Abstract
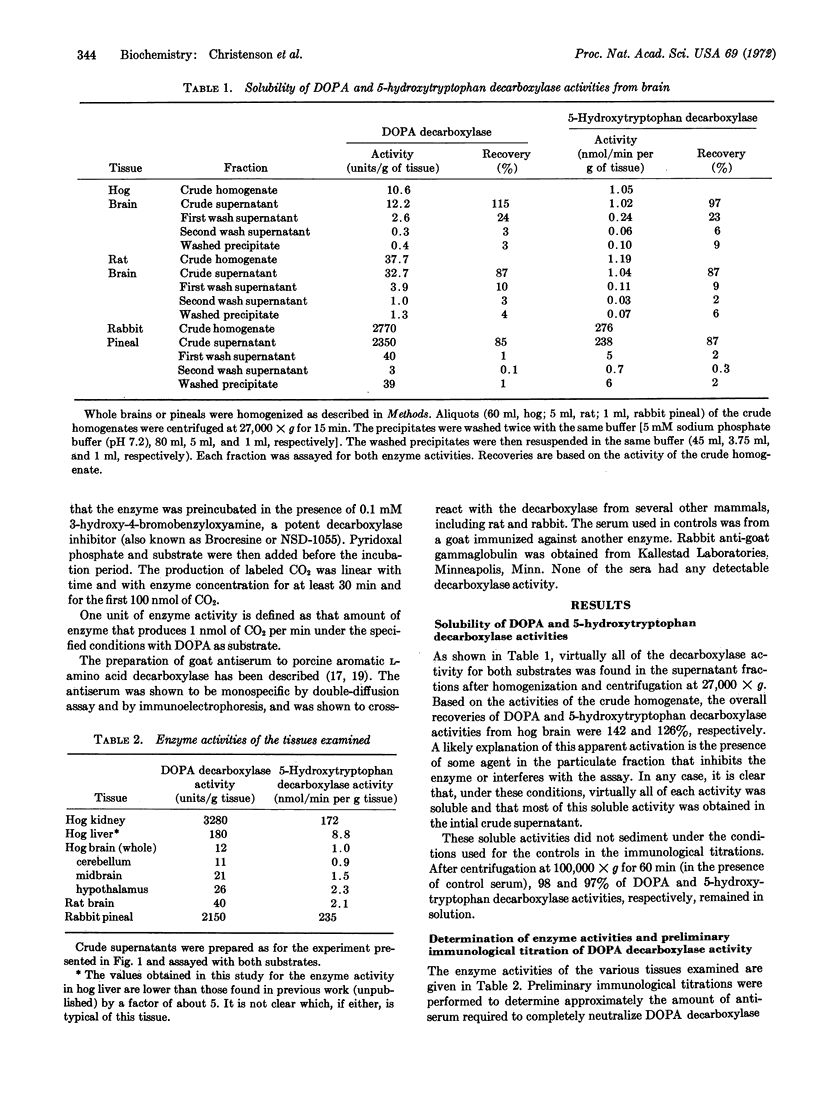
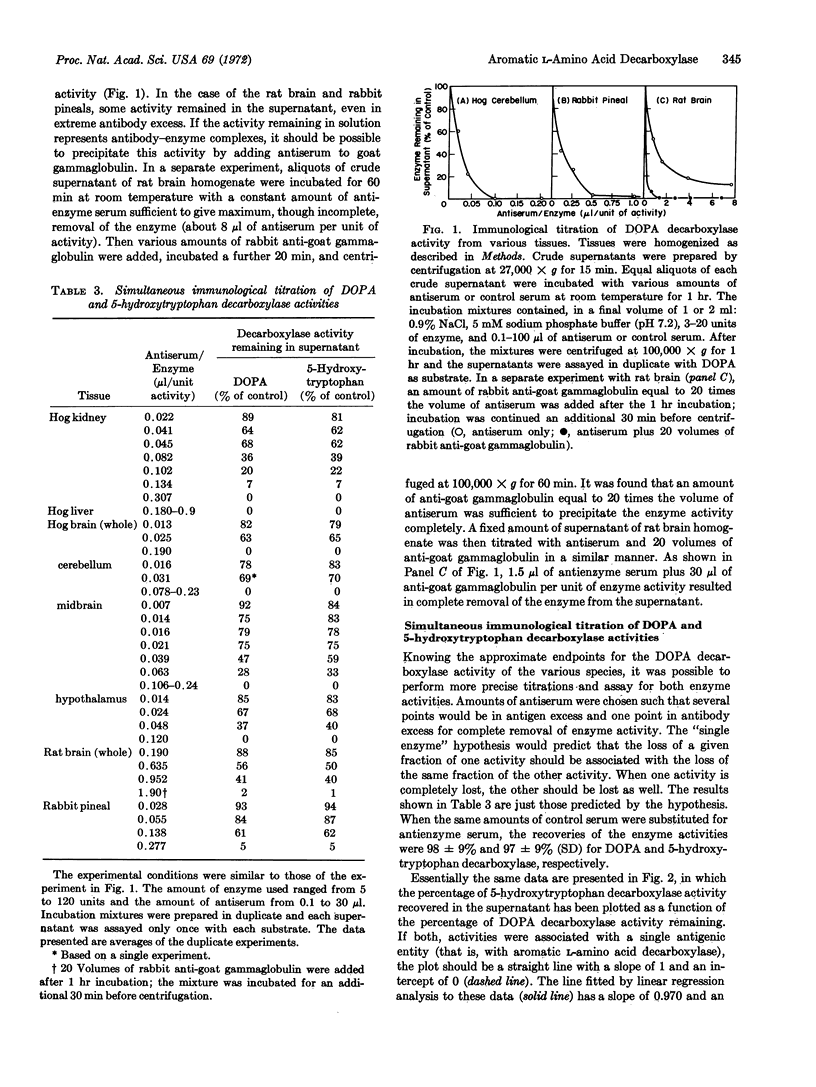
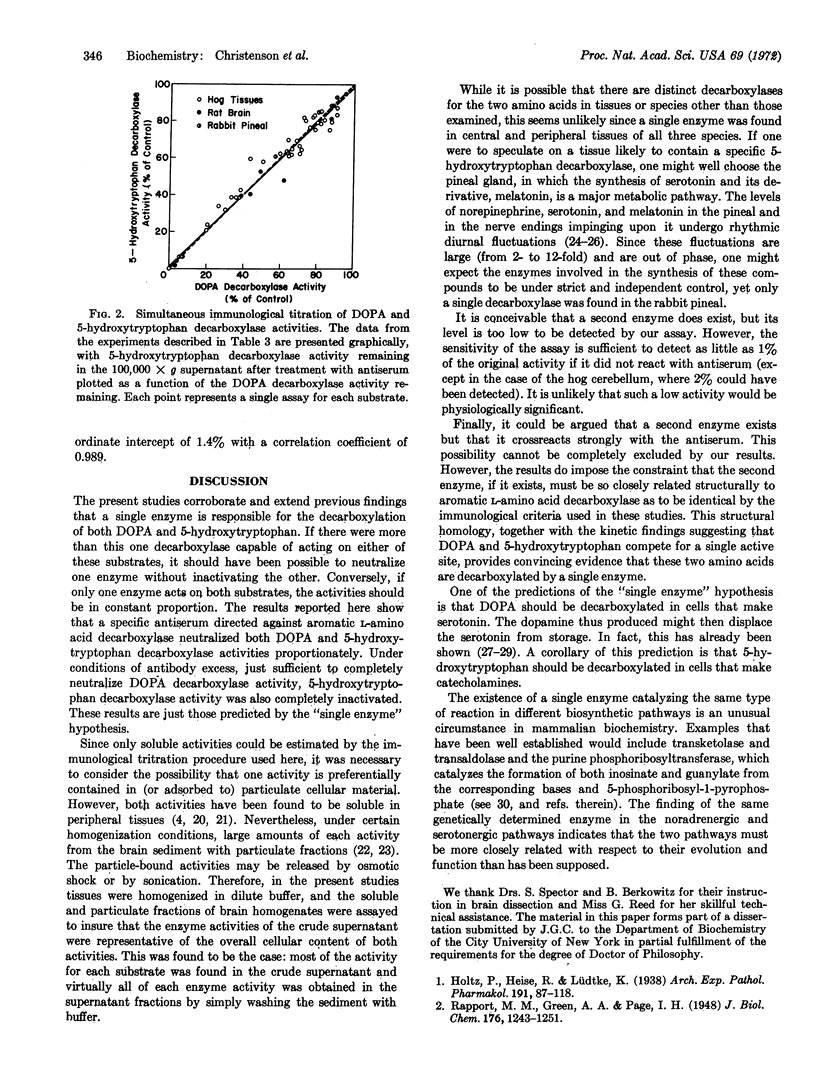
Simultaneous immunological titration of DOPA and 5-hydroxytryptophan decarboxylase activities, from a number of tissues of various species, showed that the two activities were not distinguishable with a monospecific antiserum to hog kidney decarboxylase. Together with previous findings, these data firmly establish the concept that in mammalian tissues the two enzyme activities are associated with a single protein, namely aromatic L-amino acid decarboxylase.
Keywords: immunological titration, aromatic L-amino acid decarboxylase, serotonin, dopamine, norepinephrine
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AWAPARA J., SANDMAN R. P., HANLY C. Activation of DOPA decarboxylase by pyridoxal phosphate. Arch Biochem Biophys. 1962 Sep;98:520–525. doi: 10.1016/0003-9861(62)90220-5. [DOI] [PubMed] [Google Scholar]
- BLASCHKO H., HAGEN P., WELCH A. D. Observations on the intracellular granules of the adrenal medulla. J Physiol. 1955 Jul 28;129(1):27–49. doi: 10.1113/jphysiol.1955.sp005336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholini G., Da Prada M., Pletscher A. Decrease of cerebral 5-hydroxytryptamine by 3,4-dihydroxyphenylalanine after inhibition of extracerebral decarboxylase. J Pharm Pharmacol. 1968 Mar;20(3):228–229. doi: 10.1111/j.2042-7158.1968.tb09726.x. [DOI] [PubMed] [Google Scholar]
