Abstract
The prefrontal cortex is highly vulnerable to traumatic brain injury resulting in the dysfunction of many high-level cognitive and executive functions such as planning, information processing speed, language, memory, attention, and perception. All of these processes require some degree of working memory. Interestingly, in many cases, post-injury working memory deficits can arise in the absence of overt damage to the prefrontal cortex. Recently, excess GABA-mediated inhibition of prefrontal neuronal activity has been identified as a contributor to working memory dysfunction within the first month following cortical impact injury of rats. However, it has not been examined if these working memory deficits persist, and if so, whether they remain amenable to treatment by GABA antagonism. Our findings show that working memory dysfunction, assessed using both the delay match-to-place and delayed alternation t-maze tasks, following lateral cortical impact injury persists for at least 16 weeks post-injury. These deficits were found to be no longer the direct result of excess GABA-mediated inhibition of medial prefrontal cortex neuronal activity. Golgi staining of prelimbic pyramidal neurons revealed that TBI causes a significant shortening of layer V/VI basal dendrite arbors by 4 months post-injury, as well as an increase in the density of both basal and apical spines in these neurons. These changes were not observed in animals 14 days-post-injury, a time point at which administration of GABA receptor antagonists improves working memory function. Taken together, the present findings, along with previously published reports, suggest that temporal considerations must be taken into account when designing mechanism-based therapies to improve working memory function in TBI patients.
Keywords: Golgi staining, neuronal morphology, prelimbic cortex, delayed alternation T-maze, delay match-to-place, GAD
Cognitive and behavioral dysfunctions are pervasive among people with traumatic brain injury (TBI). While these deficits often normalize within a year for many with mild TBI, they persist in approximately 10-15% of mild, 50% of moderate, and a large percentage of severe TBI victims (Kraus and Chu, 2005). One of the prominent cognitive deficits in individuals with TBI is working memory (WM) impairments, the ability to hold information on line for subsequent integration and manipulation in order to guide goal-directed behavior (Baddeley, 1992; McAllister et al., 2001; Finley et al., 2005; Dash et al., 2007). Since WM is critical for many high level cognitive functions, patients with WM deficits have difficulties with executive function, such as one's ability to organize and execute complex processes like planning. In addition, information processing speed, language, memory, attention and perception, all require some degree of WM (Baddeley, 1992; Arnsten, 1997). A number of functional imaging studies in humans and electrophysiological/ pharmacological experiments in non-human primates have demonstrated that dysfunction of the dorsolateral prefrontal cortex (DLPFC) is the main cause for WM deficit (Fuster and Alexander, 1971; D'Esposito, 2000). Consistent with this, recent functional imaging studies have indicated that people with mild TBI show more activation of the dorsolateral prefrontal cortex at low WM load, and less activation of the same region at high WM load, as compared to healthy volunteers (McAllister et al., 2001). Given the major role of the prefrontal cortex (PFC) and its circuitry in mediating WM, it has been somewhat perplexing that persistence of post-injury WM deficits often arises in the absence of overt damage to this structure. Therefore, an understanding of the cellular and molecular mechanisms underlying TBI-associated WM deficits in acute, sub-acute, and chronic stages of injury are required for designing temporally-appropriate pharmacological treatments.
As with humans, rodents exhibit WM deficits following TBI (Hamm et al., 1996; Kline et al., 2002). Recent rodent studies have demonstrated that lateral cortical impact injury causes WM dysfunction in the absence of neuronal cell death in the prelimbic (PL) region of the medial prefrontal cortex (mPFC) (Kobori et al., 2006), a structure anatomically and functionally analogous to the primate DLPFC (Kolb, 1984). Mechanistically, these studies have identified excess GABA-mediated inhibition of prefrontal neuronal activity as a major contributor to the observed TBI-associated WM deficits in these animals (Kobori and Dash, 2006). This conclusion was based upon observations that the level of the rate-limiting enzyme for GABA synthesis, glutamic acid decarboxylase 67 (GAD67), is enhanced in the prefrontal cortex for up to one month post-injury, and that administration of GABAA receptor antagonists (bicuculline, carboline) improves WM function in injured rats. It has, however, not been examined if these biochemical changes, or WM dysfunction, persist beyond 4 weeks after TBI.
In the present study, we examined if rats subjected to lateral cortical impact injury still display WM deficits 4 months after injury. In these animals, we measured the levels of GAD67 in the PL cortex, and examined the effect of bicuculline on their working memory. We also examined if TBI alters the morphology of prelimbic pyramidal neurons and if these changes occur concurrent with, or subsequent to, the previously observed increase in GABAergic signaling.
Experimental Procedures
Animals
All experiments involving the use of animals were carried out under protocols approved by the Institutional Animal Care and Use Committee in compliance with the National Institute of Health guidelines outlined in Guide for the Care and Use of Laboratory Animals. Male Sprague Dawley rats (≥300g) were purchased from Harlan Sprague Dawley (Indianapolis, IN). Rats were housed in pairs and maintained on a 12:12 light dark cycle with ad libitum access to food and water.
Controlled cortical impact injury
A controlled cortical impact device (CCI) was used to initiate a unilateral brain injury as described previously (Dixon et al., 1991; Smith et al., 1995). Briefly, animals maintained under anesthesia (4% isoflurane and 2:1 mixture of N2O/O2) were placed in a stereotaxic frame while a 7mm craniotomy (halfway between bregma and lambda, 3.5 mm lateral to midline) was performed. A heating pad was used to maintain body temperature at 37°C. Using a 6-mm-diameter impact tip, a single impact (1.7 mm deformation, 6 m/s) was delivered to the parietal association cortex at an angle of 10° from the vertical plane, such that the impact was orthogonal to the cortex surface. These parameters produce a moderate-severe injury without detectable neuronal loss within the mPFC, as assessed previously with histopathological measures (Kobori and Dash, 2006). Although a unilateral crainiectomy was performed, this magnitude of injury causes bilateral responses and neuronal dysfunction (Kline et al., 2001; Giza et al., 2002; Verbois et al., 2003; Chen et al., 2005; Enomoto et al., 2005). For this reason, sham-operated animals who received all surgical procedures except the impact, were used as controls for the behavioral, biochemical and morphological measures.
