Abstract
Endothelin-1 acts on endothelial cells to enhance mechanical stimulation-induced release of ATP, which in turn can act on sensory neurons innervating blood vessels to contribute to vascular pain, a phenomenon we have referred to as stimulus-dependent hyperalgesia (SDH). In the present study we evaluated the role of the major classes of ATP release mechanisms to SDH: vesicular exocytosis, plasma membrane associated ATP synthase, ATP-Binding Cassette (ABC) transporters, and ion channels. Inhibitors of vesicular exocytosis (i.e., monensin, brefeldin A and bafilomycin), plasma membrane associated ATPase (i.e., oligomycin and pigment epithelium-derived factor-derived peptide 34-mer) and connexin ion channels (carbenoxolone and flufenamic acid), but not ABC transporters (i.e., dipyridamole, nicardipine or CFTRinh-172) attenuated stimulus-dependent hyperalgesia. These studies support a role of ATP in SDH, and suggest novel targets for the treatment of vascular pain syndromes.
Keywords: Endothelin-1, ATP release, vascular pain, endothelium
Introduction
We have recently discovered a novel mechanism by which endothelial cells, which line the lumen of blood vessels, contribute to peripheral vascular pain mechanisms 29–32. We demonstrated two separate mechanisms by which endothelin-1 (ET-1) causes hyperalgesia; intradermal administration of ET-1 decreases nociceptive threshold to a similar magnitude when measured for the first time at 15 or 30 min after administration, however, when readings are taken every 5 min, in the interval between 15 and 30 min, there is a progressive decrease in nociceptive threshold, i.e., the mechanical stimulation of the testing itself enhanced ET-1 hyperalgesia 30. This phenomenon, referred to as stimulus-dependent hyperalgesia (SDH), is elicited by two prominent vasoactive compounds, ET-1 and epinephrine, acting at their cognate receptors on endothelial cells to produce a state in which mechanical stimulation now produces enhanced release of ATP that, in turn, acts at P2X3 receptors on sensory neurons 9. One key feature of SDH that remains to be elucidated is the mechanism mediating ATP release, in response to the mechanical stimulus in the ET-1 and epinephrine sensitized endothelial cell.
There are several mechanisms that have been described by which ATP is released from cells, including: 1) vesicular exocytosis (ATP is found in high concentration in synaptic vesicles where it is thought to function as a co-transmitter 4), 2) plasma membrane associated ATP synthase (this enzyme is found in the plasma membrane as well as mitochondrial membrane 8, 10), 3) ATP-binding cassette (ABC) transporters 41, 50, and 4) various ion channels 23, 25, 41, 55 (e.g., connexin hemichannels, pannexin channels, maxi-anion channels, volume regulated anion channels and P2X7 receptors). Several of these mechanisms have already been demonstrated to be present in endothelial cells 40, 41 and, importantly from a practical standpoint, there is a relatively well-developed pharmacology available for the study of these mechanisms. Therefore, in the present study we evaluated the role of these ATP release mechanisms in ET-1 induced SDH.
Materials and Methods
Animals
Experiments were performed on male Sprague Dawley rats (200–250 g; Charles River). Animals were housed three per cage, under a 12 h light/dark cycle, in a temperature- and humidity-controlled environment. Food and water were available ad libitum. All behavioral nociceptive testing was performed between 10:00 A.M. and 4:00 P.M. All experimental protocols were approved by the University of California, San Francisco Committee on Animal Research and conformed to National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and their suffering.
Nociceptive testing
Rats were acclimatized to the experimental environment and behavioral testing procedures, before performing experiments. To acclimatize rats to the testing environment, they were brought to the experimental area in their home cage and left in their cage for 15–30 min, after which they were placed in a restrainer (cylindrical transparent acrylic tubes that have side openings to allow extension of the hind limbs from the restrainer, for nociceptive testing). Rats were left undisturbed in the restrainer for another 15–30 min before nociceptive testing was started.
The nociceptive flexion reflex was quantified with an Ugo Basile Analgesymeter (Stoelting®), which applies a linearly increasing mechanical force to the dorsum of the rat’s hind paw. Nociceptive threshold was defined as the force, in grams, at which the rat withdrew its paw; the experimenter was not blinded to treatment group. Baseline nociceptive threshold, was defined as the mean of three readings taken at 5-min intervals, determined prior to all experiments. Hyperalgesia was defined as a decrease in mechanical nociceptive threshold, here presented as percent reduction from baseline [% reduction in threshold = (pretreatment threshold - post-treatment threshold)/(pretreatment threshold)] × 100. Each paw was treated as an independent measure; both paws of the same rat received the same treatment 2, 3, 36. Each experiment was performed on separate groups of rats. Inhibitors were injected intradermally into the dorsum of both hind paws at the site of nociceptive testing (to determine whether the inhibitors alone affected nociceptive threshold), 15 min before the administration of ET-1 and paw-withdrawal thresholds compared, before and after drug treatment. The doses used in this study are based on a very large number of studies that have used 1 μg as a screening dose for intradermal drugs 14, 29–33, all inhibitors were administered at concentrations known to inhibit their ATP release mechanisms 1, 13, 16, 17, 48, 62.
Drugs
Vesicular exocytosis mechanisms
The role of exocytosis (e.g., vesicular exocytosis) mechanisms was studied by administering monensin (Sigma Chemical Co., St. Louis, MO), which interferes with vesicle formation from the Golgi formation 4, 39, bafilomycin A1 (VWR International, Brisbane CA), an inhibitor of vacuolar H+-ATPases that produce proton gradients in endoplasmic reticulum thereby reducing the driving force for uptake of ATP into the vesicle 20 and brefeldin A (Sigma Chemical Co) which blocks the activation of a subset of ADP-ribosylation factors and thereby inhibiting vesicle trafficking 38.
