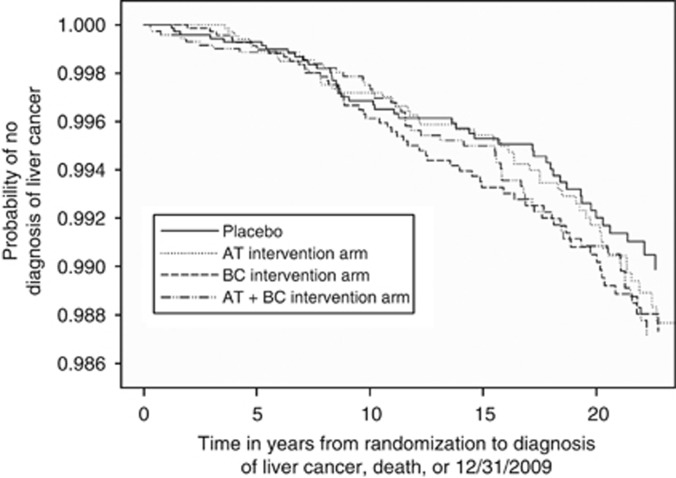
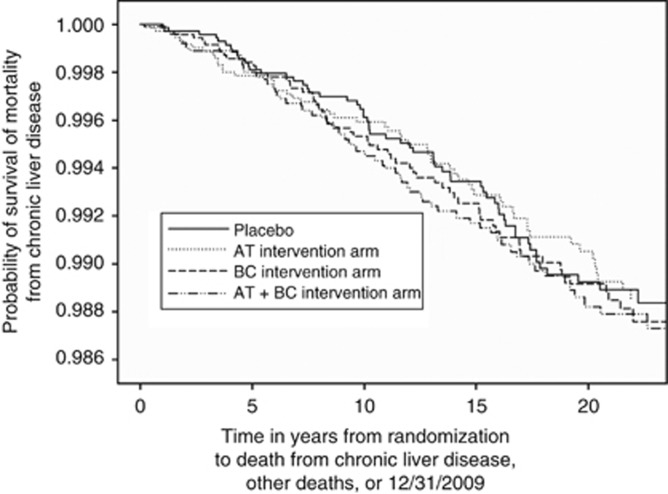
The ATBC trial was randomised, and the baseline characteristics were similar across each intervention arm (Supplementary Table 1). During follow-up, 208 incident liver cancer cases and 237 deaths from CLD occurred. We observed no effect of supplemental α-tocopherol, β-carotene, or both, relative to placebo, on liver cancer incidence (hazard ratio (HR) for α-tocopherol, 1.18; 95% confidence interval (CI), 0.79–1.75; HR for β-carotene, 1.26; 95% CI, 0.85–1.87; HR for both, 1.21, 95% CI, 0.81–1.80) or CLD mortality (HR for α-tocopherol, 0.97; 95% CI, 0.68–1.40; HR for β-carotene, 1.06; 95% CI, 0.74–1.51; HR for both, 1.01, 95% CI, 0.70–1.45). Similar results were found during the intervention and post-intervention period (Table 1; Figures 1 and 2).
Table 1. Effect of α-tocopherol and β-carotene on incident liver cancer and chronic liver disease mortality, ATBC Study.
