Abstract
Mutations in the UMOD gene encoding uromodulin (Tamm-Horsfall glycoprotein) result in the autosomal dominant transmission of progressive renal insufficiency and hypo-uricosuric hyperuricemia leading to gout at an early age. The clinical appearance is characterized by renal insufficiency and gout occurring in the late teenage years, with end-stage kidney disease characteristically developing between 40 and 70 years of age. This report provides a long-term characterization of renal functional decline in three children from one family with a novel UMOD mutation (c.891T>G, p.C297W) who received allopurinol and a low protein diet. While renal functional decline is slow in individuals with UMOD mutations, it may appear early in life and be associated with marked hyperuricemia. Anemia was also noted in this family.
Keywords: Childhood, Children, Chronic renal failure, Autosomal dominant, Hyperuricemia, Interstitial kidney, Familial juvenile hyperuricemic nephropathy, Medullary cystic kidney disease, Uromodulin, Uromodulin-associated kidney disease
Introduction
Mutations in the UMOD gene as a cause of familial juvenile hyperuricemic nephropathy [Mendelian Inheritance in Man (MIM) 162000] and medullary cystic kidney disease (MCKD) type 2 (MIM 174000) were first described in 2001 [1]. Subsequently, individuals with autosomal dominant glomerulocystic kidney disease were also described with UMOD gene mutations [2, 3]. With identification of the causative gene, it has been possible to fully characterize the clinical presentation of this disorder, and the breadth of clinical findings associated with this condition are only now being fully described. While the course of renal functional decline has been well detailed in adults, there has been little published information regarding decline in children. This report describes three children with a UMOD mutation and its effect on kidney function in childhood.
Case report
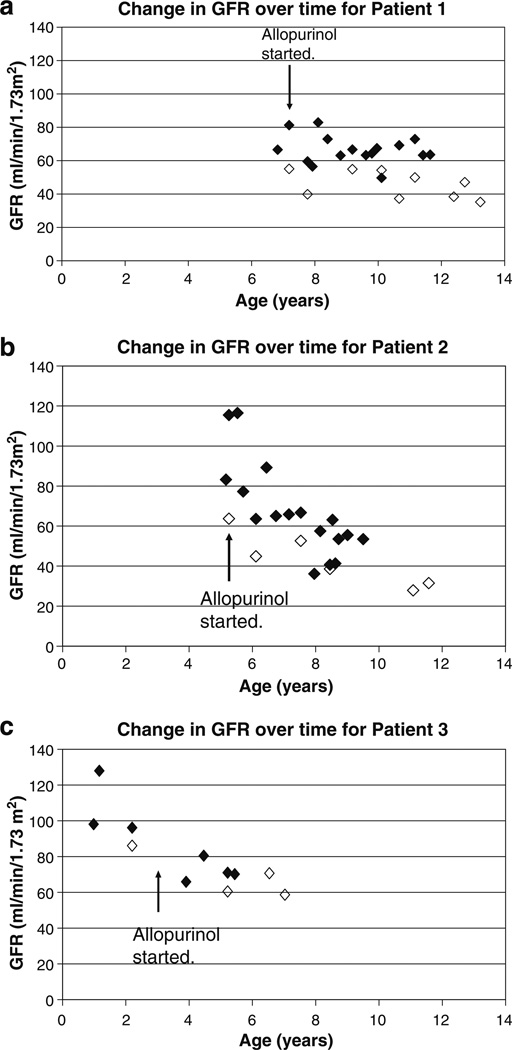
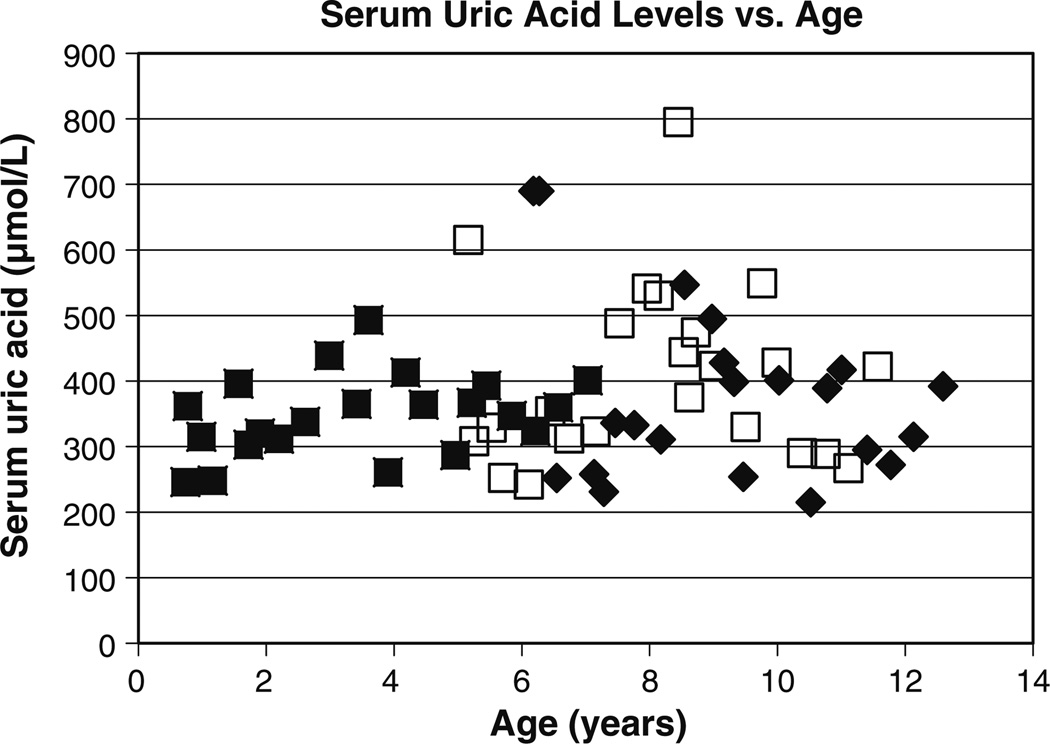
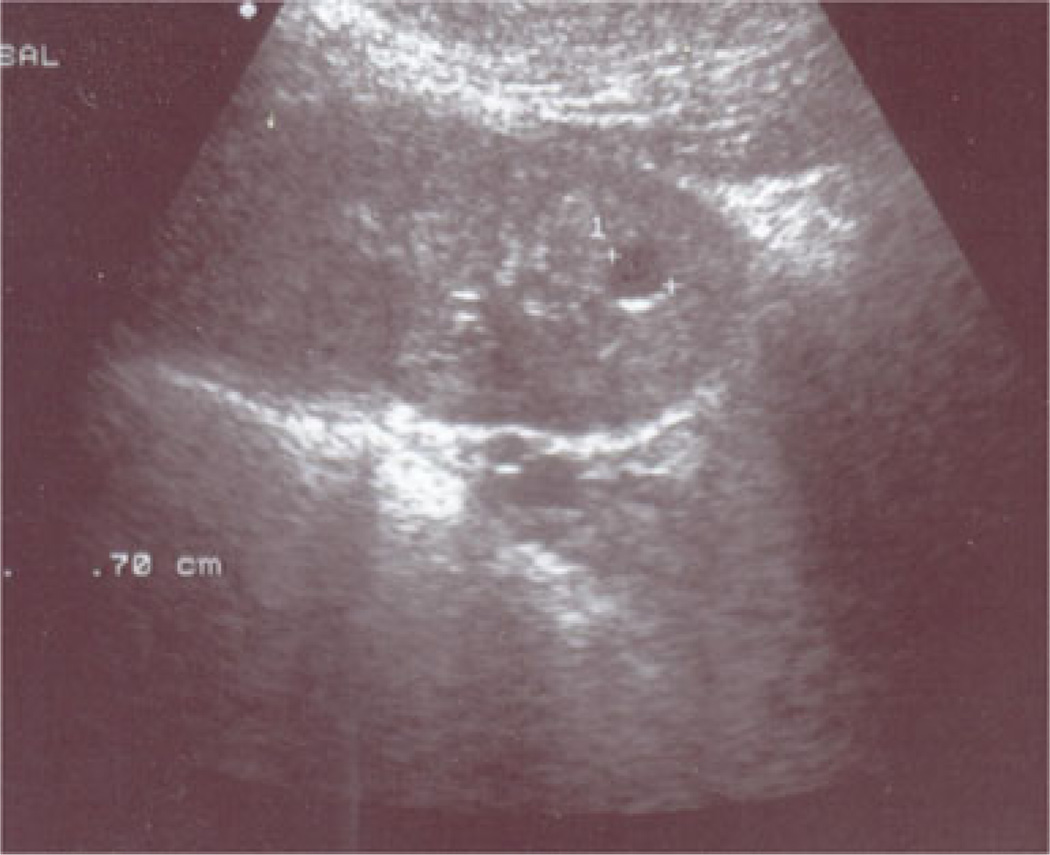