Intra-mPFC cannulae placement and drug infusion
Rats were bilaterally implanted under isoflurane anesthesia with sterile stainless-steel guide cannulae aimed at the dorsal border of the prelimbic area using a stereotaxic device (Bregma 3.2 mm, lateral +/± 0.75, and depth -2.5 mm). A bilateral infusion was performed as this has been previously employed to examine the functional consequences of intra-mPFC infusion of muscimol in prefrontal-dependent tasks (Amat et al., 2005; Blum et al., 2006; Jo et al., 2007). For drug administration, the infusion needles were inserted into awake, moving animals. The infusion needles extended 1.5 mm beyond the end of the guide cannulae, giving a total depth of 4.0 mm. Drugs were dissolved in saline and infusions were performed at a rate of 0.25 μl/min for 2 minutes. Following infusion, the needles were left in place for 2 min to allow for diffusion of the drug. For examination of the prefrontal dependency of the water version of the delayed alternation task, muscimol (1 μg/side) was infused into the prelimbic cortex 15 min prior to testing. Following the completion of the studies, cannula placement was assessed in a representative group of animals. All infusion needle tracks examined terminated within the PL cortex.
Working memory testing
All behavioral tests were performed by an experimenter blind to treatment conditions.
Delay match-to-place water maze
Approximately 120 days after injury, animals were trained in the delay match-to-place version of the Morris water maze (Hamm et al., 1996; Kline et al., 2002). The maze consisted of a circular (1m in diameter) tank in which a submerged platform (10 cm in diameter) was located. The water was maintained at 24°C and made opaque by the addition of a non-toxic water-soluble paint. Rats were initially given 5 pairs of trials to familiarize them with the task and the extramaze cues. Testing consisted of a “location” trial, in which animals were given a maximum of 60s to find the hidden platform. If an animal failed to find the platform, it was led there by the experimenter. Once on the platform, rats were allowed to rest for a period of 10s after which time they were removed from the maze. After a 5s delay, animals were returned to the maze and allowed to again search for the hidden platform. After each “location-match” pair of trials, the platform was moved to a different location and a new “location-match” pair tested. An intertrial interval of 4 min separated each pair of “location-match” trials. Each animal was tested in 5 “location-match” pairs with each pair having a novel platform location and random start site.
Delayed alternation water T-maze
Rats were trained in a delay alternation task using a version of the standard T-maze adapted for use in water. Water effectively motivated animals to explore the T-maze and find an escape platform located at the end of each arm. Training consisted of 7 days exposure to the task, during which time the animals were examined for their ability to swim within the maze, and for their ability to remain on the target platform once found. All animals used in the current study were capable of navigating the T-maze and recognized the hidden platform as an escape. For the first trial of each testing session, platforms were placed in both ends of the cross-piece of the T-maze such that the animal was allowed to choose either direction after swimming down the stem. Once the animal reached the platform, it was to remain there for 15s. After a 10s delay, the animal was returned to the maze and allowed to choose either arm, but only the arm opposite from the one chosen in the immediately preceding trial had a platform. Consequently, if an animal re-entered the arm chosen in the immediately preceding trial, he was confined to that arm with no platform for 15s and that trial was scored as incorrect. A total of 10 consecutive trials was performed for each daily session, with a total of 12 days of testing performed on each animal. The percent correct choices were calculated for each session.
Western blotting
Animals were killed and brains were dissected and submerged under ice-cold artificial cerebrospinal fluid (10 mM HEPES pH 7.2, 1.3 mM NaH2PO4, 3mM KCl, 124 mM NaCl, 10mM dextrose, 26 mM NaHCO3, and 2mM MgCl2). The prelimbic cortex ipsilateral to the side of impact was quickly removed and snap-frozen on dry ice. Sham-operated animals were used as controls. The mPFC brain tissue was homogenized in a lysis-buffer containing 10 mM Tris pH 7.4, 1 mM EGTA, 1 mM EDTA, 0.5 μM DTT, 10 μg/mL leupeptin, 10 μg/mL aprotinin, 1 mM PMSF, and 0.1 μM okadaic acid, followed by centrifugation at 10,000 × g for 10 min. The supernatant solutions were used as cytosolic fraction samples to detect GAD67. As TBI has been shown to alter the expression/levels of a wide variety of proteins including commonly used loading controls such as beta-actin and GAPDH, we use a combination of protein loading controls. First, a NanoOrange protein quantification kit (Invitrogen, Carlsbad, CA) using BSA as a standard to determine protein concentrations in each sample. Based on these numbers, equal amounts of proteins were loaded onto SDS-PAGE which are then stained with Deep Purple (GE Healthcare, Piscataway, NJ). The stained gels were quantified using ImageQuant and a BioRad FX, and the ODs were used to correct any errors in protein loading. Samples were resolved in a SDS-PAGE and transferred to an Immobilon-P membrane (Millipore, Bedford, MA), followed by blocking overnight in TBST (10 mM Tris, pH 7.5, 150 mM NaCl, and 0.05% Tween-20) plus 5% BSA. Membranes were then incubated with the anti-GAD67 antibody (0.2 μg/ml; Millipore) for 3 h at room temperature. After incubation with the primary antibody, membranes were washed three times, and immunoreactivity was assessed by an alkaline phosphatase-conjugated secondary antibody and a CDP-star chemiluminescent substrate (Cell Signaling Technology, Beverly, MA). The optical density of the immunoreactive bands was measured using NIH ImageJ software (http://rsb.info.nih.gov/ij/index.html).
Rapid Golgi staining
Golgi staining was performed using a Rapid GolgiStain kit (FD Neuro Technologies, Ellicot City, MD) following the procedures recommended by the manufacturer. The animals were killed by decapitation, the brains were quickly removed, and blood at the surface of the brain was rinsed with distilled water. The frontal lobe was sliced into 2 mm-thick sections using a Jacobwitz brain slicer and immersed in silver impregnation solution for 3 weeks in the dark, followed by immersion in “Solution C” for 2 days. 150 μm-thick serial sections were cut on a cryostat. Sections were mounted on 3% gelatin-coated slides and allowed to dry for 5 days at 37°C before staining with kit buffers “Solution D and E”. Sections were then dehydrated using an alcohol series and clarified using xylene before coverslipping with Permount (Fisher Scientific).
Dendrite quantification
Silver-impregnated layer II/III and V/VI pyramidal neurons in the prelimbic cortex were viewed on a Nikon TE2000-U microscope with a motorized z-axis stage. Images were captured using a Nikon DS-Fi1 camera using Nikon Elements software. Pyramidal neurons were identified by their characteristic triangular cell soma with a prominent apical dendrite projecting towards the superficial layers of the cortex. Layer II/III was identified as the neuron dense subcortical layer proximal to the neuron sparse layer I, whereas layer V/VI was identified by the presence of pyramidal neurons with relatively larger cell bodies. To be included in the data analysis, the dendritic trees of pyramidal cells had to fulfill the following criteria: (1) the cell had to be well impregnated and not obscured by blood vessels, astrocytes, or heavy clusters of dendrites from other cells; (2) the apical and basal dendrite arborization had to appear to be mainly intact and visible in the plane of section, and (3) the cell body had to reside roughly in the middle of the thickness of the section.