| Placebo | α-Tocopherol | β-Carotene | α-Tocopherol+β-carotene | No α-tocopherol | α-Tocopherol | No β-carotene | β-Carotene | |
|---|---|---|---|---|---|---|---|---|
|
Incident liver cancer | ||||||||
| All participantsa | ||||||||
| Cases/all | 45/7282 | 53/7280 | 56/7274 | 54/7269 | 101/14 556 | 107/14 549 | 98/14 562 | 110/14 543 |
| Entire period | 1.00 | 1.18 (0.79–1.75) | 1.26 (0.85–1.87) | 1.21 (0.81–1.80) | 1.00 | 1.06 (0.81–1.39) | 1.00 | 1.13 (0.86–1.49) |
| Cases/all | 10/7282 | 9/7280 | 8/7274 | 9/7269 | 18/14 556 | 18/14 549 | 19/14 562 | 17/14 543 |
| Trial period | 1.00 | 0.88 (0.36–2.17) | 0.76 (0.30–1.93) | 0.88 (0.36–2.15) | 1.00 | 1.00 (0.52–1.92) | 1.00 | 0.87 (0.45–1.68) |
| Cases/all | 35/6433 | 44/6412 | 48/6360 | 45/6339 | 83/12 793 | 89/12 751 | 79/12 845 | 93/12 699 |
| Post-trial period |
1.00 |
1.25 (0.80–1.95) |
1.40 (0.90–2.16) |
1.29 (0.83–2.01) |
1.00 |
1.07 (0.79–1.44) |
1.00 |
1.19 (0.88–1.61) |
| Subsetb | ||||||||
| Cases/controls | 10/98 | 14/107 | 10/83 | 11/78 | 20/181 | 25/185 | 24/205 | 21/161 |
| Without diabetes | 1.00 | 1.38 (0.58–3.32) | 1.14 (0.44–2.93) | 1.46 (0.58–3.69) | 1.00 | 1.33 (0.70–2.52) | 1.00 | 1.08 (0.57–2.03) |
| Cases/controls | 18/67 | 18/79 | 19/75 | 14/72 | 37/142 | 32/151 | 36/146 | 33/147 |
| Prediabetes | 1.00 | 0.91 (0.43–1.93) | 0.99 (0.47–2.07) | 0.70 (0.32–1.55) | 1.00 | 0.81 (0.48–1.39) | 1.00 | 0.89 (0.52–1.52) |
| Cases/controls | 6/16 | 5/11 | 7/23 | 12/12 | 13/39 | 17/23 | 11/27 | 19/35 |
| With diabetes |
1.00 |
0.94 (0.21–4.13) |
0.92 (0.25–3.45) |
3.08 (0.84–11.29) |
1.00 |
2.03 (0.81–5.09) |
1.00 |
1.70 (0.65–4.45) |
|
Chronic liver disease mortality | ||||||||
| All participantsa | ||||||||
| Cases/all | 59/7282 | 57/7280 | 62/7274 | 59/7269 | 121/14 556 | 116/14 549 | 116/14 562 | 121/14 543 |
| 1.00 | 0.97 (0.68–1.40) | 1.06 (0.74–1.51) | 1.01 (0.70–1.45) | 1.00 | 0.96 (0.75–1.24) | 1.00 | 1.05 (0.82–1.36) | |
| Cases/all | 14/7282 | 18/7280 | 16/7274 | 20/7269 | 30/14 556 | 38/14 549 | 32/14 562 | 36/14 543 |
| Trial period | 1.00 | 1.27 (0.63–2.56) | 1.12 (0.55–2.30) | 1.40 (0.71–2.78) | 1.00 | 1.26 (0.78–2.03) | 1.00 | 1.11 (0.69–1.79) |
| Cases/all | 45/6433 | 39/6412 | 46/6360 | 39/6339 | 91/12 793 | 78/12 751 | 84/12 845 | 85/12 699 |
| Post-trial period |
1.00 |
0.87 (0.57–1.33) |
1.04 (0.69–1.56) |
0.88 (0.57–1.35) |
1.00 |
0.86 (0.63–1.16) |
1.00 |
1.09 (0.81–1.47) |
| Subsetb | ||||||||
| Cases/controls | 31/98 | 14/107 | 27/83 | 19/78 | 58/181 | 33/185 | 45/205 | 46/161 |
| Without diabetes | 1.00 | 0.40 (0.20–0.80) | 1.01 (0.55–1.83) | 0.71 (0.37–1.36) | 1.00 | 0.53 (0.33–0.86) | 1.00 | 1.26 (0.79–2.00) |
| Cases/controls | 14/67 | 27/79 | 21/75 | 30/72 | 35/142 | 57/151 | 41/146 | 51/147 |
| Prediabetes | 1.00 | 1.51 (0.72–3.13) | 1.23 (0.57–2.63) | 1.96 (0.95–4.04) | 1.00 | 1.53 (0.94–2.47) | 1.00 | 1.23 (0.77–1.98) |
| Cases / controls | 10/16 | 9/11 | 8/23 | 4/12 | 18/39 | 13/23 | 19/27 | 12/35 |
| With diabetes | 1.00 | 1.59 (0.46–5.48) | 0.60 (0.18–1.96) | 0.49 (0.11–2.09) | 1.00 | 1.27 (0.51–3.14) | 1.00 | 0.46 (0.18–1.17) |
Presented as HRs and 95% CIs.
