Abstract
Juvenile hyaline fibromatosis (JHF) and infantile systemic hyalinosis (ISH) are rare, autosomal recessive disorders of the connective tissue caused by mutations in the gene encoding the anthrax toxin receptor 2 protein (ANTXR2) located on chromosome 4q21. Characteristically, these conditions present with overlapping clinical features, such as nodules and/or pearly papules, gingival hyperplasia, flexion contractures of the joints, and osteolytic bone defects. The present report describes a pair of sibs and three other JHF/ISH patients whose diagnoses were based on typical clinical manifestations and confirmed by histopathologic analyses and/or molecular analysis. A comparison of ISH and JHF, additional thoughts about new terminology (hyaline fibromatosis syndrome) and a modified grading system are also included.
Keywords: anthrax toxin receptor 2 protein, capillary morphogenesis protein-2, hyaline fibromatosis syndrome, infantile systemic hyalinosis, juvenile hyaline fibromatosis
INTRODUCTION
Juvenile hyaline fibromatosis (JHF; OMIM 228600) is a rare, autosomal recessive disorder [Ribeiro et al., 2009; El-Maaytah et al., 2010] of the connective tissue [Güldner et al., 2009] that is characterized by the abnormal growth of hyalinized fibrous tissue [El-Maaytah et al., 2010] that determines the appearance of skin lesions (nodules and/or pearly papules) and the involvement of other organs (gingiva, joints, and bones) [Antaya et al., 2007; Dhingra et al., 2008; Park et al., 2010; Slimani et al., 2011]. Infantile systemic hyalinosis (ISH; OMIM 236490) is a similar condition with an earlier age of onset, visceral involvement, and early lethality [Félix et al., 2004; Mendonça et al., 2011]. Until the molecular mechanism involved in these disorders was identified, several authors argued whether JHF and ISH are separate disorders or part of the same disease [Urbina et al., 2004; Nofal et al., 2009]. Both diagnoses, although rare [Ribeiro et al., 2009; El-Maaytah et al., 2010], should be considered in children presenting with such clinical manifestations as nodules and/or pearly papules, gingival hyperplasia, and joint contracture [Ribeiro et al., 2009; El-Maaytah et al., 2010; Park et al., 2010].
Different mutations (missense, frameshift, in-frame, nonsense, and splice-site) of the anthrax toxin receptor-2 (ANTXR2; OMIM 608041) gene, also known as the capillary morphogenesis protein gene-2 (CMG2) and located on chromosome 4q21, have been found to be responsible for both disorders [Dowling et al., 2003; Hanks et al., 2003]. ANTXR2 encodes a transmembrane protein in which the von Willebrand A (vWA) domain binds to both lamin and collagen IV, suggesting that this protein plays a role in basement-membrane matrix assembly and endothelial cell morphogenesis [Hanks et al., 2003; El-Maaytah et al., 2010]. Hanks et al. [2003] suggested that a defect in ANTXR2 can lead to extravasation of hyaline material (plasma components) through the basement membrane into the perivascular space. This phenomenon may explain the histological findings of both JHF and ISH [Hanks et al., 2003; El-Maaytah et al., 2010].
The present report describes five JHF/ISH patients, two of them siblings, whose diagnoses were based on typical clinical manifestations and subsequently confirmed by histopathologic analyses and/or molecular studies. A discussion of the similarities and differences between ISH and JHF, additional thoughts about proposed terminology (Hyaline fibromatosis syndrome), and a modified grading system are also included.
CLINICAL REPORTS
Family 1
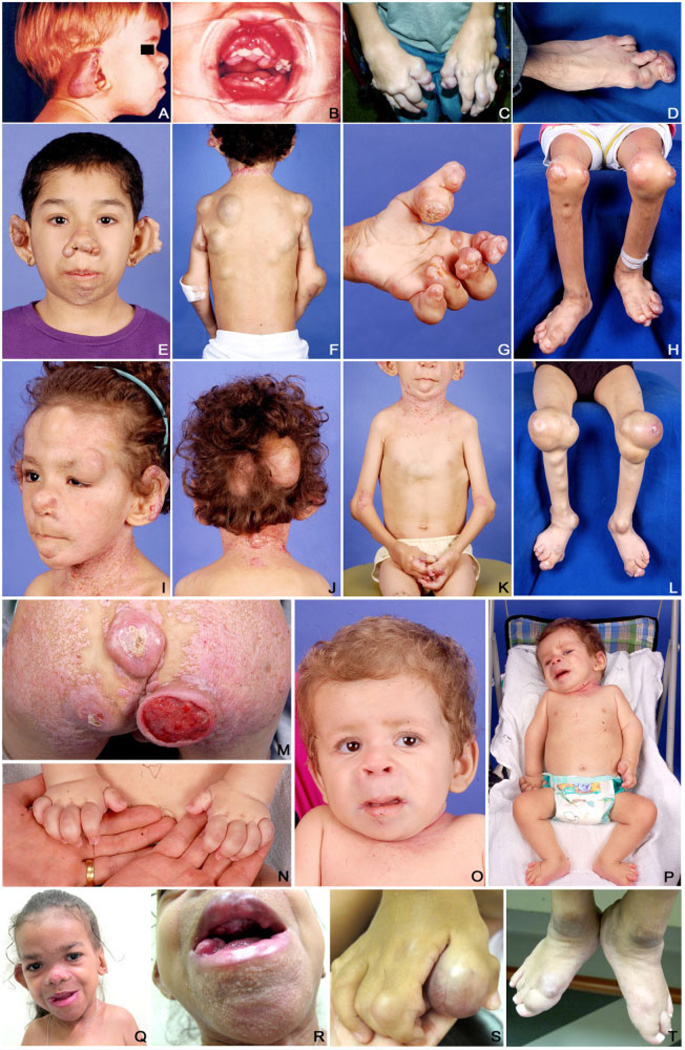
The proband is a 20-year-old man (Patient 1) who has been undergoing regular follow-up at the Institute of Plastic and Craniofacial Surgery (SOBRAPAR) for approximately 18 years. The parents, who were first cousins, reported that the patient presented with skin lesions (nodulations and pearly papules), gingival enlargement, and limited limb movement since the first months of life. The nodulations, as well as the joint involvement, had a progressive course. Pearly papules affected only the posterior cervical region. No systemic involvement was disclosed. A physical examination revealed cutaneous nodulations on the ears, thoracic wall (ulcerated), and fingers. Pearly papules were not noticed. A dental examination showed gingival hyperplasia with irregular implantation of the teeth. The wrists, elbows, shoulders, hips, knees, ankles, and fingers exhibited flexion contracture. The patient presented with an important postural deformity (deviation of the spinal axis) being in a wheelchair for many years (Fig. 1A–D).
FIG. 1.
Clinical photographs of the Patients (A–D) 1, (E–H) 2, (I–M) 3, (N–P) 4, and (Q-T) 5 showing multiple skin nodules distributed on various body regions (mainly, ear and fingers), multiple pearly papules on nose, chin, and cervical regions (mainly, Patients 2, 3, and 5), gingival hyperplasia (Patients 1 and 5), and flexion contractures of the several joints (wrists, knees, ankles, and fingers).
