Summary
The American Rare Donor Program (ARDP) was formed in 1998 to provide rare blood units for patients in need. Members of the program identify rare donors and submit donor information for entrance into a database, REGGI. Information on patients in need of rare blood is also submitted and entered into REGGI. REGGI serves to match phenotypes of registered donors with patients having the respective antibodies. A search process for available units ensues, and blood is provided to the patient. This report provides information on REGGI and its use in the ARDP.
Keywords: Blood donation, Blood products, Donor screening, Donors, Red blood cell antibodies, Red blood cell antigens
Overview
The American Rare Donor Program (ARDP) was formed in 1998 as a collaborative effort between the AABB and the American Red Cross to provide rare blood for patients in need. Both organizations began rare donor databases in the 1960s; the American Red Cross Rare Donor Registry and the AABB Rare Donor File were merged into the ARDP database, REGGI, in 1998 as a result of this effort. Currently REGGI has phenotype information on more than 59,000 active rare donors submitted by its 82 member facilities located in the USA as well as one member facility each in Italy, Kuwait, and Brazil (fig. 1). ARDP members consist of Red Cross and AABB-accredited immunology reference laboratories (IRL). Blood units collected at ARDP member facilities are available to other ARDP member facilities upon request. In addition, non-ARDP member facilities with a transfusion request for rare blood units may access the ARDP by contacting a member facility, making the services of the ARDP available to all transfusion services and to all patients, both nationally and internationally.
Fig. 1.
Geographical location of ARDP member IRLs as of December 2013 (map courtesy of Geralyn M. Meny, MD).
The ARDP is governed by a standard operating procedure (SOP) approved by members of the ARDP advisory committee. This SOP provides procedural steps for accessing the ARDP to submit donors and to request rare blood units for patients.
Submitting Rare Donors to the ARDP
The ARDP database, REGGI, is housed and maintained at the Philadelphia ARDP site. ARDP members screen and identify rare donors and complete rare donor submission forms with either full or partial donor demographic information and donor test results which are then scanned, faxed, or mailed to the Philadelphia site. According to the current ARDP SOP, there are three categories of rare donors: negative for a high-prevalence antigen, negative for multiple common antigens, or IgA-deficient. Table 1 defines the specific criteria for each.
Table 1.
Rare donor definitions per ARDP criteria
| ABO group | Criteria |
|---|---|
| All ABO groups | negative for a high-prevalence antigen (<1/1,000), such as U, Jsb, Kpb, or Yta |
| Group O and A | negative for multiple common antigens R1R1, R2R2, R0R0, or rr; and K–; and Fy(a–) or Fy(b–); and Jk(a–) or Jk(b–); and S– or s– or R1R1, R2R2, or rr; and K–; and Fy(a–b–) |
| All ABO groups | IgA deficient; <0.05 mg/dl IgA |
Testing to identify donors as antigen-negative must be performed using two different testing sources; molecular testing may be considered one source or it may be allowed as the sole source for specific antigens for which antisera are unavailable or unreliable or for which further allelic characterization is required to appropriately provide units; namely V, VS, hrB, hrS, Doa, Dob, U, Hy, Joa, Jsa, and Lua. In addition, all donors identified as being negative for a high-prevalence antigen must be tested for the common antigens: C, c, E, e, K, Fya, Fyb, Jka, Jkb, S, and s also using two testing sources. Donors submitted as IgA-deficient must be tested as < 0.05 mg/dl IgA on two different blood donations.
Submitted rare donor information is reviewed by ARDP staff at the Philadelphia site for completeness and to ensure the donor phenotype meets the criteria for being rare. Donor information is entered into REGGI in a double-blinded entry. An ARDP administrative staff member enters information to create a donor profile; the same information is entered a second time to ensure accuracy of the entry. REGGI compares the information and identifies entry discrepancies or if the donor is already in the database. Discrepancies are corrected, if applicable, and a donor proof sheet is printed for comparison with the initial rare donor submission form. Review is performed by ARDP staff. Once determined to be accurate, the donor information is reconciled in REGGI and a donor packet is printed through REGGI for return to the submitting ARDP member with subsequent distribution to the donor. For members who submitted full donor demographics, the donor packet consists of a wallet-sized card with the donor's name and phenotype, a donor-addressed letter explaining the donor's entry into the program and the importance of the donor's blood for rare patients, an ARDP informational pamphlet, and a postage-paid envelope for donor use if edits to the demographic information are needed or if the donor does not want to join the ARDP. For members who submitted donors with partial demographics, only the ARDP informational pamphlet is provided. These members have opted to distribute facility-specific donor letters and information to their donors.
Once the donor is registered in REGGI, it is important to ensure that donor demographics are current to enable contact of the donor for recruitment. Donors are contacted semi-annually through a mailed, REGGI-generated address update card or another contact mechanism with instructions to review and update address information and to send changes to the Philadelphia site. ARDP administrative staff edits the demographic information in REGGI or deactivates the donor if the address update card is received as ‘Return to Sender.’ Donors are also deleted upon notification to the ARDP of donor death or if the donor no longer wants to donate or cannot donate for medical reasons.