Presented as ORs and 95% CIs.
Figure 1.
Kaplan–Meier estimates of the probability of no diagnosis of liver cancer among participants randomised to α-tocopherol (AT), β-carotene (BC), combination or placebo arms.
Figure 2.
Kaplan–Meier estimates of the probability of survival from mortality from chronic liver disease among participants randomised to α-tocopherol (AT), β-carotene (BC), combination or placebo arms.
Results were similar across the examined subgroups previously mentioned in the methods section (data not shown) and after excluding the few (0.8% for HBsAg) HBV or (1.6% for anti-HCV) HCV-positive cases (data not shown), although they appeared to vary by diabetes status. Among a subset of participants with information on fasting insulin and glucose, an effect of α-tocopherol vs. no α-tocopherol on CLD was observed among men without diabetes (odds ratio (OR), 0.53; 95% CI, 0.33–0.86), but not among men with prediabetes (OR, 1.53; 95% CI, 0.94–2.47) or diabetes (OR, 1.27; 95% CI, 0.51–3.14; P interaction=0.009) (Table 1). A suggestive protective effect was observed in β-carotene vs. no β-carotene on CLD in men with diabetes (OR, 0.46; 95% CI, 0.18–1.17) but not in men without diabetes or with prediabetes (Pinteraction=0.09). No differences by diabetes status were observed for liver cancer (all Pinteractions >0.20) (Table 1).
Discussion
Overall, our study provides no evidence for a benefit of supplementation with α-tocopherol and/or β-carotene supplementation on liver cancer incidence or CLD mortality in middle-aged participants. Indeed, the observed HRs exceeded 1.00 for most comparisons. It is possible that intervention benefits were not detected because the numbers of incident liver cancers and deaths from CLD were limited. The lower confidence limits in the analyses do suggest that if there are risk reductions from these interventions that we could not detect because of limited sample size, they would be modest (21% and 32% lower from supplemental α-tocopherol for liver cancer and CLD mortality, respectively, and 15% and 26% lower from supplemental β-carotene).We also note that our subgroup analyses (data not shown) indicated consistent null findings across a range of other exposures.
An exception to this pattern was evidence for a benefit of α-tocopherol on CLD mortality in men without diabetes. It is not clear why the effects may differ by diabetes, and chance is a possible explanation. Although these results are consistent with recent findings from the PIVENS trial (Sanyal et al, 2010), which observed that daily supplementation with 800 IU of α-tocopherol had a beneficial effect on NASH progression over 96 weeks in patients lacking diabetes, there are substantial differences between the design of PIVENS and ATBC. A lower dose of α-tocopherol was given in our study (50 IU) and our supplementation period (5–8 years) and follow-up (24 years) were substantially longer. Also, the PIVENS trial enrolled participants with pre-existing NASH, whereas the ATBC study enrolled participants from the general population, most of who probably lacked NASH. Several other smaller trials have reported a beneficial effect of vitamin E supplementation on treating patients with NASH (Sanyal et al, 2004; Yakaryilmaz et al, 2007; Bjelakovic et al, 2011) and for other liver diseases (Bjelakovic et al, 2011). These studies, like the PIVENS trial, enrolled a specific population, that is, patients with pre-existing liver disease and supplemented with higher doses of vitamin E, which contrast notably with the general Finnish population that made up the ATBC study participants and the lower dose of α-tocopherol.
Our analyses treated deaths from other causes as independent censoring at the time of the death. Unlike analyses of cumulative incidence over a long time interval (Gray, 1988), these analyses would not be distorted by differences in the amount of competing mortality among the intervention groups. In any case, mortality rates from other causes were similar across the treatment groups, both overall and in participants with and without diabetes.
Other data on micronutrient supplementation and liver cancer are sparse. Whereas a Chinese observational cohort recently reported an inverse association of both vitamin E supplement use and dietary vitamin E intake with liver cancer (Zhang et al, 2012), no effect was observed for ‘factor D' (β-carotene (15 mg), α-tocopherol (30 mg), and selenium (50 μg)) on liver cancer mortality, relative to placebo in the Linxian General Population Nutrition Intervention Trial (Qu et al, 2007).