Family 2
An 11-year-old female (Patient 2) was referred to SOBRAPAR with a history of multiple skin lesions that were present from 3 months of age. The first signs observed by the parents were pearly skin papules on the face and small cutaneous nodules on the fingers. Rapidly and progressively, the nodulations began to affect other parts of the patient’s body. Simultaneously, the patient presented with gingival enlargement and limited knee movement. The patient suffered from recurrent diarrhea during the first 2 years of life and failure to thrive. A physical examination showed multiple skin nodules of various sizes located in several regions of the body, and pearly papules primarily in the nose and chin regions. Gingival hyperplasia and joint flexion contracture were also present, especially on the knees and fingers (Fig. 1E–H).
A 7-year-old boy (Patient 3), the younger sibling of Patient 2, has a history of skin lesions (nodules and pearly papules) that were present since 8 months of age. The parents are nonconsanguineous and have an older, healthy son. The mother reported that, at early onset, the nodules affected the scalp and ears and the pearly papules affected the neck and gluteal regions, but rapidly increased in both number and size and involved other regions of the body. Simultaneously, the patient presented with gingival enlargement and limited knee movement but showed no other related symptoms. A physical examination showed multiple cutaneous nodules of various sizes on different regions of the body. The pearly skin papules coalesced to form plaques in the neck and gluteal regions. The patient also presented with gingival hyperplasia and joint flexion contracture of the knees (predominant), elbows, and fingers (Fig. 1I–M).
Family 3
A 2-year-old boy (Patient 4), the first and only child of non-consanguineous parents, was referred to SOBRAPAR with a history of limitation of knee extension since the first months of life with painful movements of the upper limb joints. In the course, skin lesions (nodules and pearly papules) developed in the perioral and perianal regions and interphalangeal joints, as well as gingival hyperplasia (Fig. 1N–P). He developed failure to thrive and at the age of 2 years, a severe and persistent diarrhea required hospitalization. He developed septicemia, the cause of his death after 15 days in hospital.
Family 4
An 8-year-old female (Patient 5), product of nonconsanguineous parents, presented with joint movement restriction of the upper and lower limbs since 2 months of age. The cutaneous involvement and gingival hyperplasia developed latter on. She developed persistent diarrhea, requiring hospitalization at the age of 1 year. The physical examination showed growth delay, coarse facial features, gingival hyperplasia, several nodules over her occipital area, neck, inferior lip, ears, thoracic region and interphalangeal joints, multiple papules over her face and ears and flexion contractures of the knees, elbows, and interphalangeal joints (Fig. 1Q–T).
Further Evaluation
X-ray examinations of the five patients showed osteolytic lesions in the hips (Patients 1 and 5), long bones and distal phalanges (Patient 5) and generalized bone rarefaction (Patients 2, 3, and 5).Notypical abnormalities were observed in the X-rays of Patient 4 at 7 months of age. Laboratory examination and investigation of the other organs were normal for Patients 1 and 3. A duodenal biopsy was performed in Patient 2, which showed a deposition of a hyalinized material. Computed tomography scans performed in Patient 4 detected a mass in the retropharyngeal region, which partially reduced the air column and another mass in the kidney; these lesion were not biopsied.
Therapeutic Approaches
From the earliest years of life, four patients (1, 2, 3, and 5) underwent multiple surgeries (mean: 2–3 surgeries per year) for the resection of cutaneous nodules, both new and recurrent (Fig. 2). Clinical criteria for the resection of the lesions were ulceration of the lesions (risk of infection) and/or functional and aesthetic involvement.
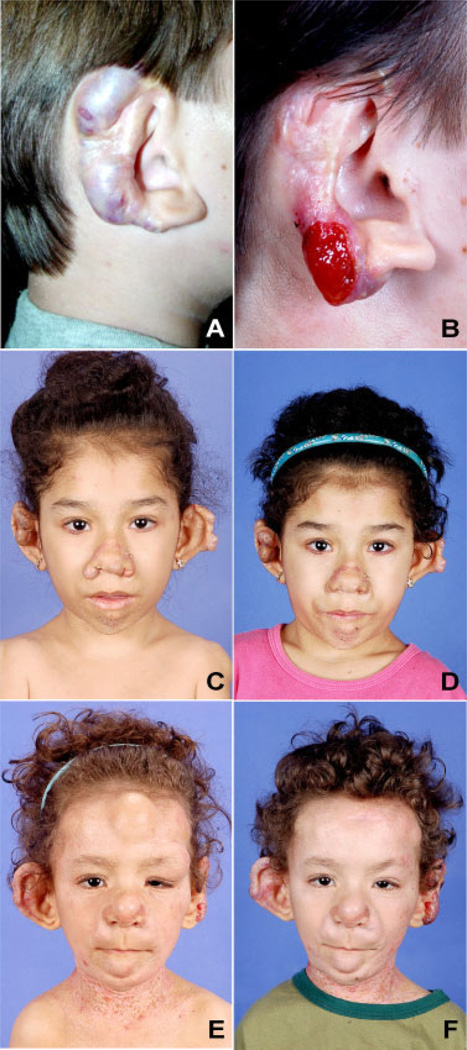
FIG. 2.
Patient 1 presenting with nodules on the right ear as a (A) 13- year-old and (B) 7 years later; note the mutilating aspect of the multiple surgeries performed in the right ear due to the recurrent nature of the lesions. Aspect of the pre-and post-surgical resection of the (C–D) nasal (Patient 2) and (E–F) forehead (Patient 3) lesions.
All five patients had difficulties in oral hygiene due to the gingival lesions. The extraction of some teeth was performed in Patient 1, and radical gingivectomy was performed in Patient 2. Patients 3 and 4 had limited access to the oral cavity, owing of the paucity of soft tissue plasticity; therefore, no surgical procedure for either dental or gingival lesions was carried out.
All patients received physical therapy (Patient 1: 6 years; Patients 2, 3, 4, and 5: several sessions), but there were no major improvements in movement. Patient 4 received steroids, without obtaining any improvement. To date, no other therapeutic approaches have been employed.
Histopathologic Examination and Genetic Analysis
The histopathologic evaluation of the surgical specimens of all patients showed compatible features of JHF/ISH (Fig. 3). The cutaneous lesions exhibited proliferation of spindle cells without atypia, with varied cellularity, forming strands in the midst of homogeneous and hyaline eosinophilic material that was more abundant in areas of less cellularity and denser around certain vessels. The hyaline material stained with periodic acid-Schiff (PAS) reagent; it was diastase-resistant and did not demonstrate the characteristics of amyloid material by Congo red staining. In certain areas, fusocellular cords were close to clear slits, simulating vascular spaces, or otherwise exhibited clear pericellular halos, providing a chondroid appearance. Some sparse, irregularly distributed macrophages were also present. The lesions extended into part of either the dermis or hypodermis and some were partially ulcerated and covered by fibrin-neutrophilic exudate, sometimes with scattered bacterial colonies. The immunohistochemistry markers S-100 protein, 1A4, and CD68 were negative in the spindle cells.