- Butcher L., Engel J., Fuxe K. L-dopa induced changes in central monoamine neurons after peripheral decarboxylase inhibition. J Pharm Pharmacol. 1970 Apr;22(4):313–316. doi: 10.1111/j.2042-7158.1970.tb08529.x. [DOI] [PubMed] [Google Scholar]
- CLARK C. T., WEISSBACH H., UDENFRIEND S. 5-Hydroxytryptophan decarboxylase: preparation and properties. J Biol Chem. 1954 Sep;210(1):139–148. [PubMed] [Google Scholar]
- Christenson J. G., Dairman W., Udenfriend S. Preparation and properties of a homogeneous aromatic L-amino acid decarboxylase from hog kidney. Arch Biochem Biophys. 1970 Nov;141(1):356–367. doi: 10.1016/0003-9861(70)90144-x. [DOI] [PubMed] [Google Scholar]
- Coulson W. F., Bender D. A., Jepson J. B. Multiple electrophoresis peaks of rat liver decarboxylases for 3,4-dihydroxyphenylalanine and 5-hydroxytryptophan. Biochem J. 1969 Dec;115(5):63P–64P. doi: 10.1042/bj1150063p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELLMAN J. H. Purification and properties of adrenal L-DOPA decarboxylase. Enzymologia. 1959 Jul 15;20:366–376. [PubMed] [Google Scholar]
- HAGEN P. Observations on the substrate specificity of DOPA decarboxylase from ox adrenal medulla, human phaeochromocytoma and human argentaffinoma. Br J Pharmacol Chemother. 