ATP-binding cassette (ABC) transporters
ATP-binding cassette (ABC) transporters have been implicated in ATP release, including the multidrug resistance gene product, MDR1 (also known as P-glycoprotein, ABCB1 59), and the cystic fibrosis transmembrane conductance regulator CFTR (ABCC7) 58. We tested for a contribution of ABC transporters to SDH by administering ABC transporter inhibitors dipyridamole (Sigma Chemical Co.), an inhibitor of MDR1/P-glycoprotein/BCRP 52, and nicardipine (Santa Cruz Biotechnology, Paso Robles, CA), a dihydropyridine in different chemical class from dipyridamole, that is another potent inhibitor of MDR1/P-glycoprotein 52, as well as breast cancer resistance protein 67. We also tested the highly selective inhibitor of CFTR, CFTRinh-172 (Santa Cruz Biotechnology), a thiazolidinone compound that acts directly on the CFTR 57, 58.
Ion channels
Finally, the role of ion channels (e.g., connexin hemichannels, pannexin channels, maxi-anion channels, and volume regulated anion channels) has been commonly studied by administering the gap junction inhibitor flufenamic acid (Sigma Chemical Company), an inhibitor of Connexin 43 22, and carbenoxolone (Sigma Chemical Company), a non-specific gap junction blocker 12.
Plasma membrane ATP synthase
In addition to its synthesis by mitochondria, ATP is also synthesized by a plasma membrane ATP synthase. The role of plasma membrane ATP synthase was evaluated by administering the ATP synthesis inhibitor oligomycin (Sigma Chemical Co.) 46 and PEDF (pigment epithelium-derived factor peptide 34-mer, Phoenix Pharmaceutical, Brisbane, CA) a ligand of cell-surface F1 ATP synthase, that only inhibits extracellular ATP synthesis 13, 47, to rule out the mitochondrion as the site of ATP synthase inhibition.
All drugs were administered intradermally (i.d.) in a volume of 5 μl using a 30-gauge hypodermic needle attached to a micro-syringe (Hamilton, Reno, NV) by PE-10 polyethylene tubing. All inhibitors were administered 15 min prior to ET-1 (Fisher Scientific, Houston, TX) and nociceptive thresholds measured (four times), at 15, 20, 25 and 30 min post ET-1. The per se effect of all the inhibitors were separately evaluated and none had a significant effect on paw-withdrawal threshold of the naïve rats (data not shown). Monensin1, oligomycin 62, PEDF 13, carbenoxolone 16, flufenamic acid 17, brefeldin A and bafilomycin 48 were given at concentrations that have been shown to inhibit ATP release or degradation.
Statistics
The dependent variable in experiments evaluating cutaneous nociceptive threshold was change in paw withdrawal threshold from the pretreatment baseline threshold. Group data are represented as mean ± SEM. Statistical significance was determined by one- or two-way repeated-measures ANOVA, followed by Dunnet’s post hoc test. p < 0.05 was considered statistically significant.
Results
Vesicular exocytosis
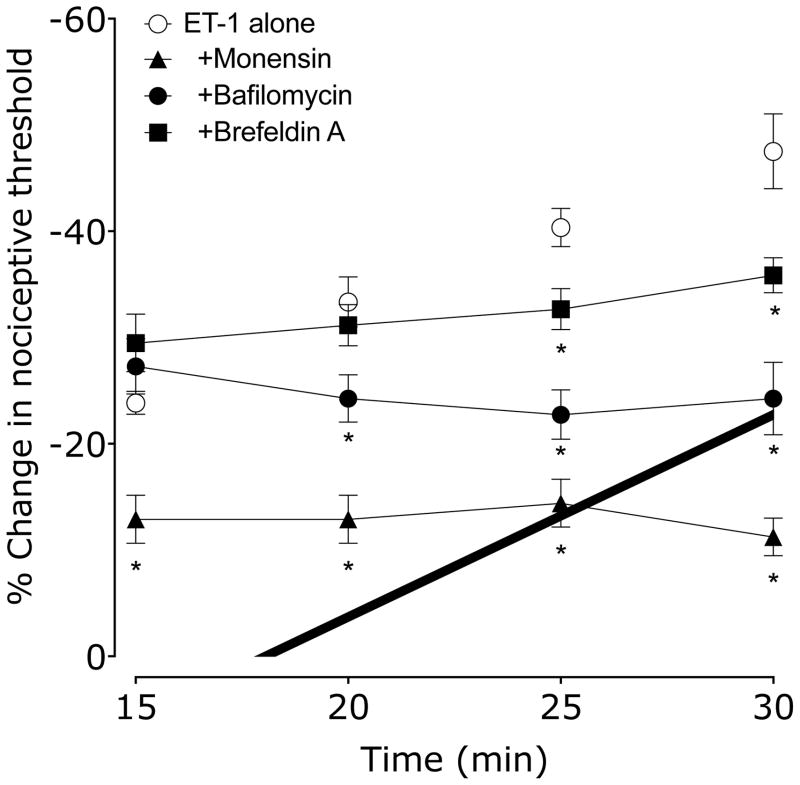
We administered three inhibitors of vesicular release mechanisms, monensin, brefeldin A and bafilomycin, to evaluate the role of this release mechanism in endothelial cell mediated ET-1-induced SDH. Rats received either vehicle (0.9% sodium chloride), monensin, bafilomycin or brefeldin A, 15 min before ET-1 administration. Nociceptive threshold was evaluated every 5 min beginning 15 min after ET-1, the standard protocol for detecting SDH 30, 31. In rats pretreated with vehicle, ET-1 hyperalgesia increased with each subsequent test of mechanical threshold, indicating the presence of SDH, as previously described 30. However, in rats pretreated with either monensin, brefeldin A or with bafilomycin, this enhancement of hyperalgesia by mechanical stimulation was abolished (Figure 1). Monensin also affects ET-1 hyperalgesia, a phenomenon we have previously observed with β2-adrenergic and 5HT1B/D receptor antagonists 31, presumably due to action on the nociceptor terminal.