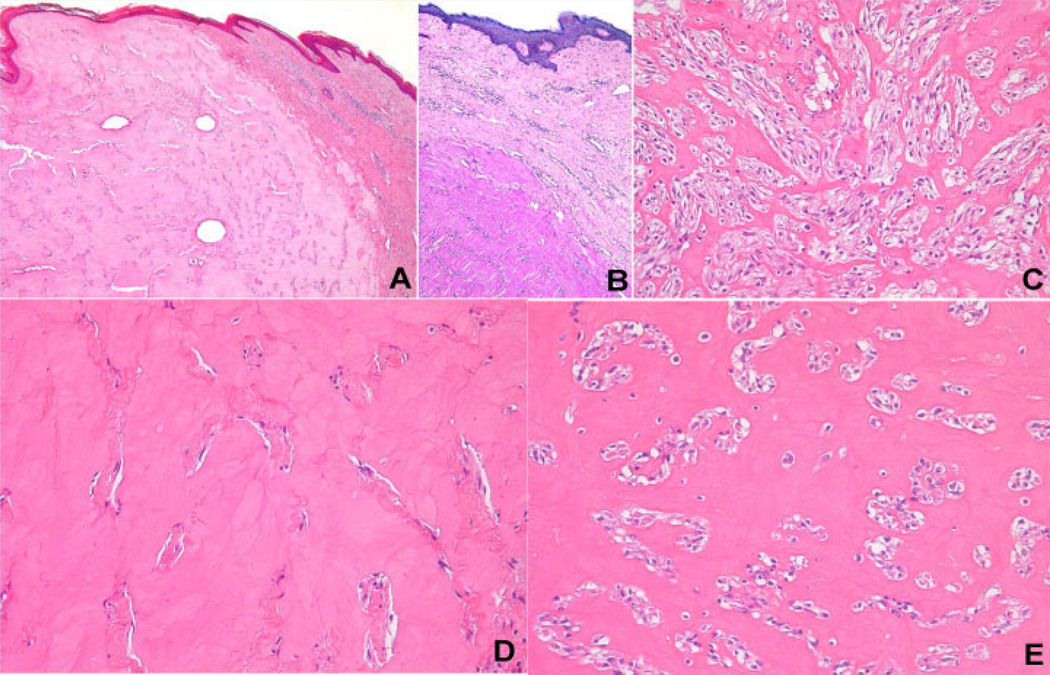
FIG. 3.
A: Lesion occupying the papillary and reticular dermis, raising the overlying epidermis (hematoxylin–eosin, original magnification: 50×). B: Dermal lesion showing hyaline material stained by periodic acid-Schiff reagent (original magnification: 100×). C: Hypercellular area displaying multiple strands of spindle cells that sometimes anastomose (hematoxylin–eosin, original magnification: 200×). D: Hypocellular area showing abundant eosinophilic material that is amorphous, homogeneous, and characteristically hyaline (hematoxylin–eosin, original magnification: 200×). E: Clear spaces around the spindle cells, which, combined with the background of hyaline material, confers a chondroid appearance to certain areas of the lesions (hematoxylin–eosin, original magnification: 200×).
Sequencing analysis of the ANTXR2 gene was performed in the siblings (Patient 2 and 3), as well as in Patients 4 and 5 (Fig. 4). Consistent with nonconsanguinity, all affected individuals were compound heterozygotes, and all had one copy of a previously reported mutation, c.1074delT [Hanks et al., 2003; Hatamochi et al., 2007; Huang et al., 2007; El-Kamah et al., 2010]. In Patient 4, the other allele was the previously reported c.1073_1074insC mutation [Hanks et al., 2003; Lee et al., 2005; Shieh et al., 2006]. In the other two families novel mutations that affect splicing were identified. In Patient 5, the mother transmitted a novel mutation located in intron 5 (c.487-2A>G) that was predicted to affect splicing and cause skipping of exon 6 (p.Ala163_Gln185del). This transcript was observed in cDNA from the patient along with another transcript where only six nucleotides of exon 6 were skipped (p.Ala163_Lys164del). Thus, nucleotides 5 and 6 of exon 6 can function as a splice acceptor site when the normal splice acceptor site of intron 5 is mutated. Genomic DNA analysis did not reveal the mutation from the father of the siblings (Patients 2 and 3), but cDNA analysis of the father and affected children demonstrated skipping of exons 15 and 16, c.1180_1428del/p.Val394_Glu476del, also a novel gene alteration. The father and children are most likely heterozygous for a partial gene deletion with breakpoints in introns 14 and 16.
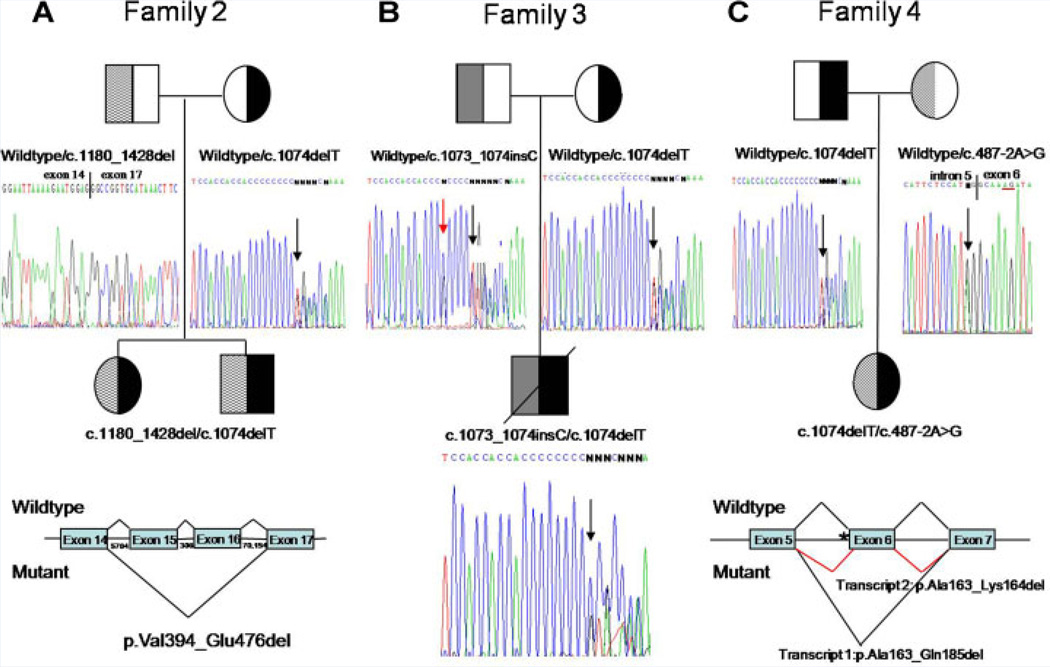
FIG. 4.