Five pyramidal neurons in the prelimbic cortex ipsilateral to the injury per animal (n=3 for both injured and sham animals) were randomly chosen for analysis. Serial photographs throughout the z-axis plane (2.5 μm steps) were taken at 200X magnification and the dendrites on each plane traced using NikonElements. The traced images were stacked to yield a single projection for each dendritic arbor to be quantified. High magnification microscopy (900X) verified that all visible dendrites were present in the resultant projection. The projections were then used for measuring dendritic arborization by the concentric ring method of Sholl (Sholl, 1956). For the counting of the basal dendrites, the hubs of the rings (increasing diameters in 20μm increments) were placed at the center of the cell body and the number of dendrites which crossed each ring counted. For the evaluation of the terminal arborization of the apical dendrite, the centers of the rings were placed at the distal end of the dendritic shaft. Only those branches that emerged from the distal end of the dendritic shaft were used in the analysis.
To determine the total number of basal and apical dendrites for each cell, the terminals of the dendritic branches were counted. The dendrite numbers for five cells were average for each animal. Cumulative dendrite length was measured using Nikon Elements, calibrated using a slide micrometer. First-order dendrites were marked and measured to their terminus, followed by measurement of secondary, tertiary, etc. dendritic branches. Total dendrite length (basal or apical) was calculated by summing the length of the individual branches.
For presentation purposes, threshold adjusted z-stacked projections were traced using the Live Trace plugin for Adobe Illustrator CS3. Adjustments to these traces were made by hand to ensure the fidelity of the traces. These traces were not used in the data analysis and were provided for presentation purposes only.
Measurement of spine density
Spine counts were made by an experimenter blind to the group designation of the subjects. To evaluate spine density, at least 3 tertiary apical dendrites and 4 secondary basal dendrites per cell were chosen for counting. The selection of neurons was based on the criteria described under histological analysis. Dendrites to be counted had to be located within the area of interest, had to be unbroken, and had to be sufficiently isolated for an unobstructed view. Dendrites that met these criteria were counted at 1000X magnification using an oil emersion objective. The length of the dendrite segment was measured, and the spine density calculated as spines/10μm. Individual dendrites were averaged for each neuron, with at least 5 neurons counted and averaged for each animal.
Statistical analyses
All data was initially subjected to a Kolmogorov-Smirnov normality test and an equal variance test. Behavioral and morphological data were evaluated using repeated measures two-way ANOVAs. Significant differences were determined at P < 0.05 for either group main effects or interactions. The points at which differences were observed were identified by post-hoc analysis using the Holm-Sidak method for multiple comparisons. Western blots were analyzed using a Student's t-test for unpaired variables.
Results
Cortical impact injury causes WM deficits which persist for at least 4 months
In order to assess the persistence of WM deficits following cortical impact injury, we employed two WM tasks, the delay match-to-place version of the Morris water maze, and a water version of the delayed alternation T-maze (Runyan et al., 2005; Locchi et al., 2007). In the delay match-to-place task, animals are required to find a submerged, hidden platform in two consecutive trials separated by a brief delay. Five pairs of platform positions (each separated by a 4 min inter-trial interval) are tested for each rat with the position of the platform randomized between each pair (Figure 1A). The difference in latency between the first trial (location trial) and the second trial (match trial) of each pair is used as a measure of WM. The delayed alternation T-maze requires that animals alternate between left and right turns to find the location of a submerged platform. The number of correct alternations out of 10 consecutive trials is used as the measure of WM function.
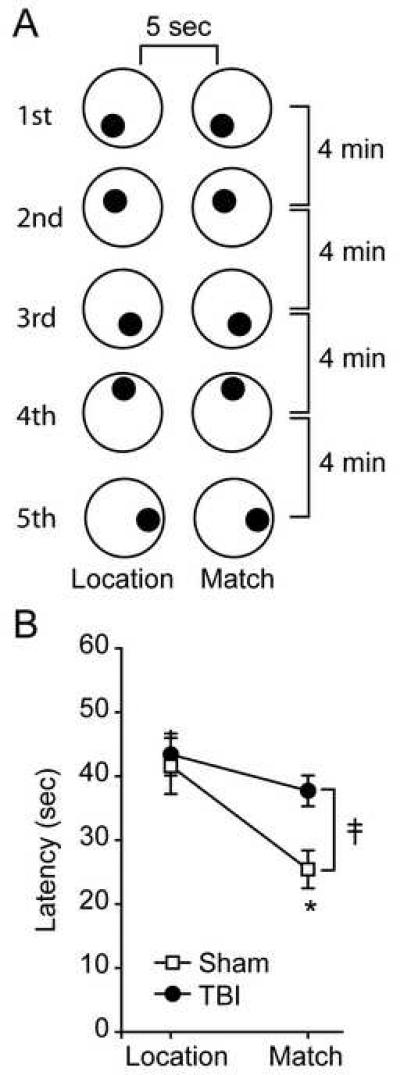
Figure 1. Four month post-TBI rats display working memory deficits in the delay match-to-place task.
A) Schematic depiction of the delay match-to-place testing paradigm. Each location-match pair of trials is carried out with the hidden platform (filled circle) in a novel location within the water tank (large, open circle). A 5-second delay is used between the location and match trials. Rats were tested using at least five different platform positions, with each location-match pair separated by a 4 min resting period. B) Summary data for performance in the delay match-to-place task. Data are presented as the mean ± SEM. ǂ, group main effect by two-way ANOVA. *, significant difference between match trials for sham and injured groups.
Figure 1B shows that sham operated animals (n=6) have significantly shorter latencies to find the hidden platform in the match trial than they do in the location trial, indicating intact WM (latency (sec) location: 41.60 ± 4.81 sec, match: 25.43 ± 3.25, P = 0.005). In contrast, TBI animals (n=10) do not show a difference in latency between the location and match trials (latency (sec) location: 43.40 ± 3.85 sec, match: 37.72 ± 2.69, p = 0.175). A two-way repeated measures ANOVA comparing the performance of the sham and injured animals revealed a significant interaction between group and trial (ǂ, F(1,14) = 6.22, P = 0.026), with a post-hoc analysis indicating that there was a significant difference in match trial performance between the sham and injured groups (*, P = 0.017).