Figure 1. Effect of bafilomycin (vacuolar H-ATPase inhibitor), monensin (inhibitor of vesicle formation) and brefeldin A (inhibitor of vesicle transport) on ET-1 induced mechanical hyperalgesia and stimulus-dependent hyperalgesia (SDH).
15 min before ET-1, rats received vehicle (5 μl), bafilomycin, monensin or brefeldin A (each 1 μg in 5 μl/paw). Paw withdrawal thresholds were measured 15, 20, 25 & 30 min after ET-1 administration. Bafilomycin, monensin and brefeldin A each significantly inhibited ET-1–induced SDH compared to vehicle treated controls, and monensin also significantly inhibited ET-1 hyperalgesia (*P < 0.001, two-way repeated measures ANOVA, followed by Bonferroni post test, N = 6).
ATP-binding cassette (ABC) transporters
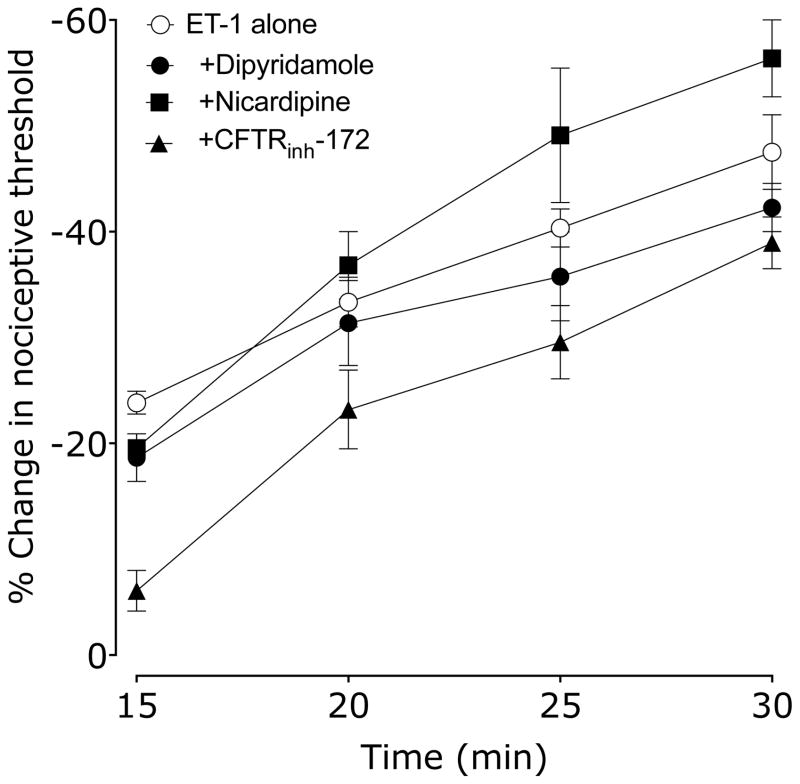
We administered inhibitors of three ATP-binding cassette (ABC) transporters, dipyridamole, nicardipine and CFTRinh-172, to evaluate the role of ABC transporters in endothelial cell mediated SDH. Rats received vehicle (0.9% sodium chloride, or 10% DMSO in 0.9% saline for CFTRinh-172), dipyridamole, nicardipine or CFTRinh-172 15 min before ET-1. Nociceptive threshold was evaluated every 5 min beginning 15 min after ET-1. Neither dipyridamole, nicardipine nor CFTRinh-172 affected the development of SDH, but CFTRinh-172 significantly attenuated ET-1–induced hyperalgesia (Figure 2).
Figure 2. Effect of dipyridamole, nicardipine and CFTRinh-172 (ABC transport inhibitors) on ET-1 induced mechanical hyperalgesia and SDH.
15 min before ET-1, rats received vehicle (5 μl), dipyridamole, nicardipine or CFTRinh-172 (all 1 μg in 5 μl/paw). Paw withdrawal thresholds were measured 15, 20, 25 & 30 min after ET-1 administration. Neither dipyridamole, nicardipine nor CFTRinh-172 affected ET-1 SDH (P=N.S., two-way repeated measures ANOVA, N = 6), however CFTRinh-172 significantly attenuated ET-1 hyperalgesia (2-way ANOVA with Dunnet’s post hoc test, *P<0.05). Note that the ET-1 alone data is the same group as in Figure 1.
Ion channels
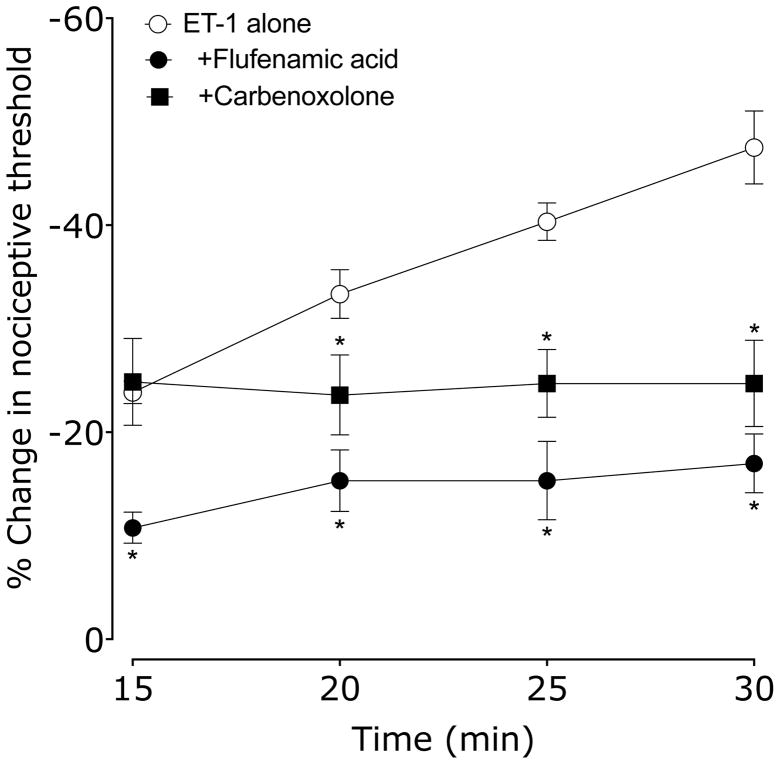
We administered two ion channel inhibitors, flufenamic acid (a voltage gated sodium channel blocker) and carbenoxolone (an interneuronal gap junction blocker), to evaluate the role of ion channels in endothelial cell mediated SDH. Rats received vehicle (0.9% sodium chloride), flufenamic acid or carbenoxolone 15 min before ET-1. Nociceptive threshold was evaluated every 5 min beginning 15 min after ET-1. Flufenamic acid and carbenoxolone pretreatment completely prevented the development of SDH, and flufenamic acid attenuated ET-1 hyperalgesia (Figure 3).