1962 Feb;18:175–182. doi: 10.1111/j.1476-5381.1962.tb01161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson J. F., Brox L. W., Kelley W. N., Rosenbloom F. M., Seegmiller J. E. Kinetic studies of hypoxanthine-guanine phosphoribosyltransferase. J Biol Chem. 1968 May 25;243(10):2514–2522. [PubMed] [Google Scholar]
- LOVENBERG W., WEISSBACH H., UDENFRIEND S. Aromatic L-amino acid decarboxylase. J Biol Chem. 1962 Jan;237:89–93. [PubMed] [Google Scholar]
- Laduron P., Belpaire F. Tissue fractionation and catecholamines. II. Intracellular distribution patterns of tyrosine hydroxylase, dopa decarboxylase, dopamine-beta-hydroxylase, phenylethanolamine N-methyltransferase and monoamine oxidase in adrenal medulla. Biochem Pharmacol. 1968 Jul;17(7):1127–1140. doi: 10.1016/0006-2952(68)90048-8. [DOI] [PubMed] [Google Scholar]
- Lynch H. J. Diurnal oscillations in pineal melatonin content. Life Sci I. 1971 Jul 15;10(14):791–795. doi: 10.1016/0024-3205(71)90033-6. [DOI] [PubMed] [Google Scholar]
- Ng K. Y., Chase T. N., Colburn R. W., Kopin I. J. L-Dopa-induced release of cerebral monoamines. Science. 1970 Oct 2;170(3953):76–77. doi: 10.1126/science.170.3953.76. [DOI] [PubMed] [Google Scholar]
- ROSENGREN E. Are dihydroxphenylalanine decarboxylase and 5-hydroxytrptophan decarboxylase individual enzymes? Acta Physiol Scand. 1960 Aug 25;49:364–369. doi: 10.1111/j.1748-1716.1960.tb01958.x. [DOI] [PubMed] [Google Scholar]