To further examine WM performance in the injured animals, the rats tested in the delay match-to-place task were next evaluated in a water version of the delayed alternation task. Rats were given 7 days pre-exposure to the T-maze in order to familiarize themselves with the task. During testing (1 session of 10 consecutive trials per day), sham-operated animals learned to correctly alternate directions, with animals reaching a performance of approximately 80% correct choices by day 10 of training (Figure 2A). Injured animals, by comparison, only made 65% correct choices after the same amount of training. When the performances of the two groups were compared using a repeated measures twoway ANOVA, a group main effect was found (ǂ, F(1,14) = 35.37, P < 0.001). Post-hoc analysis revealed that the performance of the two groups was different on several days throughout training (*, Figure 2A). In order to corroborate that performance in this task was dependent on mPFC activity, the GABA agonist muscimol was bilaterally infused into the mPFC of trained, uninjured rats. Figure 2B shows that rats receiving 1 μg/side muscimol (n=8) had significantly impaired performance in this task as compared to vehicle-infused controls (n=7) (ǂ, interaction between group and trial F(1,4) = 11.25, P = 0.028). Interestingly, performance was reduced to below 50% (chance level) in the muscimol-infused rats. This appeared to be a result of not alternating sides (e.g. LRLR), but rather perseverating to one side (e.g. LLLR).
Figure 2. Four month post-TBI rats display working memory deficits in the water version of the delayed alternation task.
A) Summary data for the performance of sham-operated and injured animals in the delayed alternation T-maze task. The percent correct alternations for each animal was calculated as the average of two days of testing. Data are presented as the mean ± SEM. ǂ, group main effect by repeated measures two-way ANOVA. *, significant difference between between sham and injured groups. B) Intra-mPFC infusion of 1.0 μg/side muscimol impairs performance in the delayed alternation task. Data are presented as the mean ± SEM. ǂ, interaction between group and condition by repeated measures two-way ANOVA. *, significant difference between vehicle and muscimol groups.
GABAA receptor antagonism does not improve WM performance in 4 month post-injury animals
Our previous studies have shown that systemic administration (i.p.) of GABAA receptor antagonists 14 days post-TBI restores WM performance in the delay match-to-place task (Kobori and Dash, 2006). To test if GABA-mediated inhibition also contributes to WM deficits observed at 4 months post-TBI, the injured animals were given systemic injections of either 0.5 mg/kg bicuculline (a GABAA receptor antagonist) or an equal volume of vehicle (n=5/group). This dose and route of administration of bicucilline, although insufficient to influence the behavior of normal rats, has been previously demonstrated to restore working memory function in injured rats when tested 14 days post-injury (Kobori and Dash, 2006). Twenty minutes following the injection, rats were tested for their ability to perform the delay match-to-place task. Figure 3A shows that bicuculline did not improve the performance of the 4 month post-injury animals compared to vehicle-treated controls (group main effect: F(1,14) = 0.129, P = 0.727), suggesting a normalization of GABA signaling.
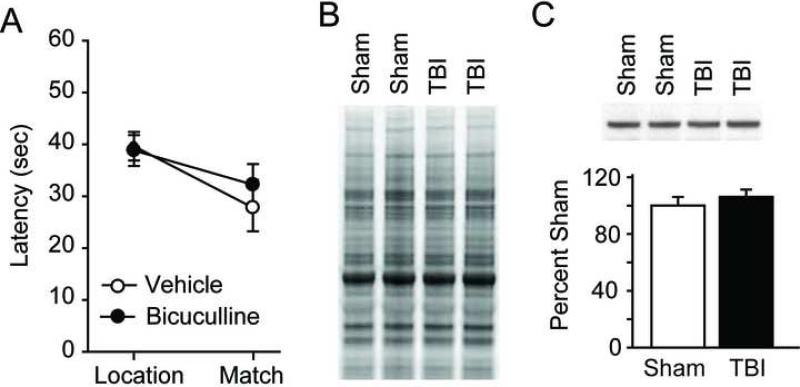
Figure 3. Working memory deficits are not alleviated by GABAA antagonism in 4 month post-TBI animals.
A) Summary data for performance in the delay match-to-place task for injured animals i.p. injected with 0.5 mg/kg bicuculline or vehicle. B) Representative Deep Purple stained gel illustrating the equality of loading for the samples used in C). C) Representative western blots and summary data for GAD67 immunoreactivity within the mPFC for sham-operated and 4 month post-TBI animals. Data are presented as the mean ± SEM.
To test this possibility, animals were killed and prelimbic cortices removed for the evaluation of GAD67, the rate-limiting enzyme in the production of GABA, levels. We have previously demonstrated that TBI causes a protracted increase in GAD67 levels that persists for at least one month post-injury (Kobori and Dash, 2006). Figure 3B shows a representative Deep Purple stained gel (2 of 5 rats/group shown) illustrating the equality of loading. The representative western blots (identical samples to that shown in Figure 3B) and summary results shown in figure 3C (n=5/group) show that, consistent with a lack of improvement by GABAA receptor antagonist, 4 month post-TBI animals have GAD67 levels that are not significantly different from those detected in sham-operated controls (2-tailed t-test, vehicle: 100.0 ± 6.0%; bicuculline: 106.2 ± 5.1%; P = 0.452).