Figure 3. Effect of flufenamic acid (voltage gated sodium channel blocker) and carbenoxolone (interneuronal gap junction blocker) on ET-1 induced mechanical hyperalgesia and SDH.
15 min before ET-1, rats received vehicle (5 μl), flufenamic acid or carbenoxolone (both 1 μg in 5 μl/paw). Paw withdrawal thresholds were measured 15, 20, 25 & 30 min after ET-1 administration. Both flufenamic acid and carbenoxolone significantly inhibited SDH; flufenamic acid, but not carbenoxolone, significantly attenuated ET-1 induced hyperalgesia (*P < 0.001, two-way repeated measures ANOVA, followed by Bonferroni post test, N = 6). Note that the ET-1 alone data is the same group as in Figure 1.
Plasma membrane associated ATP synthase
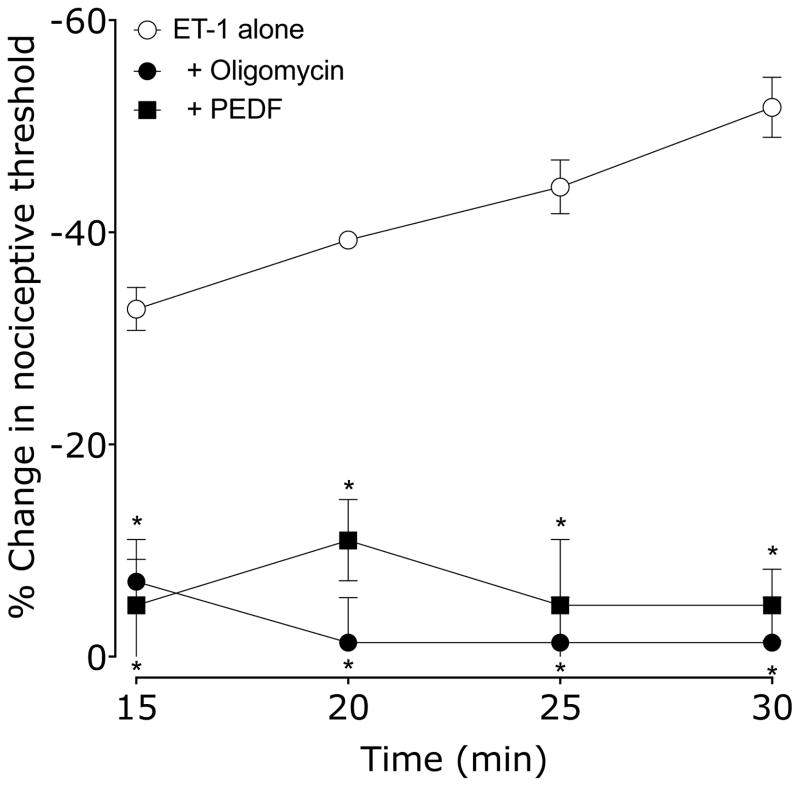
Finally, we administered two inhibitors of plasma membrane associated ATP synthase, oligomycin and PEDF, to evaluate the role of ATP synthase in endothelial cell mediated SDH. Rats received either vehicle (0.9% sodium chloride or 10% DMSO in 0.9% saline for oligomycin), oligomycin or PEDF 15 min before ET-1. Nociceptive threshold was evaluated every 5 min beginning 15 min after ET-1. Both oligomycin and PEDF pretreatment prevented both ET-1 hyperalgesia and the development of ET-1–induced SDH (Figure 4).
Figure 4. Effect of oligomycin A (ATP synthase inhibitor) or PEDF-34-mer (extracellular ATP synthase inhibitor) on ET-1 induced mechanical hyperalgesia and SDH.
15 min before ET-1, rats received vehicle (5 μl), oligomycin or PEDF-34-mer (both 1 μg in 5 μl/paw). Paw withdrawal thresholds were measured 15, 20, 25 & 30 min after ET-1 administration. Both oligomycin and PEDF-34-mer significantly inhibited SDH as well as ET-1 hyperalgesia compared to vehicle treated controls (*P < 0.05, two-way repeated measures ANOVA, followed by Bonferroni post test, N = 6).
Discussion
The intradermal administration of ET-1 produces mechanical hyperalgesia, which is further enhanced by repeated testing with a threshold nociceptive intensity mechanical stimulus, a phenomenon that we have termed stimulus dependent hyperalgesia (SDH) 30. While ET-1 hyperalgesia is mediated by its action at ET receptors on the primary afferent nociceptors 29, we hypothesized that SDH is mediated via ET receptors on endothelial cells. Since a role of the endothelial cell in vascular pain has been postulated 6, 44, 51, 56, we used a method employed in the cardiovascular and renal vascular literature to impair endothelial function: administration of octoxynol-9 to the luminal side of blood vessels, in vitro 11, 26 and in vivo 54. We observed that this method of impairing endothelial function eliminates ET-1–induced SDH while leaving ET-1 hyperalgesia undiminished 29, 31.