Pedigree and mutation analyses for Families 2–4. Blackened symbols indicate individuals who have the c.1074delT mutation. A: Pedigree for Family 2 showing that the parents are heterozygous for different ANTXR2 mutations. The children are compound heterozygotes. Below the mother is a portion of exon 13 from the mother, demonstrating that she is heterozygous for a known single base deletion (shown by the arrow), c.1074delT. Below the father is a sequence result from cDNA from the father, showing skipping of exons 15 and 16, c.1180_1428del. This is predicted to result in a protein with an in-frame deletion of 83 amino acids, p.Val394_Glu476del. The father and children most likely have a partial gene deletion involving exons 15 and 16. The size (in bp) of the introns is shown below the horizontal lines designating the introns. B: Pedigree for Family 3 showing that the mother is a carrier of the common mutation, c.1074delT. The father is a carrier of another known mutation, c.1073_1074insC (black arrow). The child is a compound heterozygote, with the position of both mutations indicated by a black arrow. In addition the father is heterozygous for a known SNP, rs12647691 (red arrow). C: Pedigree for Family 4 showing that the father is a carrier of the common mutation, c.1074delT. The mother has a novel mutation, c.487-2A>G, that affects the splice acceptor site of intron 5 (shown by black arrow). cDNA analysis demonstrated two transcripts from the mutant allele. Transcript 1 skips exon 6, potentially producing an in frame deletion of 23 amino acids (p.Ala163_Gln185del). A second transcript was produced that only lacked the first 6 nucleotides of exon 6, potentially producing an in frame deletion of 2 amino acids (p.Ala163_Lys164del). As shown on the electropherogram of the mother’s sequence, nucleotides 5 and 6 of exon 6 can function as a splice acceptor site (red underline) when the normal splice acceptor site of intron 5 is mutated. At the bottom is a portion of the ANTXR2 gene showing normal splicing (above the genomic structure) and splicing observed in the presence of the c.487-2A>G splice acceptor mutation (indicated by asterisk).
Summary of demographic, clinical, molecular characteristics as well as grading identification of the five patients are included in Table I.
TABLE I.
Clinical and Molecular Findings of Hyaline Fibromatosis Syndrome Patients
| Findings | Family 1 Patient 1 |
Family 2 |
Family 3 Patient 4 |
Family 4 Patient 5 |
|
|---|---|---|---|---|---|
| Patient 2 | Patient 3 | ||||
| Sex | M | F | M | M | F |
| Age (years) | 20 | 11 | 7 | 2 | 8 |
| Failure to thrive/short stature | − | + | + | + | + |
| Joint flexion contracture | + | + | + | + | + |
| Skin nodules | + | + | + | + | + |
| Pearly papules | + | + | + | + | + |
| Gingival hyperplasia | + | + | + | + | + |
| Persistent diarrhea | − | + | _ | + | + |
| Recurrent infection | − | − | − | − | − |
| Bone lesions | + | + | + | − | + |
| Visceral involvement | − | + | − | ? | ? |
| Prolonged survival | + | + | + | − | + |
| Diagnosis | JHF | ISH | ISH | ISH | ISH |
| Grading systema | 2 | 3 | 2 | 4 | 3 |
| Consanguinity | + | − | − | − | − |
| ANTXR2 gene mutation | NA | c.1074delT/ c.1180_1428del |
c.1074delT/ c.1073_1074insC |
c.1074delT/ c.487-2A>G |
|
M, male; F, female; JHF, juvenile hyaline fibromatosis; ISH, infantile systemic hyalinosis; ?, no histological studies performed.
Proposed modified grading system.
DISCUSSION
JHF was first described in 1873 as molluscum fibrosum [Lim et al., 2005; Güldner et al., 2009]. In 1973, the current nomenclature (JHF) was proposed [Ribeiro et al., 2009; Park et al., 2010]. To date, only about 70 cases have been reported worldwide [Ribeiro et al., 2009; El-Maaytah et al., 2010; Park et al., 2010]. ISH is a disorder even more rare [Félix et al., 2004; Urbina et al., 2004; Antaya et al., 2007; Mendonça et al., 2011]. To the best of our knowledge, the cases reported here are five of the very few reports on JHF/ISH in Brazilian patients. A comprehensive literature review found eight Brazilian patients [Félix et al., 2004; Muniz et al., 2006; Brandão et al., 2009; Ribeiro et al., 2009; Mendonça et al., 2011].
The pathogenesis of these disorders is incompletely understood [Rahman et al., 2002; Muniz et al., 2006; El-Maaytah et al., 2010] but is thought to involve either an abnormality in type IV or VI (α1, α2, and α 3 chains) collagen [El-Maaytah et al., 2010] or a defect in the formation of glycosaminoglycans [Muniz et al., 2006; Ribeiro et al., 2009].
Patients with JHF/ISH are typically diagnosed during childhood [Lim et al., 2005; Mallet et al., 2010; Slimani et al., 2011], and neurocognitive development is usually normal [Urbina et al., 2004; Muniz et al., 2006; Güldner et al., 2009], similar to the patients reported herein.
Skin lesions (nodules and/or pearly papules) are the most common feature [Dhingra et al., 2008; Ribeiro et al., 2009; El-Maaytah et al., 2010]. The nodules, although unsightly and disfiguring [Güldner et al., 2009; Park et al., 2010], are asymptomatic, either hard or soft, and either mobile or adherent to the underlying fascia [Muniz et al., 2006; Ribeiro et al., 2009]. They may increase in number, reaching large dimensions [Antaya et al., 2007; Dhingra et al., 2008; Mallet et al., 2010] and become ulcerated (predisposed to infection) [Lim et al., 2005; Muniz et al., 2006].
Extracutaneous findings include gingival hyperplasia (nodular or papillomatous) [Lim et al., 2005; El-Maaytah et al., 2010], which may result in poor oral hygiene, inability to feed, and dental infections [Lim et al., 2005; Park et al., 2010]; joint involvement (flexion contractures) [Dhingra et al., 2008; Slimani et al., 2011]; and bone lesions [Antaya et al., 2007; Slimani et al., 2011]. Flexion contracture, particularly of large joints, is the most debilitating problem, causing most teenagers and adults to become bedridden with time [Dhingra et al., 2008; Slimani et al., 2011]. Radiological evaluation shows lytic lesions of the long bones, phalanges, and pubic synthesis, as well as generalized osteoporosis [Antaya et al., 2007; Slimani et al., 2011].
The sequence and severity of the involvement of each organ (skin, gingiva, bones, and joints) are variable [Gilaberte et al., 1993; El-Maaytah et al., 2010; Slimani et al., 2011]. Joint involvement (shoulders, elbows, hips, knees, and fingers) was more exacerbated in Patient 1, who was confined to a wheelchair since childhood, compared to the two siblings whose skin disorders were more severe. Early in disease onset, all five patients took on a ‘‘frog-leg’’ position due to flexion contracture of the lower limbs and consequently four patients (1, 2, 3, and 5) presented with secondary muscle atrophy, which has also been described elsewhere [Lim et al., 2005].
The histopathologic findings of JHF/ISH may vary from normal epidermis to minor changes and few inflammatory cells in the dermis [Urbina et al., 2004; El-Maaytah et al., 2010] as well as abundant deposition of amorphous, homogeneous, hyaline material, particularly in the papillary and reticular dermis, accompanied by a proliferation of spindle cells without atypia [Urbina et al., 2004; Antaya et al., 2007; El-Maaytah et al., 2010]. This hyaline substance is eosinophilic and stains positive with PAS [Urbina et al., 2004; El-Maaytah et al., 2010; Mallet et al., 2010]. Electron microscopy sometimes reveals a banding pattern with expanded Golgi complexes that are filled with a microfibrillar and fine granular layer [Urbina et al., 2004; El-Maaytah et al., 2010].