TBI causes delayed morphological changes within layer V/VI neurons of the prelimbic cortex
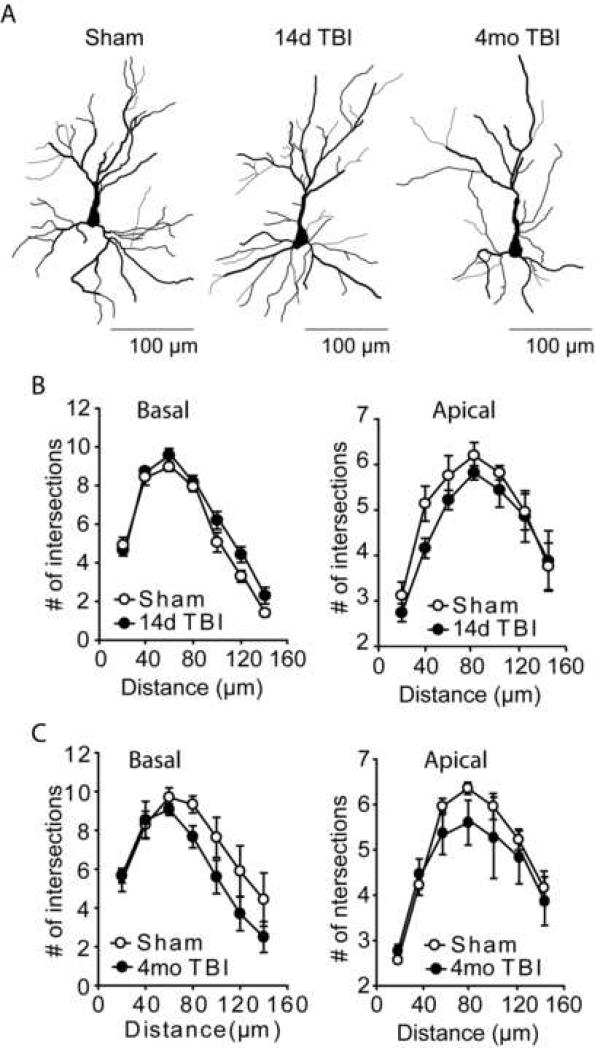
A number of previous studies have reported that WM deficits and morphological changes occur in disorders such as schizophrenia or chronic stress (Glantz and Lewis, 2000; Brown et al., 2005). In order to examine if the WM deficits we observed at 14 days (n=5/group) or 4 months (n=3/group) following TBI are associated with morphological changes of prelimbic pyramidal neurons, a rapid Golgi stain was employed. Figure 4A shows drawings of Golgi-stained pyramidal neurons from within layer II/III of the prelimbic cortex from sham, 14 day post-TBI and 4 month post-TBI animals. Layer II/III neurons were identified and selected based on their distance from the pial surface, and their proximity to the neuron sparse layer I. The basilar and apical dendritic arbors for the cells chosen for analysis were contained within a single section, with no dendrites present in the outer 20μm of the section. Sholl analysis did not reveal any significant differences in neuronal arborization of layer II/III pyramidal neurons at the 14 day post-TBI time point for either basal (two-way repeated measures for interaction: F(6,48) = 1.658, P = 0.152) or apical (two-way repeated measures for interaction: F(6,48) = 0.409, P = 0.869) dendrites (Figure 4B). Similarly, no significant difference in either prelimbic basal (two-way repeated measures for interaction: F(6,24) = 0.928, P = 0.493) or apical (two-way repeated measures for interaction: F(6,24) = 0.316, P = 0.922) dendritic arbors was detected in the 4 month post-TBI animals.
Figure 4. Dendritic arbors of layer II/III pyramidal neurons are not altered as a result of TBI.
A) Computer assisted traces of layer II/III pyramidal neurons from sham and 14 day and 4 month post-injury rats. B) Sholl analysis revealed no significant difference in either basilar or apical dendritic arbors from 14 day post-injury animals compared to sham-operated controls. C) Similarly, no significant changes in arborization were detected in basal or apical dendrites from layer II/III pyramidal neurons at the 4 month post-injury time point. Data are presented as the mean ± SEM.
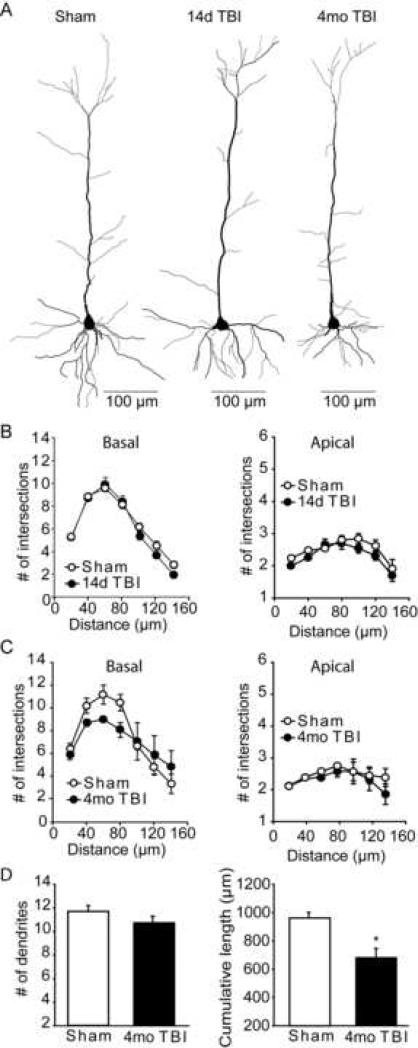
Figure 5A shows traces of layer V/VI pyramidal neurons of the prelimbic cortex from sham, 14 day post-TBI and 4 month post-injury animals. Similar to that observed for layer II/III pyramidal neurons, no significant differences in arborization were detected for layer V/VI basal (two-way repeated measures for interaction: F(6,48) = 1.767, P = 0.126) or apical (two-way repeated measures for interaction: F(6,48) = 0.636, P = 0.701) dendrites at the 14 day post-injury time point (Figure 5B). Although not significant, a trend towards increased intersections of the 40-80μm shells was observed in the basal dendrites of the 4 month post-TBI animals (two-way repeated measures for interaction: F(6,24) = 2.471, P = 0.053), suggesting either an increase in dendrite numbers or an overall shortening of existing dendrites (Figure 5C). Based on this finding, the total number of basal branches, as well as their cumulative length, was quantified. Figure 5D shows that while the total number of dendritic branches did not significantly change as a result of injury (two-tailed t-test: P=0.245), a significant overall shortening of these dendrites was detected (two-tailed t-test: P=0.019).
Figure 5. Layer V/VI pyramidal neurons from 4 month injured animals have reduced basal dendrite lengths.
A) Computer assisted traces of layer V/VI pyramidal neurons from sham and 14 day and 4 month post-injury rats. B) No significant difference in dendrite arbors was detected for either the basal or apical dendrites between the sham and 14 day injured groups. C) Although not significant by two-way repeated measures ANOVA (p=0.053), a trend towards reduced basal dendrite arborization was found in the layer V/VI pyramidal neurons of 4 month post-injury rats. D) The total number and cumulative length of basal dendrites for layer V/VI pyramidal neurons. Data are presented as the mean ± SEM. ǂ, group main effect by repeated measures two-way ANOVA. *, significant difference in dendrite number between sham and 4 month post-TBI animals.