- SNYDER S. H., ZWEIG M., AXELROD J., FISCHER J. E. CONTROL OF THE CIRCADIAN RHYTHM IN SEROTONIN CONTENT OF THE RAT PINEAL GLAND. Proc Natl Acad Sci U S A. 1965 Feb;53:301–305. doi: 10.1073/pnas.53.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streffer C. Eine Methode zur Bestimmung der Dekarboxylase aromatischer Aminosäuren. Biochim Biophys Acta. 1967 May 16;139(1):193–195. doi: 10.1016/0005-2744(67)90132-5. [DOI] [PubMed] [Google Scholar]
- Udenfriend S. Biosynthesis of the sympathetic neurotransmitter, norepinephrine. Harvey Lect. 1966;60:57–83. [PubMed] [Google Scholar]
- WERLE E., AURES D. [On the purification and specificity of DOPA-decarboxylase]. Hoppe Seylers Z Physiol Chem. 1959 Sep 30;316:45–60. doi: 10.1515/bchm2.1959.316.1.45. [DOI] [PubMed] [Google Scholar]
- WESTERMANN E., BALZER H., KNELL J. Hemmung der Serotoninbildung durch alpha-Methyl-Dopa. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1958;234(3):194–205. [PubMed] [Google Scholar]
- Wurtman R. J., Axelrod J. A 24-hour rhythm in the content of norepinephrine in the pineal and salivary glands of the rat. Life Sci. 1966 Apr;5(7):665–669. doi: 10.1016/0024-3205(66)90298-0. [DOI] [PubMed] [Google Scholar]
- YUWILER A., GELLER E., EIDUSON S. Studies on 5-hydroxy-tryptophan decarboxylase. II. Additional inhibition studies and suggestions on the nature of the enzymic site. Arch Biochem Biophys. 1960 Jul;89:143–147. doi: 10.1016/0003-9861(60)90025-4. [DOI] [PubMed] [Google Scholar]