JHF and ISH share many similarities, including clinical characteristics [Dowling et al., 2003; Urbina et al., 2004; Muniz et al., 2006; Mallet et al., 2010], identical histopathologic patterns [Mallet et al., 2010; Park et al., 2010; Slimani et al., 2011], and mutation in the same gene (ANTXR2) [Dowling et al., 2003; Hanks et al., 2003], demonstrating that both entities represent different degrees of severity of the same disorder [Urbina et al., 2004; Nofal et al., 2009]. ISH would be the most severe form [Mancini et al., 1999; Urbina et al., 2004; Antaya et al., 2007] presenting with early onset (first weeks or months of life), failure to thrive, short stature, diffuse thickening of the skin, hyperpigmented plaques on bony prominences, systemic involvement (visceral), persistent diarrhea, recurrent infections, and death before 2 years of age [Félix et al., 2004; Mendonça et al., 2011]. On the other hand, JHF would be the milder form [Urbina et al., 2004; El-Maaytah et al., 2010] because it is usually less severe [Nofal et al., 2009; El-Maaytah et al., 2010] with later onset (3 months to 4 years of age) [Muniz et al., 2006], and most patients survive until the fourth decade of life [Slimani et al., 2011].
After localization of the gene responsible for JHF/ISH on 4q21 [Rahman et al., 2002] and identification of the ANTXR2 gene as responsible for both disorders [Dowling et al., 2003; Hanks et al., 2003], attempts at a genotype–phenotype correlation were undertaken [Hanks et al., 2003]. The wide phenotypic variability can be related, at least in part, to the underlying mutational spectrum. Primarily, patients harboring missense and truncating mutations in the vWA domain have been associated with a more severe phenotype, more compatible with a diagnosis of ISH. This was also observed in individuals harboring at least one insertion/deletion mutations resulting in a translational frameshift. On the other hand, in-frame and missense mutations in another domain—the cytoplasmic domain—lead to a milder phenotype [Hanks et al., 2003]. However, this correlation is not always straightforward, suggesting that other modifying genes and/or environmental factors may also play important roles in. As shown in Table II, 28 different mutations have been reported in JHF/ISH [El-Kamah et al., 2010; Deuquet et al., 2011]; the c.1074delT mutation found in all of the patients reported here is the second most frequent. Three of our patients had Portuguese, Italian, and Spanish ancestry. This mutation has been described in a homozygous state in two cases of ISH (Egyptian and Kuwaiti patients) and as a compound heterozygote in cases of both ISH (Chinese patient) and JHF (Japanese patient). The difference in phenotype may be explained by the nature and location of the mutation in the second allele in these patients [El-Kamah et al., 2010]. The Chinese patient with ISH had 2 frameshift mutations while the Japanese patient had a frameshift and a missense mutation. In our patients the mutation in the second allele involved either splicing (Patients 2, 3, and 5) or a frameshift (Patient 4). In Family 4, the novel mutation of the splice acceptor site of intron 5 (c.487-2A>G) resulted in two transcipts: one that removed only two amino acids (p.Ala163_Lys164del) and one that skipped all of exon 6, resulting in protein with an in-frame deletion of 23 amino acids (p.Ala163_Gln185del). The relative quantity of each transcript was not determined. cDNA analysis of the father and affected children in Family 2 revealed skipping of exons 15 and 16, c.1180_1428del, which is predicted to result in an in-frame deletion of 83 amino acids (p.Val394_Glu476del). Sequencing of intron 15 (306 bp) did not reveal any mutations. Most likely the father and affected children are heterozygous for a partial gene deletion with breakpoints in introns 14 (5,704 bp) and 16 (70,154 bp). The second allele identified in Patient 4, c.1073_1074insC, is the most common ANTXR2 mutation reported and has been described in the homozygous state in 2 probands with ISH (US Hispanic and Taiwanese) and in the compound heterozygous state in 5 ISH probands (Swiss, Chinese, Puerto Rican/African American, and Mexican). As shown in Table II, eight of the nine compound heterozygous patients have one allele involving the c.1073-1074 nucleotides. Interestingly, in two cases the second mutation could not be identified. This may reflect an interstitial deletion of the ANTXR2 gene as postulated for Family 2 of this study.
TABLE II.
Molecular Findings in ANTRX2 Gene and its Distribution According to Phenotype, Degree of Clinical Severity and Ethnicity of Hyaline Fibromatosis Syndrome Patients
| Mutationb | Location | Domain | Predicted effect | Phenotype/grading systema |
Ethnicity | Reference |
|---|---|---|---|---|---|---|
| c.2T>G | Exon 1 | Signal peptide | p.0?/p.Met1Arg/p.Met1_Glu77del | JHF/3 | Dominican Republican | Antaya |
| c.134T>C | Exon 1 | vWFA | p.Leu45Pro | ISH/3d(family R) | Bedouin | Hanksc |
| c.304_305insA | Exon 4 | vWFA | p.Ile102Asnfs*12 | ISH/2 | Chineseg | Huang |
| c.314G>A | Exon 4 | vWFA | p.Gly105Asp | JHF/3 (family JHF1) | Turkish | Dowling |
| c.353C>A | Exon 4 | vWFA | p.Thr118Lys | ISH/4 | Mexican | Lindvall |
| c.487-2A>G | Intron 5 | vWFA | p.Ala163_Gln185del;p.Ala163_Lys164del | ISH/3 (family 4) | Braziliang | This report |
| c.495_496insA | Exon 6 | vWFA | p.Ser166Ilefs*7 | ISH/3d(family N) | Moroccan | Hanks |
| c.566T>C | Exon 7 | vWFA | p.Ile189Thr | ISH/2 (family ISH2) | Swissg | Dowling |
| ISH/3 (family L) | European/Swissg | Hanks | ||||
| c.652T>C | Exon 8 | Membrane proximal domain |
p.Cys218Arg | ISH/3d(family K) | Fiji/East Indian | Hanks |
| c.658G>T | Exon 8 | Membrane proximal domain |
p.E220* | ISH/4 (family ISH1) | Turkish | Dowling |
| c.697+1G>A | Intron 8 | Membrane proximal domain |
Presumed splice defect | ISH/3d(family I) | European/Canadiang | Hanks |
| c.796+2T>C | Intron 9 | Membrane proximal domain |
Presumed splice defect | JHF/2 (family E)f | European | Hanks |
| c.876_877insCAA | Exon 11 | Membrane proximal domain |
p.Asp292_Val293insGln | JHF/2 (family G) | East Turkish | Hanks |
| c.989T>G | Exon 12 | Transmembrane | p.Leu330Arg | JHF/1 (family JHF2) | African American | Dowling |
| c.1073_1074insC | Exon 13 | Cytoplasmic | p.Ala359Cysfs*13 | ISH/2 (family ISH2) | Swissg | Dowling |
| ISH/3d(family J)f | Chinese | Hanks | ||||