A signal feature of SDH is that it is produced by repeated mechanical stimulation. Since endothelial cells are activated by mechanical stimuli to release ATP 4, 6, 43, 65, and ATP acts on P2X3 receptors on nociceptors to mediate pain, we hypothesized that ET-1 acts on endothelial cells to enhance mechanical stimulation-induced ATP release, giving rise to SDH. In this study, we examined the contribution of ATP release mechanisms to SDH.
We observed that inhibitors of ATP release via vesicular mechanisms (bafilomycin A, monensin, brefeldin A), and ion channel-dependent mechanisms (flufenamic acid, carbenoxolone) significantly attenuate SDH. We also observed that inhibition of ATP synthase activity with oligomycin A, an inhibitor of the ATP synthase, as well as by PEDF-34-mer, which is specific for ecto F1-ATP synthase on the plasma membrane 47. However, we did not observe attenuation in ET-1–induced SDH with dipyridamole, a potent inhibitor of the ABC transporters, including P-glycoprotein (ABCB1) 60, multidrug resistance-associated protein (ABCC1) 28, and breast cancer resistance protein (ABCG2) 64, or by nicardipine (a dihydropyridine, different chemical class from dipyridamole) another potent inhibitor of P-glycoprotein 52 and breast cancer resistance protein 67. CFTRinh-172, a potent and highly selective antagonist for the CFTR transport mechanism, also failed to attenuate ET-1 induced SDH. However, CFTRinh-172 attenuation of ET-1 hyperalgesia may be related to the role CFTR plays in mediating the release of ATP from dorsal root ganglia (DRG) 34; presence of CFTRinh-172 may decrease the release of ATP from DRG in response to increased extracellular levels of ATP 42.
Since the drugs used in this study have actions other than inhibition of a specific ATP release mechanism, we used more than one class of drug for each release mechanism (i.e. bafilomycin A, monensin and brefeldin A for vesicular release, and flufenamic acid and carbenoxolone for ion channel-dependent release). This approach is used routinely in the endothelial cell literature to establish the role of these mechanisms in ATP release 4, 15, 17, 24, 40, 41, 63. Of note in this regard, specifically identified following high throughput screening of 50,000 molecules, CFTRinh-172 is a potent and highly selective antagonist for the CFTR transport mechanism 35. Our observations implicate multiple ATP release mechanisms in response to mechanical stimulation, as seen in other cell types 20, 39, 61.
While release of ATP from endothelial cells in response to mechanical stress is well-established 4, 5, 40, 43, 65, the specific mechanisms mediating release are still under investigation 41. Our observation that monensin, bafilomycin and brefeldin A inhibit ET-1–induced SDH support the hypothesis that vesicular release mechanism, presumably ATP release based on our previous P2X3 antagonist studies 31, is involved in the expression of SDH. This hypothesis is consistent with histological evidence that ATP is present in vesicles in endothelial cells, and release of ATP from cultured endothelial cells exposed to a shear stress is blocked by monensin 4. Of note, it is also possible that the compounds used in this study to inhibit ATP release, may have action on cell types in the periphery other than the endothelial cell. For example, gene products of glial cells, such as Schwann cells, have been hypothesized to play a role in nerve injury and sensory nerve function 7, 19, while a role for immune cells (e.g. neutrophils, macrophages, T cells) in acute and chronic inflammatory and neuropathic pain is well-established 21, 45, 53. Other cell types, such as keratinocytes 18, 37 and mast cells 27, 49 are also implicated in peripheral nociceptive mechanisms. It is likely that actions of mediators, released from these cell types, on nociceptors contribute to regulating nociceptor function in chronic pain.
We also observed that inhibition of ATP synthesis by oligomycin or PEDF-derived peptide 34-mer (which is selective for extracellular ATPase), prevented both ET-1 induced hyperalgesia as well as SDH. While it might be assumed that inhibition of extracellular ATP synthesis would be independent of mechanical stimulation-induced release of ATP, it has previously been shown that inhibition of cell surface ATP synthase with angiostatin, piceatannol, or anti-ATP synthase antibody (none of which cross the plasma membrane, and so have no effect on mitochondrial ATP synthase activity) markedly decrease mechanical stimulation-induced ATP release 66.
Taken together with our previous observations that ET-1 induced SDH is attenuated by a selective antagonist, for the P2X3 receptor, A-317491, present on the nociceptor and activated by ATP 29, our data provide additional evidence for mechanical stimulation-induced release of ATP from endothelial cells via vesicular and ion channel-dependent mechanisms mediating SDH.
Perspective.
Endothelin-1 acts on endothelial cells to produce mechanical stimulation-induced hyperalgesia. Inhibitors of three different ATP release mechanisms attenuated this stimulus-dependent hyperalgesia. These studies provide support for a role of ATP in stimulus-dependent hyperalgesia, and suggest novel targets for the treatment of vascular pain syndromes.