In apparent contrast with our results for α-tocopherol and β-carotene supplementation, micronutrient status, as assessed by serum levels, has been associated with lower risk of liver cancer in several prospective cohort studies (Knekt et al, 1991; Yu et al, 1995; Ito et al, 2006; Yuan et al, 2006), including in the current one (companion paper). Within ATBC, we observed that higher baseline levels of serum β-carotene and retinol were associated with a reduced risk of incident liver cancer and CLD mortality, whereas higher baseline α-tocopherol levels were associated with a lower risk of CLD mortality but not incident liver cancer. Such contrasting results do not necessarily conflict. Serum micronutrient status reflects on the long-term dietary intake and metabolism. Micronutrient supplementation during middle age may miss the important window of liver disease and liver cancer development. Alternatively, serum micronutrient levels may serve as a proxy for another aspect of diet or lifestyle, and may not actually have a direct role in the development of liver disease and liver cancer.
Our study has several strengths, including large size, randomised design, evaluation of α-tocopherol or β-carotene supplementation separately, long-term follow-up over 24 years, minimal loss to follow-up, and information on major liver cancer risk factors including HBV, HCV, alcohol, and diabetes. Limitations included the modest numbers of liver cancer cases and deaths from CLD; the primary purpose of the ATBC Study was to evaluate whether these supplements could reduce the lung cancer risk. We lacked information about fibrosis and NASH at baseline and also about incident liver disease. However, ATBC excluded subjects with a baseline history of cirrhosis or alcoholism. As ATBC was conducted among Finnish male smokers, our results may not apply to other populations. Finally, liver cancer incidence and mortality from CLD were not primary end points of ATBC, and we examined numerous subgroups. Thus findings in subgroups may well be due to chance.
In summary, in a large randomised trial, α-tocopherol and β-carotene supplementation had no overall effect on the incidence of liver cancer or on mortality from CLD over the course of 24 years of follow-up either during the intervention period or in the post-intervention follow-up period. Effects were noted in certain subgroups of baseline diabetes status, but these findings were based on a small number of cases. Future studies are needed to examine these subgroup findings, as they may be due to chance.
Acknowledgments
We thank all individuals for their participation in this study. This research was supported by the Intramural Research Program, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department of Health and Human Services and US Public Health Service contracts (N01-CN-45165, N01-RC-45035, and N01-RC-37004). The ATBC Study was registered with clinicaltrials.gov (#NCT00342992).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
Disclaimer
The sponsor reviewed and approved final submission but had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation of the manuscript.
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Bjelakovic G, Gluud LL, Nikolova D, Bjelakovic M, Nagorni A, Gluud C. Antioxidant supplements for liver diseases. Cochrane Database Syst Rev. 2011;3:CD007749. doi: 10.1002/14651858.CD007749.pub2. [DOI] [PubMed] [Google Scholar]
- Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55 (6:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- Clemente C, Elba S, Buongiorno G, Berloco P, Guerra V, Di Leo A. Serum retinol and risk of hepatocellular carcinoma in patients with child-Pugh class A cirrhosis. Cancer Lett. 2002;178 (2:123–129. doi: 10.1016/s0304-3835(01)00843-6. [DOI] [PubMed] [Google Scholar]
- Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann Stat. 1988;16 (3:1141–1154. [Google Scholar]
- Ito Y, Suzuki K, Ishii J, Hishida H, Tamakoshi A, Hamajima N, Aoki K. A population-based follow-up study on mortality from cancer or cardiovascular disease and serum carotenoids, retinol and tocopherols in Japanese inhabitants. Asian Pac J Cancer Prev. 2006;7 (4:533–546. [PubMed] [Google Scholar]