| ISH/3d(family M)f | Puerto Rican + African American | Hanks | ||||
| ISH/3d(family P) | US Hispanic | Hanks | ||||
| ISH/3 | Taiwanese | Lee | ||||
| ISH/4 (patient 1) | Mexicang | Shiehc | ||||
| ISH/4 (family 3) | Braziliang | This report | ||||
| c.1073_1074insCC | Exon 13 | Cytoplasmic | p.Ala359Leufs*51 | ISH/3d(family L) | European/Swiss | Hanks |
| c.1074delT | Exon 13 | Cytoplasmic | p.Ala359Hisfs*50 | ISH/3 (family Q) | Kuwaiti | Hanks |
| ISH/3 | Chineseg | Huang | ||||
| JHF/3 | Japaneseg | Hatamochi | ||||
| ISH/4 (family 1) | Egyptian | El-Kamah | ||||
| ISH (families 2– 4)h | Braziliang | This report | ||||
| c.1086+1G>A | Intron 13 | Cytoplasmic | p.Val394Ilefs*6 | JHF/2 (families C and F) | Turkish/European | Hanks |
| c.1087_1706dele | Introns 13– 17 | Cytoplasmic | Presumed deletion | ISH/4 (patient 2) | Mexican | Shieh |
| c.1142A>G | Exon 14 | Cytoplasmic | p.Tyr381Cys | JHF/1 (family D) | Moroccan | Hanks |
| c.1150C>T | Exon 14 | Cytoplasmic | p.Arg384* | ISH/3d(family I) | European/Canadiang | Hanks |
| c.1156G>T | Exon 14 | Cytoplasmic | p.Val386Phe | JHF/2 | Turkish | Hakki |
| c.1179G>A | Exon 14 | Cytoplasmic | p.Glu363_Glu393del | JHF/2 (families A and B) | Indian | Hanks |
| c.1181T>C | Exon 14 | Cytoplasmic | p.Val394Ala | JHF/3 | Japaneseg | Hatamochi |
| c.1179+5G>T | Intron 14 | Cytoplasmic | Presumed splice defect | JHF/2 (family H) | Turkish | Hanks |
| c.1180_1428del | Introns 14–16 | Cytoplasmic | p.Val394_Glu476del | ISH/3 (patient 2), 2 (patient 3) | Braziliang | This report |
| c.1294C>T | Exon 15 | Cytoplasmic | p.Arg432* | ISH/2 (patient 3) | Mexicang | Shieh |
| c.1340delC | Exon 15 | Cytoplasmic | p.Pro447Glnfs*13 | JHF/2 (family 2) | Egyptian | El-Kamah |
vWFA, von Willebrand factor type A; JHF, juvenile hyaline fibromatosis; ISH, infantile systemic hyalinosis.
Proposed modified grading system.
Numbering assumes the A of the ATG start codon as nucleotide 1 with NM_058172.5 as reference sequence.
Numbering in study did not begin with the A of the ATG start codon as nucleotide 1.
The severity score is at least a 3. Information was not provided to be able to determine if there was severe clinical decompensation.
This is presumed since the patient DNA could not be amplified for this region of the gene.
Compound heterozygote with only 1 mutation identified.
The nine compound heterozygotes are c.304_305insA/c.1074delT (Huang), c.487-2A>G/c.1074delT (this report), c.566T>C/c.1073_1074insC (Dowling), c.566T>C/c.1073_1074insCC (Hanks), c.697+1G>A/c.1150C>T (Hanks), c.1073_1074insC/c.1074delT (this report), c.1074delT/c.1181T>C (Hatmochi), c.1074delT/c.1180_1130del (this report), and c.1074delT/c.1294C>T (Shieh).
All three families were compound heterozygotes. The severity score is listed with the second allele c.487-2A>G (family 4), c.1073_1074insC (family 3), and c.1180_1428del (family 2).
JHF/ISH should be distinguished from other similar disorders, including infantile myofibromatosis (congenital generalized fibromatosis) [Ribeiro et al., 2009], Winchester syndrome [Mallet et al., 2010], Farber lipogranulomatosis [Güldner et al., 2009], juvenile idiopathic arthritis [Ribeiro et al., 2009], neurofibromatosis type 1 [Lim et al., 2005], and mucopolysaccharidoses [Urbina et al., 2004].
In this study, all diagnoses (JFH [Gilaberte et al., 1993] and ISH [Urbina et al., 2004; Antaya et al., 2007; Dhingra et al., 2008]) were based on clinical manifestations combined with histopathologic characteristics [Gilaberte et al., 1993; Mancini et al., 1999; Muniz et al., 2006; Güldner et al., 2009; Park et al., 2010; Slimani et al., 2011]. Interestingly, Patient 2 showed failure to thrive and gastro-intestinal involvement, leading to a diagnosis of a more severe phenotype, but without early lethality. Nevertheless, this patient’s brother, who has the same ANTXR2 mutations, had a more benign course. This particular family highlights the difficulty of classifying a patient into a single category. Thus, we favor the use of the broader term, hyaline fibromatosis syndrome (HFS), which comprises both disorders as proposed by Nofal et al. [2009].
Nofal et al. [2009] classified HFS into three grades (mild, moderate, and severe). He defined severe (grade 3) as the occurrence of manifestation resulting from organ involvement. Patients 2 and 5 of this study as well as others from the literature have systemic involvement without mortality [Urbina et al., 2004; Antaya et al., 2007; Dhingra et al., 2008; El-Maaytah et al., 2010]. Access to adequate healthcare might explain the absence of clinical decompensation and mortality in these patients. Thus, we propose that a patient (e.g., Patients 2 and 5) who has organ involvement but does not present with clinical decompensation (organ failure and/or septicemia) may not fit into the highest severity (grade 3 of Nofal’s classification system). In this disorder, death is a consequence of a severe clinical decompensation (septicemia [Mancini et al., 1999; Muniz et al., 2006; Dhingra et al., 2008; Mallet et al., 2010] and/or organ failure [Gilaberte et al., 1993; Dhingra et al., 2008; Park et al., 2010]); therefore, we propose a modification of the current Nofal et al. grading system (Table III), which includes patients with internal organ involvement/infiltration (intestine, esophagus, stomach, spleen, trachea, heart, thyroid, adrenal glands, lymph nodes, and/or others) with or without clinical manifestations (persistent diarrhea and/or recurrent infections and/or other), except the criteria 1 and 2 without severe clinical decompensation as a modified grade 3 (severe). Grade 4 (new grade; lethal) would encompass all patients with severe clinical decompensation. Thus, Patients 2, 5, and others [Urbina et al., 2004; Antaya et al., 2007; Dhingra et al., 2008; El-Maaytah et al., 2010] would be classified as grade 3, and patients (e.g., Patient 4) with severe clinical decompensation would be classified as grade 4, with the highest probability of fatal end. Future studies are important to identify predictors of clinical decompensation and to elucidate potential clinical features that may also lead to death. We hypothesize that the absence of adequate healthcare (access to routine physical therapy and psychosocial assistance) may rapidly worsen the prognosis, leading a grade 3 disorder to become a grade 4.
TABLE III.