Footnotes
Disclosures: This work was supported by NIH grant AR063312. The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akopova I, Tatur S, Grygorczyk M, Luchowski R, Gryczynski I, Gryczynski Z, Borejdo J, Grygorczyk R. Imaging exocytosis of ATP-containing vesicles with TIRF microscopy in lung epithelial A549 cells. Purinergic Signal. 2012;8:59–70. doi: 10.1007/s11302-011-9259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aley KO, Levine JD. Multiple receptors involved in peripheral alpha 2, mu, and A1 antinociception, tolerance, and withdrawal. J Neurosci. 1997;17:735–744. doi: 10.1523/JNEUROSCI.17-02-00735.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aley KO, Levine JD. Role of protein kinase A in the maintenance of inflammatory pain. J Neurosci. 1999;19:2181–2186. doi: 10.1523/JNEUROSCI.19-06-02181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodin P, Burnstock G. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J Cardiovasc Pharmacol. 2001;38:900–908. doi: 10.1097/00005344-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Burnstock G. Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat. 1999;194:335–342. doi: 10.1046/j.1469-7580.1999.19430335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnstock G. Purinergic regulation of vascular tone and remodelling. Auton Autacoid Pharmacol. 2009;29:63–72. doi: 10.1111/j.1474-8673.2009.00435.x. [DOI] [PubMed] [Google Scholar]
- 7.Campana WM. Schwann cells: activated peripheral glia and their role in neuropathic pain. Brain Behav Immun. 2007;21:522–527. doi: 10.1016/j.bbi.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Champagne E, Martinez LO, Collet X, Barbaras R. Ecto-F1Fo ATP synthase/F1 ATPase: metabolic and immunological functions. Curr Opin Lipidol. 2006;17:279–284. doi: 10.1097/01.mol.0000226120.27931.76. [DOI] [PubMed] [Google Scholar]
- 9.Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- 10.Chi SL, Pizzo SV. Cell surface F1Fo ATP synthase: a new paradigm? Ann Med. 2006;38:429–438. doi: 10.1080/07853890600928698. [DOI] [PubMed] [Google Scholar]
- 11.Connor HE, Feniuk W. Influence of the endothelium on contractile effects of 5-hydroxytryptamine and selective 5–HT agonists in canine basilar artery. Br J Pharmacol. 1989;96:170–178. doi: 10.1111/j.1476-5381.1989.tb11797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Groot JR, Veenstra T, Verkerk AO, Wilders R, Smits JP, Wilms-Schopman FJ, Wiegerinck RF, Bourier J, Belterman CN, Coronel R, Verheijck EE. Conduction slowing by the gap junctional uncoupler carbenoxolone. Cardiovasc Res. 2003;60:288–297. doi: 10.1016/j.cardiores.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Deshpande M, Notari L, Subramanian P, Notario V, Becerra SP. Inhibition of tumor cell surface ATP synthesis by pigment epithelium-derived factor: implications for antitumor activity. Int J Oncol. 2012;41:219–227. doi: 10.3892/ijo.2012.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dina OA, Joseph EK, Levine JD, Green PG. Mechanisms mediating vibration-induced chronic musculoskeletal pain analyzed in the rat. J Pain. 2010;11:369–377. doi: 10.1016/j.jpain.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faigle M, Seessle J, Zug S, El Kasmi KC, Eltzschig HK. ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia. PLoS One. 2008;3:e2801. doi: 10.1371/journal.pone.0002801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Godecke S, Roderigo C, Rose CR, Rauch BH, Godecke A, Schrader J. Thrombin-induced ATP release from human umbilical vein endothelial cells. Am J Physiol Cell Physiol. 2012;302:C915–C923. doi: 10.1152/ajpcell.00283.2010. [DOI] [PubMed] [Google Scholar]
- 17.Gomes P, Srinivas SP, Van Driessche W, Vereecke J, Himpens B. ATP release through connexin hemichannels in corneal endothelial cells. Invest Ophthalmol Vis Sci. 2005;46:1208–1218. doi: 10.1167/iovs.04-1181. [DOI] [PubMed] [Google Scholar]
- 18.Gopinath P, Wan E, Holdcroft A, Facer P, Davis JB, Smith GD, Bountra C, Anand P. Increased capsaicin receptor TRPV1 in skin nerve fibres and related vanilloid receptors TRPV3 and TRPV4 in keratinocytes in human breast pain. BMC Womens Health. 2005;5:2. doi: 10.1186/1472-6874-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gosselin RD, Suter MR, Ji RR, Decosterd I. Glial cells and chronic pain. Neuroscientist. 2010;16:519–531. doi: 10.1177/1073858409360822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grygorczyk R, Furuya K, Sokabe M. Imaging and characterization of stretch-induced ATP release from alveolar A549 cells. J Physiol. 2013 doi: 10.1113/jphysiol.2012.244145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guillot X, Semerano L, Decker P, Falgarone G, Boissier MC. Pain and immunity. Joint Bone Spine. 2012;79:228–236. doi: 10.1016/j.jbspin.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Harks EG, de Roos AD, Peters PH, de Haan LH, Brouwer A, Ypey DL, van Zoelen EJ, Theuvenet AP. Fenamates: a novel class of reversible gap junction blockers. J Pharmacol Exp Ther. 2001;298:1033–1041. [PubMed] [Google Scholar]
- 23.Hattori F, Ohshima Y, Seki S, Tsukimoto M, Sato M, Takenouchi T, Suzuki A, Takai E, Kitani H, Harada H, Kojima S. Feasibility study of B16 melanoma therapy using oxidized ATP to target purinergic receptor P2X7. Eur J Pharmacol. 2012;695:20–26. doi: 10.1016/j.ejphar.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Hisadome K, Koyama T, Kimura C, Droogmans G, Ito Y, Oike M. Volume-regulated anion channels serve as an auto/paracrine nucleotide release pathway in aortic endothelial cells. J Gen Physiol. 2002;119:511–520. doi: 10.1085/jgp.20028540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Islam MR, Uramoto H, Okada T, Sabirov RZ, Okada Y. Maxi-anion channel and pannexin 1 hemichannel constitute separate pathways for swelling-induced ATP release in murine L929 fibrosarcoma cells. Am J Physiol Cell Physiol. 2012;303:C924–C935. doi: 10.1152/ajpcell.00459.2011. [DOI] [PubMed] [Google Scholar]