- Knekt P, Aromaa A, Maatela J, Alfthan G, Aaran RK, Nikkari T, Hakama M, Hakulinen T, Teppo L. Serum micronutrients and risk of cancers of low incidence in Finland. Am J Epidemiol. 1991;134 (4:356–361. doi: 10.1093/oxfordjournals.aje.a116097. [DOI] [PubMed] [Google Scholar]
- Korhonen P, Malila N, Pukkala E, Teppo L, Albanes D, Virtamo J. The Finnish Cancer Registry as follow-up source of a large trial cohort—accuracy and delay. Acta Oncol. 2002;41 (4:381–388. doi: 10.1080/028418602760169442. [DOI] [PubMed] [Google Scholar]
- Lirussi F, Azzalini L, Orando S, Orlando R, Angelico F. Antioxidant supplements for non-alcoholic fatty liver disease and/or steatohepatitis. Cochrane Database Syst Rev. 2007;1:CD004996. doi: 10.1002/14651858.CD004996.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedde T, Schwabe RF. NF-kappaB in the liver—linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011;8 (2:108–118. doi: 10.1038/nrgastro.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52 (1:79–104. doi: 10.1002/hep.23623. [DOI] [PubMed] [Google Scholar]
- Pan WH, Wang CY, Huang SM, Yeh SY, Lin WG, Lin DI, Liaw YF. Vitamin A, Vitamin E or beta-carotene status and hepatitis B-related hepatocellular carcinoma. Annals Epidemiol. 1993;3 (3:217–224. doi: 10.1016/1047-2797(93)90022-v. [DOI] [PubMed] [Google Scholar]
- Poli G. Pathogenesis of liver fibrosis: role of oxidative stress. Mol Aspects Med. 2000;21 (3:49–98. doi: 10.1016/s0098-2997(00)00004-2. [DOI] [PubMed] [Google Scholar]
- Qu CX, Kamangar F, Fan JH, Yu B, Sun XD, Taylor PR, Chen BE, Abnet CC, Qiao YL, Mark SD, Dawsey SM. Chemoprevention of primary liver cancer: a randomized, double-blind trial in Linxian, China. J Natl Cancer Inst. 2007;99 (16:1240–1247. doi: 10.1093/jnci/djm084. [DOI] [PubMed] [Google Scholar]
- Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362 (18:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal AJ, Mofrad PS, Contos MJ, Sargeant C, Luketic VA, Sterling RK, Stravitz RT, Shiffman ML, Clore J, Mills AS. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2004;2 (12:1107–1115. doi: 10.1016/s1542-3565(04)00457-4. [DOI] [PubMed] [Google Scholar]
- The ATBC Cancer Prevention Study Group The alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. Ann Epidemiol. 1994;4 (1:1–10. doi: 10.1016/1047-2797(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Yakaryilmaz F, Guliter S, Savas B, Erdem O, Ersoy R, Erden E, Akyol G, Bozkaya H, Ozenirler S. Effects of vitamin E treatment on peroxisome proliferator-activated receptor-alpha expression and insulin resistance in patients with non-alcoholic steatohepatitis: results of a pilot study. Intern Med J. 2007;37 (4:229–235. doi: 10.1111/j.1445-5994.2006.01295.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Yamashita S, Fujisawa A, Kokura S, Yoshikawa T. Oxidative stress in patients with hepatitis, cirrhosis, and hepatoma evaluated by plasma antioxidants. Biochem Biophys Res Commun. 1998;247 (1:166–170. doi: 10.1006/bbrc.1998.8752. [DOI] [PubMed] [Google Scholar]
- Yu MW, Chiu YH, Chiang YC, Chen CH, Lee TH, Santella RM, Chern HD, Liaw YF, Chen CJ. Plasma carotenoids, glutathione S-transferase M1 and T1 genetic polymorphisms, and risk of hepatocellular carcinoma: independent and interactive effects. Am J Epidemiol. 1999;149 (7:621–629. doi: 10.1093/oxfordjournals.aje.a009862. [DOI] [PubMed] [Google Scholar]
- Yu MW, Hsieh HH, Pan WH, Yang CS, CH CJ. Vegetable consumption, serum retinol level, and risk of hepatocellular carcinoma. Cancer Res. 1995;55 (6:1301–1305. [PubMed] [Google Scholar]
- Yuan JM, Gao YT, Ong CN, Ross RK, Yu MC. Prediagnostic level of serum retinol in relation to reduced risk of hepatocellular carcinoma. J Natl Cancer Inst. 2006;98 (7:482–490. doi: 10.1093/jnci/djj104. [DOI] [PubMed] [Google Scholar]
- Zhang W, Shu XO, Li H, Yang G, Cai H, Ji BT, Gao J, Gao YT, Zheng W, Xiang YB. Vitamin intake and liver cancer risk: a report from two cohort studies in china. J Natl Cancer Inst. 2012;104 (15:1174–1182. doi: 10.1093/jnci/djs277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.