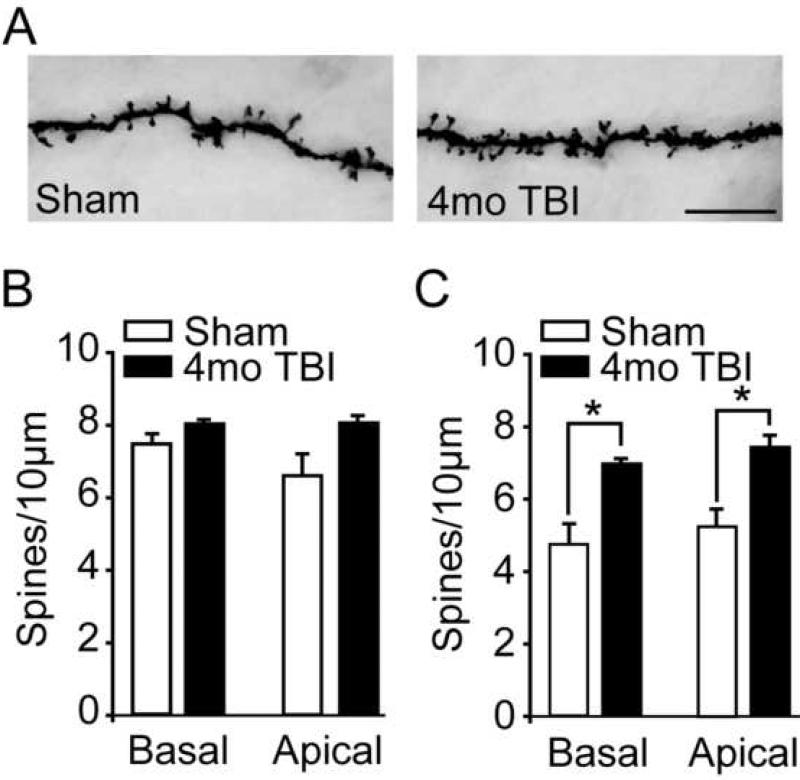
To determine if TBI is associated with a change in the number of potential prelimbic synapses, dendritic spines from sham and 4 month injured animals were counted. For basal dendrites, the number of spines from 2nd-order dendritic branches was counted, the branches measured, and the number of spines/10μm calculated. For apical dendrites, the number of spines present on 3rd-order dendritic branches were counted and normalized to give the number of spines/10 μm. Figure 6A shows representative photomicrographs of dendritic spines from sham and 4 month post-TBI animals (3rd-order apical dendrites from layer V/VI shown). Quantification of spine number revealed that although the number of spines on layer II/III pyramidal neurons did not significantly differ between the sham and injured groups (two-way repeated measures ANOVA group main effect F(1,4) = 3.004, P = 0.158), an overall increase in spines was present on layer V/VI neurons of the 4 month post-TBI animals (two-way repeated measures ANOVA group main effect F(1,4) = 20.423, P = 0.011). Post-hoc analysis revealed that this group difference was due to increased spine density for both basal and apical dendrites.
Figure 6. Layer V/VI pyramidal neurons from 4 month injured animals have increased spine densities.
A) Representative photomicrographs of 3rd order layer V/VI apical dendrites from sham and 4 month post-injury rats. Scale bar = 10μm. B) Quantification of layer II/III spine density (spines/10 μm) from 2nd order basal and 3rd order apical dendrites from sham and 4 month post-injury rats. C) The spine densities of both 2nd order basal and 3rd order apical dendrites from layer V/VI pyramidal neurons were found to be significantly increased (group main effect by repeated measures two-way ANOVA) in 4 month post-injured animals compared to age-matched sham controls.
Discussion
It is well established that traumatic brain injury (TBI) in humans can produce pronounced WM dysfunction that can persist for months (in mild TBI cases) or longer (in moderate-severe TBI cases) (McAllister et al., 2001). Although damage to the prefrontal cortex, both in human subjects and in experimental lesion models, has been demonstrated to be sufficient to perturb proper WM function, the mechanisms by which TBI causes WM deficits in the absence of overt damage to the prefrontal cortex is only beginning to be understood (Ramos et al., 2003). The present investigation into the mechanisms of TBI-associated WM deficits revealed three key findings: (1) similar to that observed in human TBI, controlled cortical impact injury to rodents elicits WM deficits that can persists for several months, (2) the molecular mechanisms previously demonstrated to cause WM dysfunction in the acute phase (e.g. excessive inhibitory neurotransmission) do not appear to contribute to the observed chronic deficits, and (3) chronic WM deficits are associated with TBI-induced morphological changes of layer V/VI pyramidal neurons within the prelimbic cortex of the mPFC. Taken together, the present findings, along with the previously published reports, indicate that the cellular and molecular mechanisms underlying TBI-associated WM dysfunction change over time. An understanding of these temporal changes is critical for designing mechanism-based therapies to treat TBI-associated WM dysfunction.
A few previous studies have reported WM dysfunction in the initial days-to-weeks following experimental TBI in rodents (Hamm et al., 1996; Kline et al., 2002; Kobori and Dash, 2006). However, clinical studies examining therapeutic interventions are often performed in patients who are months removed from their injury (McAllister et al., 2004; Siddall, 2005; Bramlett and Dietrich, 2007). In light of the possibility that the underlying mechanism for WM deficits may change over time, the objective of the current study was to determine whether or not rats subjected to TBI would have persistent WM dysfunction, and if so, would the previously identified mechanisms contribute at later time points. Recent work from our laboratory revealed that lateral cortical impact injury, in which the impact is centered between the lambda and bregma, does not cause detectable cell death in the prelimbic cortex, but is associated with WM deficits that persist for at least 4 weeks post-injury (Kobori and Dash, 2006). This dysfunction was found to be due to, at least in part, a marked increase in GAD67 expression in the mPFC. Consistent with this, GABAA receptor antagonists and inverse agonists significantly improved the WM performance of TBI animals (Kobori and Dash, 2006). In the present study, we extend these findings and demonstrate that cortical impact injury causes WM dysfunction that remains detectable four months after the injury. This deficit was evidenced by poor performance in two working memory tasks: the delay match-to-place task, and the delayed alternation T-maze. However, in contrast to our previous observations, the expression of GAD67 in the prelimbic cortex was found to have returned to sham levels. Consistent with this apparent normalization of GABAergic neurotransmission, GABAA receptor antagonists were no longer capable of restoring WM function. These findings suggest that a different, perhaps longer-lasting, mechanism may underlie the WM impairments observed in this chronic phase of injury.