In addition, monthly donor activity reports are generated through REGGI for those ARDP members who have submitted new donors or for whom changes to donor demographics or phenotype information were made. Annual ARDP member reports are also generated through REGGI. These consist of a summary of the member's activity for the year (table 2) as well as a complete list of the member's current, active donor list with demographic and phenotype information for each donor. The summary report is sent to the ARDP member annually. The complete donor list is sent at the request of the ARDP member.
Table 2.
ARDP member information provided on Annual ARDP Summary Report through REGGI
| Number of donors submitted for the year |
| Total number of registered, active donors |
| Number of times member was contacted |
| Number of rare units shipped by member |
| Number of requests for rare units |
| Number of rare units received |
Searching for Rare Blood Units
ARDP members identify patients requiring rare blood units using the same rare criteria as for donor submission; patients must have the respective antibodies. Completed requests are faxed to the ARDP laboratory staff in Philadelphia. The ARDP staff search REGGI for previous requests for this patient as each patient is given a unique case number and all subsequent requests for this patient (recorded in REGGI as incidents) are contained under this case number. Patient information is entered into REGGI, creating a new case number and/or an incident. Once created, REGGI can perform a search to identify ARDP members that have registered donors who match the phenotype desired for the patient request. REGGI provides the name of the ARDP members as well as the number of registered donors at each facility. Further drilling down in REGGI shows the name and demographic information on these registered donors; this information is helpful when requesting recruitment.
Identified ARDP members are then contacted for unit availability. Currently ARDP members are contacted through a separate fax process that uses phenotype-defined speed dial groups on ARDP-dedicated fax machines. These speed dial groups were created using donor phenotype information entered into REGGI upon donor submission. Previously, all contact was made by phone. Contacting ARDP members through the use of speed dial groups allows for more widespread, expeditious contact of members and their subsequent reply regarding the availability of units. If units are available, the ARDP member faxes unit information and member contact information back to the Philadelphia site which then serves as the liaison between the requesting and shipping facilities by relaying unit availability and member contact information. Shipping facility information is entered into REGGI for the respective patient incident; namely, the ARDP member providing the units, number of units provided, and whether the units are liquid or frozen. It is this information that is captured in the Annual ARDP Summary Report for each ARDP member.
If units are not readily available through the fax search process, ARDP members with registered donors are requested to recruit their donors. If these efforts do not produce units, an international search may be ensued requiring adherence to Food and Drug Administration (FDA) guidelines.
Effectiveness of the ARDP
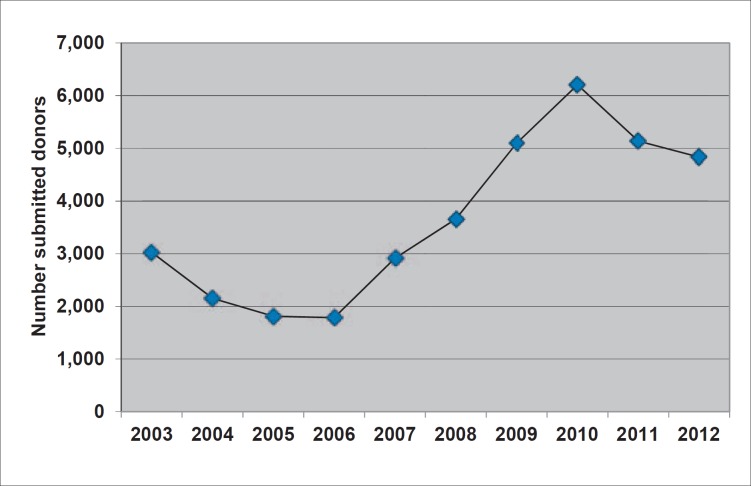
Although REGGI currently stores information on more than 59,000 active rare donors, RBC units are not always available at the member facilities when needed to fill a transfusion request. Figure 2 shows the effectiveness of the ARDP in filling 6,995 patient requests from January 1, 2005 through December 31, 2012. Of the 4,517 requests for high-prevalence antigen-negative units, 7.9% were unfilled (i.e., no units were provided), while 1.8% of the 2,317 requests for multiple common negative antigen-negative and 1.2% of the 161 requests for IgA-deficient units were unfilled. Unfilled requests can be attributed to an inadequate number of identified donors as well as to inadequate donor retention. Dwindling sources of licensed and unlicensed antisera for certain low-prevalence antigens, such as Jsa and Lua, and for high-incidence antigens, such as Hy and Joa, as well as the variant nature of certain antigens, such as hrB and hrS, made donor screening a challenge. Toward increasing the effectiveness of the ARDP, the Red Cross National Molecular Laboratory began screening donors using commercially available array-based platforms in 2007, enabling the mass screening of donors for up to 35 different antigens with a consequent increase in rare donor submissions to the ARDP (fig. 3.)
Fig. 2.
Unfilled versus filled requests*.
Fig. 3.
Donor submissions to the ARDP*.
Although the ARDP is able to fill 94–95% of the requests, there are still unfilled requests and patients not receiving rare blood. Data generated through REGGI aids in identifying unfilled requests and the associated donors needed to fill these requests. The ARDP continues to assess testing methodologies and processes to better identify rare donors in a continued effort to provide rare units for patients in need.
Source
American Rare Donor Program, Standard Operating Procedure, Version Date: 4/1/2013.
Disclosure Statement
The author has no conflict of interests.