- 26.Jamal A, Bendeck M, Langille BL. Structural changes and recovery of function after arterial injury. Arterioscler Thromb. 1992;12:307–317. doi: 10.1161/01.atv.12.3.307. [DOI] [PubMed] [Google Scholar]
- 27.Jankowski MP, Koerber HR. In: Translational Pain Research: From Mouse to Man. Kruger L, Light AR, editors. Chapter 2. CRC Press; Boca Raton, FL: 2010. [PubMed] [Google Scholar]
- 28.Janneh O, Jones E, Chandler B, Owen A, Khoo SH. Inhibition of P-glycoprotein and multidrug resistance-associated proteins modulates the intracellular concentration of lopinavir in cultured CD4 T cells and primary human lymphocytes. J Antimicrob Chemother. 2007;60:987–993. doi: 10.1093/jac/dkm353. [DOI] [PubMed] [Google Scholar]
- 29.Joseph EK, Green PG, Bogen O, Alvarez P, Levine JD. Vascular endothelial cells mediate mechanical stimulation-induced enhancement of endothelin hyperalgesia via activation of P2X2/3 receptors on nociceptors. J Neurosci. 2013;33:2849–2859. doi: 10.1523/JNEUROSCI.3229-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joseph EK, Gear RW, Levine JD. Mechanical stimulation enhances endothelin-1 hyperalgesia. Neuroscience. 2011;178:189–195. doi: 10.1016/j.neuroscience.2011.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joseph EK, Levine JD. Role of endothelial cells in antihyperalgesia induced by a triptan and beta-blocker. Neuroscience. 2012;232:83–89. doi: 10.1016/j.neuroscience.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joseph EK, Levine JD. Sexual dimorphism in endothelin-1 induced mechanical hyperalgesia in the rat. Exp Neurol. 2012;233:505–512. doi: 10.1016/j.expneurol.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joseph EK, Reichling DB, Levine JD. Shared mechanisms for opioid tolerance and a transition to chronic pain. J Neurosci. 2010;30:4660–4666. doi: 10.1523/JNEUROSCI.5530-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanno T, Nishizaki T. CFTR mediates noradrenaline-induced ATP efflux from DRG neurons. Mol Pain. 2011;7:72. doi: 10.1186/1744-8069-7-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly M, Trudel S, Brouillard F, Bouillaud F, Colas J, Nguyen-Khoa T, Ollero M, Edelman A, Fritsch J. Cystic fibrosis transmembrane regulator inhibitors CFTR(inh)-172 and GlyH-101 target mitochondrial functions, independently of chloride channel inhibition. J Pharmacol Exp Ther. 2010;333:60–69. doi: 10.1124/jpet.109.162032. [DOI] [PubMed] [Google Scholar]
- 36.Khasar SG, Miao FJ, Janig W, Levine JD. Vagotomy-induced enhancement of mechanical hyperalgesia in the rat is sympathoadrenal-mediated. J Neurosci. 1998;18:3043–309. doi: 10.1523/JNEUROSCI.18-08-03043.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khodorova A, Navarro B, Jouaville LS, Murphy JE, Rice FL, Mazurkiewicz JE, Long-Woodward D, Stoffel M, Strichartz GR, Yukhananov R, Davar G. Endothelin-B receptor activation triggers an endogenous analgesic cascade at sites of peripheral injury. Nat Med. 2003;9:1055–1061. doi: 10.1038/nm885. [DOI] [PubMed] [Google Scholar]
- 38.Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knight GE, Bodin P, De Groat WC, Burnstock G. ATP is released from guinea pig ureter epithelium on distension. Am J Physiol Renal Physiol. 2002;282:F281–F288. doi: 10.1152/ajprenal.00293.2000. [DOI] [PubMed] [Google Scholar]
- 40.Koyama T, Kimura C, Hayashi M, Watanabe M, Karashima Y, Oike M. Hypergravity induces ATP release and actin reorganization via tyrosine phosphorylation and RhoA activation in bovine endothelial cells. Pflugers Arch. 2009;457:711–719. doi: 10.1007/s00424-008-0544-z. [DOI] [PubMed] [Google Scholar]
- 41.Lohman AW, Billaud M, Isakson BE. Mechanisms of ATP release and signalling in the blood vessel wall. Cardiovasc Res. 2012;95:269–280. doi: 10.1093/cvr/cvs187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsuka Y, Ono T, Iwase H, Mitrirattanakul S, Omoto KS, Cho T, Lam YY, Snyder B, Spigelman I. Altered ATP release and metabolism in dorsal root ganglia of neuropathic rats. Mol Pain. 2008;4:66. doi: 10.1186/1744-8069-4-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milner P, Kirkpatrick KA, Ralevic V, Toothill V, Pearson J, Burnstock G. Endothelial cells cultured from human umbilical vein release ATP, substance P and acetylcholine in response to increased flow. Proc Biol Sci. 1990;241:245–248. doi: 10.1098/rspb.1990.0092. [DOI] [PubMed] [Google Scholar]
- 44.Mironidou-Tzouveleki M, Tsartsalis S, Tomos C. Vascular endothelial growth factor (VEGF) in the pathogenesis of diabetic nephropathy of type 1 diabetes mellitus. Curr Drug Targets. 2011;12:107–114. doi: 10.2174/138945011793591581. [DOI] [PubMed] [Google Scholar]
- 45.Molliver DC, Radeke MJ, Feinstein SC, Snider WD. Presence or absence of TrkA protein distinguishes subsets of small sensory neurons with unique cytochemical characteristics and dorsal horn projections. J Comp Neurol. 1995;361:404–416. doi: 10.1002/cne.903610305. [DOI] [PubMed] [Google Scholar]
- 46.Nicholls DG, Ferguson S. Bioenergetics. 4. 2013. p. 434. [Google Scholar]