A number of studies have shown that structural abnormalities of PFC neurons are associated with the WM deficits observed as a result of stress, schizophrenia, and mood disorders (Kalus et al., 2000; Rajkowska, 2000; Cerqueira et al., 2007). For example, Cook and Wellman (2004) demonstrated that rodents experiencing chronic stress had elevated levels of serum corticosterone that was associated with a significant reduction in mean apical branch number and length of layer II/III PFC neurons (Cook and Wellman, 2004). Similarly, a rodent model of schizophrenia (neonatal ventral hippocampus lesions) that induces WM dysfunction was found to be associated with a significant decrease in the length of basilar dendrites on layer III pyramidal neurons (Flores et al., 2005). In the present study, we found that TBI causes a significant shortening of overall basilar dendrite length of layer V/VI pyramidal neurons, the output layer of the prelimbic cortex, at the 4 month post-injury time point. Associated with this was a significant increase in the density of both basal and apical dendritic spines. It remains to be determined if the observed increase in spine density represents a compensatory change in response to the retraction of the basal dendrites, if the retraction is compensatory for the increase in spine density, or if the two phenomena are unrelated. While the significance of these morphological changes is not clear at present, these data suggest that TBI may give rise to long-lasting behavioral impairments by altering neuronal morphology.
As layer V/VI receives dense catecholaminergic innervations, and we have previously reported that the catecholamine content and tyrosine hydoxylase fibers within the mPFC are increased following injury (Emson and Koob, 1978; Descarries et al., 1987; Kobori et al., 2006), it is interesting to speculate that the morphological changes we observed may represent a response to altered catecholamine signaling. This relationship would be consistent with previous studies that have demonstrated a link between dopaminergic modulation and neuronal morphology in the mPFC. For example, 6-hydroxydopamine (6-OHDA) lesion of the ventral tegmental area of adult rats causes a decrease in the basal dendritic length and spine density of layer V/VI pyramidal neurons within the prelimbic cortex (Wang and Deutch, 2008). D1 receptor priming of rats with neonatal 6-OHDA lesions, a model of D1 receptor sensitization, results in layer II/III pyramidal neurons with truncated and disorganized apical dendrites (Papadeas et al., 2008). Although the relationship, if any, between the initial neurochemical imbalances that occur as a result of injury and the morphological changes we observed remains to be determined, the results from this study demonstrate that TBI-induced WM deficits are persistent, and that the contributing mechanisms change/evolve over time. These findings suggest that pharmacological agents useful in the early phase(s) of injury may not be well-suited to treat chronic WM dysfunction. These and other issues need to be taken into consideration when designing mechanism-based therapies for the treatment of TBI-associated WM deficits (Kochanek et al., 2008).
Acknowledgements
The authors would like to thank Tim Graham and Melanie Moody for technical assistance. The work performed in the authors’ laboratories was made possible by grants from NIH.
Abbreviations
- DLPFC
dorsolateral prefrontal cortex
- GABA
gamma-aminobutyric acid
- GAD67
glutamic acid decarboxylase 67kDa
- mPFC
medial prefrontal cortex
- PFC
prefrontal cortex
- PL
prelimbic cortex
- TBI
traumatic brain injury
- WM
working memory
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. (Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. (Catecholamine regulation of the prefrontal cortex. J Psychopharmacol. 1997;11:151–162. doi: 10.1177/026988119701100208. [DOI] [PubMed] [Google Scholar]
- Baddeley A. (Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Blum S, Hebert AE, Dash PK. (A role for the prefrontal cortex in recall of recent and remote memories. Neuroreport. 2006;17:341–344. doi: 10.1097/01.wnr.0000201509.53750.bc. [DOI] [PubMed] [Google Scholar]
- Bramlett HM, Dietrich WD. (Progressive damage after brain and spinal cord injury: pathomechanisms and treatment strategies. Prog Brain Res. 2007;161:125–141. doi: 10.1016/S0079-6123(06)61009-1. [DOI] [PubMed] [Google Scholar]
- Brown SM, Henning S, Wellman CL. (Mild, short-term stress alters dendritic morphology in rat medial prefrontal cortex. Cereb Cortex. 2005;15:1714–1722. doi: 10.1093/cercor/bhi048. [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Mailliet F, Almeida OF, Jay TM, Sousa N. (The prefrontal cortex as a key target of the maladaptive response to stress. J Neurosci. 2007;27:2781–2787. doi: 10.1523/JNEUROSCI.4372-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Li Y, Kline AE, Dixon CE, Zafonte RD, Wagner AK. (Gender and environmental effects on regional brain-derived neurotrophic factor expression after experimental traumatic brain injury. Neuroscience. 2005;135:11–17. doi: 10.1016/j.neuroscience.2005.05.041. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. (Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- D'Esposito M. (Functional neuroimaging of cognition. Semin Neurol. 2000;20:487–498. doi: 10.1055/s-2000-13182. [DOI] [PubMed] [Google Scholar]
- Dash PK, Moore AN, Kobori N, Runyan JD. (Molecular activity underlying working memory. Learn Mem. 2007;14:554–563. doi: 10.1101/lm.558707. [DOI] [PubMed] [Google Scholar]
- Descarries L, Lemay B, Doucet G, Berger B. (Regional and laminar density of the dopamine innervation in adult rat cerebral cortex. Neuroscience. 1987;21:807–824. doi: 10.1016/0306-4522(87)90038-8. [DOI] [PubMed] [Google Scholar]
- Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. (A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Emson PC, Koob GF. (The origin and distribution of dopamine-containing afferents to the rat frontal cortex. Brain Res. 1978;142:249–267. doi: 10.1016/0006-8993(78)90634-0. [DOI] [PubMed] [Google Scholar]
- Enomoto T, Osugi T, Satoh H, McIntosh TK, Nabeshima T. (Pre-Injury magnesium treatment prevents traumatic brain injury-induced hippocampal ERK activation, neuronal loss, and cognitive dysfunction in the radial-arm maze test. J Neurotrauma. 2005;22:783–792. doi: 10.1089/neu.2005.22.783. [DOI] [PubMed] [Google Scholar]