Proposed Grading System of Hyaline Fibromatosis Syndrome
| Grade | Skin and/or gingival involvement |
Joint and/or bone involvement |
Internal organ involvement with or without clinical manifestationsa |
Severe clinical decompensation (organ failure and/or septicemia) |
|---|---|---|---|---|
| 1 (Mild) | + | − | − | − |
| 2 (Moderate) | + | + | − | − |
| 3 (Severe) | + | ± | + | − |
| 4 (Lethal) | + | ± | ± | + |
Clinical manifestations = persistent diarrhea, and/or recurrent infections, and/or other except criteria 1 and 2; Grade 3 = modified criteria; Grade 4 = new criteria; ± = manifestation can be present or not, since there are minimal characteristics (clinical, radiological, histopathological, and genetics) for a correct diagnosis of hyaline fibromatosis syndrome.
Currently, there is no treatment for this debilitating disorder [Urbina et al., 2004; Dhingra et al., 2008; Ribeiro et al., 2009]. Surgical excision is considered the main form of treatment for both skin [Lim et al., 2005; Antaya et al., 2007; Güldner et al., 2009; Slimani et al., 2011] and oral lesions [Dhingra et al., 2008; Mallet et al., 2010; Park et al., 2010].
According to some authors, resection of skin nodules should be carried out early [Urbina et al., 2004; Antaya et al., 2007; Mallet et al., 2010; Park et al., 2010] because the lesions may grow and ulcerate, causing extreme discomfort to the patient [El-Maaytah et al., 2010; Park et al., 2010]. However, as in this study, local recurrence has been reported [Muniz et al., 2006; Dhingra et al., 2008; Ribeiro et al., 2009; El-Maaytah et al., 2010; Slimani et al., 2011]. Furthermore, in studies whose patients were also followed for long periods (15 and 19 years, respectively [Woyke et al., 1984; Quintal and Jackson, 1985]), while surgical excision was effective, multiple attempts at treatment can sometimes be as crippling as the disorder itself (exemplified in Fig. 2). The four Patients (1, 2, 3, and 5) continue to be evaluated in our clinic, and their skin lesions were resected only when they became ulcerated (risk of infection) or presented with some aesthetic and/or functional conditions. This procedure has also been adopted by others [Muniz et al., 2006; Dhingra et al., 2008].
For the treatment of gingival hyperplasia, a partial or radical gingivectomy is recommended and can be repeated as needed [Urbina et al., 2004; Lim et al., 2005; El-Maaytah et al., 2010]. The extraction of decayed and unstable teeth is also advised [Lim et al., 2005; El-Maaytah et al., 2010]. Physiotherapy [El-Maaytah et al., 2010; Mallet et al., 2010], treatment with cortisone [El-Maaytah et al., 2010] or d-penicillamine [Dhingra et al., 2008; Park et al., 2010], and capsulotomy [Urbina et al., 2004; Ribeiro et al., 2009] may provide temporary relief for symptoms of joint involvement.
Recent new insights on the genetic basis of this disorder may positively influence genetic counseling in families whose children are born with HFS [Rahman et al., 2002; Dowling et al., 2003; Hanks et al., 2003; Antaya et al., 2007]. Moreover, such an understanding can enable the future development of potential genetic therapies for this devastating disorder [Lim et al., 2005; Muniz et al., 2006; Park et al., 2010; Slimani et al., 2011]. In this scenario, partial rescue of CMG2 was obtained in the HFS patient-derived fibroblasts by proteasome inhibitors, indicating that these compounds are potential therapeutic drugs for this disorder [Deuquet et al., 2011].
CONCLUSION
HFS is a broader term to describe rare, connective tissue disorders with a progressive relapsing nature and poor prognosis (stigmatizing, debilitating, and potentially lethal), including both JHF and ISH. A diagnosis of HFS, made by combining clinical history findings with histopathologic and genetic analyses, should be considered in patients with skin lesions (nodules and/or pearly papules), gingival hyperplasia, and joint contracture. Surgical excision, despite crippling over time, is the most accepted treatment option for skin lesions because it substantially improves both the function and aesthetic conditions of the patients.
Footnotes
Sources of funding: None/Conflict of interest: None
REFERENCES
- Antaya RJ, Cajaiba MM, Madri J, Lopez MA, Ramirez MC, Martignetti JA, Reyes-Múgica M. Juvenile hyaline fibromatosis and infantile systemic hyalinosis overlap associated with a novel mutation in capillary morphogenesis protein-2 gene. Am J Dermatopathol. 2007;29:99–103. doi: 10.1097/01.dad.0000245636.39098.e5. [DOI] [PubMed] [Google Scholar]
- Brandão FV, Silva CM, Gontijo B, Guedes AC. Juvenile hyaline fibromatosis and infantile systemic hyalinosis. Case for diagnosis. An Bras Dermatol. 2009;84:677–679. doi: 10.1590/s0365-05962009000600017. [DOI] [PubMed] [Google Scholar]
- Deuquet J, Lausch E, Guex N, Abrami L, Salvi S, Lakkaraju A, Ramirez MC, Martignetti JA, Rokicki D, Bonafe L, Superti-Furga A, van der Goot FG. Hyaline fibromatosis syndrome inducing mutations in the ecto-domain of anthrax toxin receptor 2 can be rescued by proteasome inhibitors. EMBO Mol Med. 2011;3:208–221. doi: 10.1002/emmm.201100124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra M, Amladi S, Savant S, Nayak C. Juvenile hyaline fibromatosis and infantile systemic hyalinosis: Divergent expressions of the same genetic defect? Indian J Dermatol Venereol Leprol. 2008;74:371–374. doi: 10.4103/0378-6323.42913. [DOI] [PubMed] [Google Scholar]
- Dowling O, Difeo A, Ramirez MC, Tukel T, Narla G, Bonafe L, Kayserili H, Yuksel-Apak M, Paller AS, Norton K, Teebi AS, Grum-Tokars V, Martin GS, Davis GE, Glucksman MJ, Martignetti JA. Mutations in capillary morphogenesis gene-2 result in the allelic disorders juvenile hyaline fibromatosis and infantile systemic hyalinosis. Am J Hum Genet. 2003;73:957–966. doi: 10.1086/378781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Kamah GY, Fong K, El-Ruby M, Afifi HH, Clements SE, Lai-Cheong JE, Amr K, El-Darouti M, McGrath JA. Spectrum of mutations in the ANTXR2 (CMG2) gene in infantile systemic hyalinosis and juvenile hyaline fibromatosis. Br J Dermatol. 2010;163:213–215. doi: 10.1111/j.1365-2133.2010.09769.x. [DOI] [PubMed] [Google Scholar]