- 47.Notari L, Arakaki N, Mueller D, Meier S, Amaral J, Becerra SP. Pigment epithelium-derived factor binds to cell-surface F(1)-ATP synthase. FEBS J. 2010;277:2192–2205. doi: 10.1111/j.1742-4658.2010.07641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Grady SM, Patil N, Melkamu T, Maniak PJ, Lancto C, Kita H. ATP release and Ca2+ signalling by human bronchial epithelial cells following Alternaria aeroallergen exposure. J Physiol. 2013;591:4595–4609. doi: 10.1113/jphysiol.2013.254649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parada CA, Tambeli CH, Cunha FQ, Ferreira SH. The major role of peripheral release of histamine and 5-hydroxytryptamine in formalin-induced nociception. Neuroscience. 2001;102:937–944. doi: 10.1016/s0306-4522(00)00523-6. [DOI] [PubMed] [Google Scholar]
- 50.Patak P, Hermann DM. ATP-binding cassette transporters at the blood-brain barrier in ischaemic stroke. Curr Pharm Des. 2011;17:2787–2792. doi: 10.2174/138161211797440195. [DOI] [PubMed] [Google Scholar]
- 51.Pate M, Damarla V, Chi DS, Negi S, Krishnaswamy G. Endothelial cell biology: role in the inflammatory response. Adv Clin Chem. 2010;52:109–130. [PubMed] [Google Scholar]
- 52.Sakurai A, Onishi Y, Hirano H, Seigneuret M, Obanayama K, Kim G, Liew EL, Sakaeda T, Yoshiura K, Niikawa N, Sakurai M, Ishikawa T. Quantitative structure--activity relationship analysis and molecular dynamics simulation to functionally validate nonsynonymous polymorphisms of human ABC transporter ABCB1 (P-glycoprotein/MDR1) Biochemistry. 2007;46:7678–7693. doi: 10.1021/bi700330b. [DOI] [PubMed] [Google Scholar]
- 53.Sandler SG, Tobin W, Henderson ES. Vincristine-induced neuropathy. A clinical study of fifty leukemic patients. Neurology. 1969;19:367–374. doi: 10.1212/wnl.19.4.367. [DOI] [PubMed] [Google Scholar]
- 54.Sun ZW, Wang XD, Deng XM, Wallen R, Gefors L, Hallberg E, Andersson R. The influence of circulatory and gut luminal challenges on bidirectional intestinal barrier permeability in rats. Scand J Gastroenterol. 1997;32:995–1004. doi: 10.3109/00365529709011216. [DOI] [PubMed] [Google Scholar]
- 55.Tan C, Voss U, Svensson S, Erlinge D, Olde B. High glucose and free fatty acids induce beta cell apoptosis via autocrine effects of ADP acting on the P2Y(13) receptor. Purinergic Signal. 2012 doi: 10.1007/s11302-012-9331-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Triggle CR, Samuel SM, Ravishankar S, Marei I, Arunachalam G, Ding H. The endothelium: influencing vascular smooth muscle in many ways. Can J Physiol Pharmacol. 2012;90:713–738. doi: 10.1139/y2012-073. [DOI] [PubMed] [Google Scholar]
- 57.Tu J, Le G, Ballard HJ. Involvement of the cystic fibrosis transmembrane conductance regulator in the acidosis-induced efflux of ATP from rat skeletal muscle. J Physiol. 2010;588:4563–4578. doi: 10.1113/jphysiol.2010.195255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tu J, Lu L, Cai W, Ballard HJ. cAMP/protein kinase A activates cystic fibrosis transmembrane conductance regulator for ATP release from rat skeletal muscle during low pH or contractions. PLoS One. 2012;7:e50157. doi: 10.1371/journal.pone.0050157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valverde MA, Diaz M, Sepulveda FV, Gill DR, Hyde SC, Higgins CF. Volume-regulated chloride channels associated with the human multidrug-resistance P-glycoprotein. Nature. 1992;355:830–833. doi: 10.1038/355830a0. [DOI] [PubMed] [Google Scholar]
- 60.Verstuyft C, Strabach S, El-Morabet H, Kerb R, Brinkmann U, Dubert L, Jaillon P, Funck-Brentano C, Trugnan G, Becquemont L. Dipyridamole enhances digoxin bioavailability via P-glycoprotein inhibition. Clin Pharmacol Ther. 2003;73:51–60. doi: 10.1067/mcp.2003.8. [DOI] [PubMed] [Google Scholar]
- 61.Wang EC, Lee JM, Ruiz WG, Balestreire EM, von Bodungen M, Barrick S, Cockayne DA, Birder LA, Apodaca G. ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. J Clin Invest. 2005;115:2412–2422. doi: 10.1172/JCI24086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang T, Chen Z, Wang X, Shyy JY, Zhu Y. Cholesterol loading increases the translocation of ATP synthase beta chain into membrane caveolae in vascular endothelial cells. Biochim Biophys Acta. 2006;1761:1182–1190. doi: 10.1016/j.bbalip.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 63.Woodward HN, Anwar A, Riddle S, Taraseviciene-Stewart L, Fragoso M, Stenmark KR, Gerasimovskaya EV. PI3K, Rho, and ROCK play a key role in hypoxia-induced ATP release and ATP-stimulated angiogenic responses in pulmonary artery vasa vasorum endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;297:L954–L964. doi: 10.1152/ajplung.00038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu H, Kulkarni KH, Singh R, Yang Z, Wang SW, Tam VH, Hu M. Disposition of naringenin via glucuronidation pathway is affected by compensating efflux transporters of hydrophilic glucuronides. Mol Pharm. 2009;6:1703–1715. doi: 10.1021/mp900013d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamamoto K, Furuya K, Nakamura M, Kobatake E, Sokabe M, Ando J. Visualization of flow-induced ATP release and triggering of Ca2+ waves at caveolae in vascular endothelial cells. J Cell Sci. 2011;124:3477–3483. doi: 10.1242/jcs.087221. [DOI] [PubMed] [Google Scholar]
- 66.Yamamoto K, Shimizu N, Obi S, Kumagaya S, Taketani Y, Kamiya A, Ando J. Involvement of cell surface ATP synthase in flow-induced ATP release by vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2007;293:H1646–H1653. doi: 10.1152/ajpheart.01385.2006. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y, Gupta A, Wang H, Zhou L, Vethanayagam RR, Unadkat JD, Mao Q. BCRP transports dipyridamole and is inhibited by calcium channel blockers. Pharm Res. 2005;22:2023–2034. doi: 10.1007/s11095-005-8384-4. [DOI] [PubMed] [Google Scholar]