- Finley JW, Sigrid-Keck A, Robbins RJ, Hintze KJ. (Selenium Enrichment of Broccoli: Interactions between Selenium and Secondary Plant Compounds. J Nutr. 2005;135:1236–1238. doi: 10.1093/jn/135.5.1236. [DOI] [PubMed] [Google Scholar]
- Flores G, Alquicer G, Silva-Gomez AB, Zaldivar G, Stewart J, Quirion R, Srivastava LK. (Alterations in dendritic morphology of prefrontal cortical and nucleus accumbens neurons in post-pubertal rats after neonatal excitotoxic lesions of the ventral hippocampus. Neuroscience. 2005;133:463–470. doi: 10.1016/j.neuroscience.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE. (Neuron activity related to short-term memory. Science. 1971;173:652–654. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- Giza CC, Prins ML, Hovda DA, Herschman HR, Feldman JD. (Genes preferentially induced by depolarization after concussive brain injury: effects of age and injury severity. J Neurotrauma. 2002;19:387–402. doi: 10.1089/08977150252932352. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. (Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Hamm RJ, Temple MD, Pike BR, O'Dell DM, Buck DL, Lyeth BG. (Working memory deficits following traumatic brain injury in the rat. J Neurotrauma. 1996;13:317–323. doi: 10.1089/neu.1996.13.317. [DOI] [PubMed] [Google Scholar]
- Jo YS, Park EH, Kim IH, Park SK, Kim H, Kim HT, Choi JS. (The medial prefrontal cortex is involved in spatial memory retrieval under partial-cue conditions. J Neurosci. 2007;27:13567–13578. doi: 10.1523/JNEUROSCI.3589-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalus P, Muller TJ, Zuschratter W, Senitz D. (The dendritic architecture of prefrontal pyramidal neurons in schizophrenic patients. Neuroreport. 2000;11:3621–3625. doi: 10.1097/00001756-200011090-00044. [DOI] [PubMed] [Google Scholar]
- Kline AE, Massucci JL, Marion DW, Dixon CE. (Attenuation of working memory and spatial acquisition deficits after a delayed and chronic bromocriptine treatment regimen in rats subjected to traumatic brain injury by controlled cortical impact. J Neurotrauma. 2002;19:415–425. doi: 10.1089/08977150252932370. [DOI] [PubMed] [Google Scholar]
- Kline AE, Yu J, Horvath E, Marion DW, Dixon CE. (The selective 5-HT(1A) receptor agonist repinotan HCl attenuates histopathology and spatial learning deficits following traumatic brain injury in rats. Neuroscience. 2001;106:547–555. doi: 10.1016/s0306-4522(01)00300-1. [DOI] [PubMed] [Google Scholar]
- Kobori N, Clifton GL, Dash PK. (Enhanced catecholamine synthesis in the prefrontal cortex after traumatic brain injury: implications for prefrontal dysfunction. J Neurotrauma. 2006;23:1094–1102. doi: 10.1089/neu.2006.23.1094. [DOI] [PubMed] [Google Scholar]
- Kobori N, Dash PK. (Reversal of brain injury-induced prefrontal glutamic acid decarboxylase expression and working memory deficits by D1 receptor antagonism. J Neurosci. 2006;26:4236–4246. doi: 10.1523/JNEUROSCI.4687-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek PM, Berger RP, Bayir H, Wagner AK, Jenkins LW, Clark RS. (Biomarkers of primary and evolving damage in traumatic and ischemic brain injury: diagnosis, prognosis, probing mechanisms, and therapeutic decision making. Curr Opin Crit Care. 2008;14:135–141. doi: 10.1097/MCC.0b013e3282f57564. [DOI] [PubMed] [Google Scholar]
- Kolb B. (Functions of the frontal cortex of the rat: a comparative review. Brain Res. 1984;320:65–98. doi: 10.1016/0165-0173(84)90018-3. [DOI] [PubMed] [Google Scholar]
- Kraus J, Chu LD. Neuropsychiatry of Traumatic Brain Injury. American Psychiatric press; Washington, DC: 2005. [Google Scholar]
- Locchi F, Dall'Olio R, Gandolfi O, Rimondini R. (Water T-maze, an improved method to assess spatial working memory in rats: Pharmacological validation. Neurosci Lett. 2007;422:213–216. doi: 10.1016/j.neulet.2007.06.023. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Flashman LA, Sparling MB, Saykin AJ. (Working memory deficits after traumatic brain injury: catecholaminergic mechanisms and prospects for treatment -- a review. Brain Inj. 2004;18:331–350. doi: 10.1080/02699050310001617370. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Sparling MB, Flashman LA, Guerin SJ, Mamourian AC, Saykin AJ. (Differential working memory load effects after mild traumatic brain injury. Neuroimage. 2001;14:1004–1012. doi: 10.1006/nimg.2001.0899. [DOI] [PubMed] [Google Scholar]
- Papadeas ST, Halloran C, McCown TJ, Breese GR, Blake BL. (Changes in apical dendritic structure correlate with sustained ERK1/2 phosphorylation in medial prefrontal cortex of a rat model of dopamine D1 receptor agonist sensitization. J Comp Neurol. 2008;511:271–285. doi: 10.1002/cne.21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G. (Histopathology of the prefrontal cortex in major depression: what does it tell us about dysfunctional monoaminergic circuits? Prog Brain Res. 2000;126:397–412. doi: 10.1016/S0079-6123(00)26026-3. [DOI] [PubMed] [Google Scholar]
- Ramos BP, Birnbaum SG, Lindenmayer I, Newton SS, Duman RS, Arnsten AF. (Dysregulation of protein kinase a signaling in the aged prefrontal cortex: new strategy for treating age-related cognitive decline. Neuron. 2003;40:835–845. doi: 10.1016/s0896-6273(03)00694-9. [DOI] [PubMed] [Google Scholar]
- Runyan JD, Moore AN, Dash PK. (A role for prefrontal calcium-sensitive protein phosphatase and kinase activities in working memory. Learn Mem. 2005;12:103–110. doi: 10.1101/lm.89405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholl DA. (The measurable parameters of the cerebral cortex and their significance in its organization. Prog Neurobiol. 1956:324–333. [PubMed] [Google Scholar]
- Siddall OM. (Use of methylphenidate in traumatic brain injury. Ann Pharmacother. 2005;39:1309–1313. doi: 10.1345/aph.1E637. [DOI] [PubMed] [Google Scholar]
- Smith DH, Soares HD, Pierce JS, Perlman KG, Saatman KE, Meaney DF, Dixon CE, McIntosh TK. (A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J Neurotrauma. 1995;12:169–178. doi: 10.1089/neu.1995.12.169. [DOI] [PubMed] [Google Scholar]
- Verbois SL, Hopkins DM, Scheff SW, Pauly JR. (Chronic intermittent nicotine administration attenuates traumatic brain injury-induced cognitive dysfunction. Neuroscience. 2003;119:1199–1208. doi: 10.1016/s0306-4522(03)00206-9. [DOI] [PubMed] [Google Scholar]
- Wang HD, Deutch AY. (Dopamine depletion of the prefrontal cortex induces dendritic spine loss: reversal by atypical antipsychotic drug treatment. Neuropsychopharmacology. 2008;33:1276–1286. doi: 10.1038/sj.npp.1301521. [DOI] [PMC free article] [PubMed] [Google Scholar]