- El-Maaytah M, Jerjes W, Shah P, Upile T, Murphy C, Ayliffe P. Gingival hyperplasia associated with juvenile hyaline fibromatosis: A case report and review of the literature. J Oral Maxillofac Surg. 2010;68:2604–2608. doi: 10.1016/j.joms.2009.09.060. [DOI] [PubMed] [Google Scholar]
- Félix TM, Puga AC, Cestari T, Cartell A, Cerski M. Infantile systemic hyalinosis: Report of three unrelated Brazilian children and review of the literature. Clin Dysmorphol. 2004;13:231–236. [PubMed] [Google Scholar]
- Gilaberte Y, González-Mediero I, López Barrantes V, Zambrano A. Juvenile hyaline fibromatosis with skull-encephalic anomalies: A case report and review of the literature. Dermatology. 1993;187:144–148. doi: 10.1159/000247227. [DOI] [PubMed] [Google Scholar]
- Güldner K, Hendricks C, Schaller J, Kunze J. Juvenile hyaline fibromatosis. Hautarzt. 2009;60:740–742. doi: 10.1007/s00105-008-1698-5. [DOI] [PubMed] [Google Scholar]
- Hakki SS, Ataoglu T, Avunduk MC, Erdemli E, Gunhan O, Rahman N. Periodontal treatment of two siblings with juvenile hyaline fibromatosis. J Clin Periodontol. 2005;32:1016–1021. doi: 10.1111/j.1600-051X.2005.00760.x. [DOI] [PubMed] [Google Scholar]
- Hanks S, Adams S, Douglas J, Arbour L, Atherton DJ, Balci S, Bode H, Campbell ME, Feingold M, Keser G, Kleijer W, Mancini G, McGrath JA, Muntoni F, Nanda A, Teare MD, Warman M, Pope FM, Superti-Furga A, Futreal PA, Rahman N. Mutations in the gene encoding capillary morphogenesis protein 2 cause juvenile hyaline fibromatosis and infantile systemic hyalinosis. Am J Hum Genet. 2003;73:791–800. doi: 10.1086/378418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatamochi A, Sasaki T, Kawaguchi T, Suzuki H, Yamazaki S. A novel point mutation in the gene encoding capillary morphogenesis protein 2 in a Japanese patient with juvenile hyaline fibromatosis. Br J Dermatol. 2007;157:1037–1039. doi: 10.1111/j.1365-2133.2007.08147.x. [DOI] [PubMed] [Google Scholar]
- Huang YC, Xiao YY, Zheng YH, Jang W, Yang YL, Zhu XJ. Infantile systemic hyalinosis: A case report and mutation analysis in a Chinese infant. Br J Dermatol. 2007;156:602–604. doi: 10.1111/j.1365-2133.2006.07701.x. [DOI] [PubMed] [Google Scholar]
- Lee JY, Tsai YM, Chao SC, Tu YF. Capillary morphogenesis gene-2 mutation in infantile systemic hyalinosis: Ultrastructural study and mutation analysis in a Taiwanese infant. Clin Exp Dermatol. 2005;30:176–179. doi: 10.1111/j.1365-2230.2004.01698.x. [DOI] [PubMed] [Google Scholar]
- Lim AA, Kozakewich HP, Feingold M, Padwa BL. Juvenile hyaline fibromatosis: Report of a case and comparison with infantile systemic hyalinosis. J Oral Maxillofac Surg. 2005;63:271–274. doi: 10.1016/j.joms.2004.06.055. [DOI] [PubMed] [Google Scholar]
- Lindvall LE, Kormeili T, Chen E, Ramirez MC, Grum-Tokars V, Glucksman MJ, Martignetti JA, Zaragoza MV, Dyson SW. Infantile systemic hyalinosis: Case report and review of the literature. J Am Acad Dermatol. 2008;58:303–307. doi: 10.1016/j.jaad.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Mancini GM, Stojanov L, Willemsen R, Kleijer WJ, Huijmans JG, van Diggelen OP, de Klerk JB, Vuzevski VD, Oranje AP. Juvenile hyaline fibromatosis: Clinical heterogeneity in three patients. Dermatology. 1999;198:18–25. doi: 10.1159/000018058. [DOI] [PubMed] [Google Scholar]
- Mallet S, Boye T, Hesse S, Fournier B, Guennoc B, Carsuzaa F. Juvenile hyaline fibromatosis. Ann Dermatol Venereol. 2010;137:364–368. doi: 10.1016/j.annder.2010.02.019. [DOI] [PubMed] [Google Scholar]
- Mendonça JA, Marini R, Schincariol NB, Laurindo IM, Appenzeller S. Ultrasound findings in infantile systemic hyalinosis. Rheumatol Int. 2011;31:1393–1395. doi: 10.1007/s00296-010-1666-0. [DOI] [PubMed] [Google Scholar]
- Muniz ML, Lobo AZ, Machado MC, Valente NY, Kim CA, Lourenço SV, Nico MM. Exuberant juvenile hyaline fibromatosis in two patients. Pediatr Dermatol. 2006;23:458–464. doi: 10.1111/j.1525-1470.2006.00283.x. [DOI] [PubMed] [Google Scholar]
- Nofal A, Sanad M, Assaf M, Nofal E, Nassar A, Almokadem S, Attwa E, Elmosalamy K. Juvenile hyaline fibromatosis and infantile systemic hyalinosis: A unifying term and a proposed grading system. J Am Acad Dermatol. 2009;61:695–700. doi: 10.1016/j.jaad.2009.01.039. [DOI] [PubMed] [Google Scholar]
- Park KT, Chang DY, Sung MW. Juvenile hyaline fibromatosis. Clin Exp Otorhinolaryngol. 2010;3:102–106. doi: 10.3342/ceo.2010.3.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintal D, Jackson R. Juvenile hyaline fibromatosis. A 15-year follow-up. Arch Dermatol. 1985;121:1062–1063. [PubMed] [Google Scholar]
- Rahman N, Dunstan M, Teare MD, Hanks S, Edkins SJ, Hughes J, Bignell GR, Mancini G, Kleijer W, Campbell M, Keser G, Black C, Williams N, Arbour L, Warman M, Superti-Furga A, Futreal PA, Pope FM. The gene for juvenile hyaline fibromatosis maps to chromosome 4q21. Am J Hum Genet. 2002;71:975–980. doi: 10.1086/342776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro SL, Guedes EL, Botan V, Barbosa A, Freitas EJ. Juvenile hyaline fibromatosis: A case report and review of literature. Acta Reumatol Port. 2009;34:128–133. [PubMed] [Google Scholar]
- Shieh JT, Swidler P, Martignetti JA, Ramirez MC, Balboni I, Kaplan J, Kennedy J, Abdul-Rahman O, Enns GM, Sandborg C, Slavotinek A, Hoyme HE. Systemic hyalinosis: A distinctive early childhood-onset disorder characterized by mutations in the anthrax toxin receptor 2 gene (ANTRX2) Pediatrics. 2006;118:e1485–e1492. doi: 10.1542/peds.2006-0824. [DOI] [PubMed] [Google Scholar]
- Slimani S, Haddouche A, Haid S, Ladjouze-Rezig A. Juvenile hyaline fibromatosis: Focus on radiographic features in adulthood. Rheumatol Int. 2011;31:273–276. doi: 10.1007/s00296-010-1583-2. [DOI] [PubMed] [Google Scholar]
- Urbina F, Sazunic I, Murray G. Infantile systemic hyalinosis or juvenile hyaline fibromatosis? Pediatr Dermatol. 2004;21:154–159. doi: 10.1111/j.0736-8046.2004.21214.x. [DOI] [PubMed] [Google Scholar]
- Woyke S, Domagala W, Markiewicz C. A 19-year follow-up of multiple juvenile hyaline fibromatosis. J Pediatr Surg. 1984;19:302–304. doi: 10.1016/s0022-3468(84)80192-x. [DOI] [PubMed] [Google Scholar]