A 6-year-old girl (Patient 1) was evaluated for chronic anemia and fatigue. She had no other symptoms and did not suffer from enuresis. The patient was the product of a normal gestation and birth. At presentation, her height and weight were 115 cm (44th percentile) and 19 kg (33rd percentile), respectively. Her blood pressure was 103/54 mmHg, and the rest of the physical examination was unremarkable. Laboratory studies revealed a hemoglobin level of 98 g/L, blood urea nitrogen (BUN) of 12.7 mmol/L, and serum creatinine of 84 µmol/L. Further hematologic studies revealed iron deficiency. A urinalysis was negative for protein. A 24-h urine collection revealed a creatinine clearance of 55.1 ml/min/1.73 m2 (Fig. 1a). The serum uric acid level was 690 µmol/L (Fig. 2). The fractional excretion of uric acid was very low at 3.3% (normal >10%). The patient underwent a renal ultrasound (US) which revealed a simple 0.7-cm cyst in one kidney (Fig. 3); no remarkable findings were evident on the US scan of the other kidney. Given the markedly elevated serum uric acid level, the patient was placed on a low purine, low protein diet supplemented with essential amino acids and keto-analogs. She was also started on allopurinol, 100 mg per day. With this therapy, the serum uric acid level decreased to 310 µmol/L. The patient’s serum uric acid levels then remained well controlled with further adjustment of allopurinol up to 200 mg/day as the patient grew. Unfortunately, renal function declined over time, with a slow progression of renal insufficiency (Fig. 1). Anemia also persisted, with hemoglobin values between 96 and 110 g/L over the next 4 years despite the treatment of iron deficiency. Over time, the blood pressure rose to 125/70 mmHg at age 11 years. There was minimal urinary protein over the 8 years of follow-up, with a maximum urinary protein of 44 mg/24 h on one of the eight 24-h collections.
Fig. 1.
Change in estimated glomerular filtration rate over time for affected children. Filled diamond Schwartz formula, open diamond creatinine clearance, GFR glomerular filtration rate
Fig. 2.
Serum uric acid levels in affected children. Filled diamond Patient 1, open square patient 2, filled square patient 3
Fig. 3.
Renal ultrasound of Patient 1
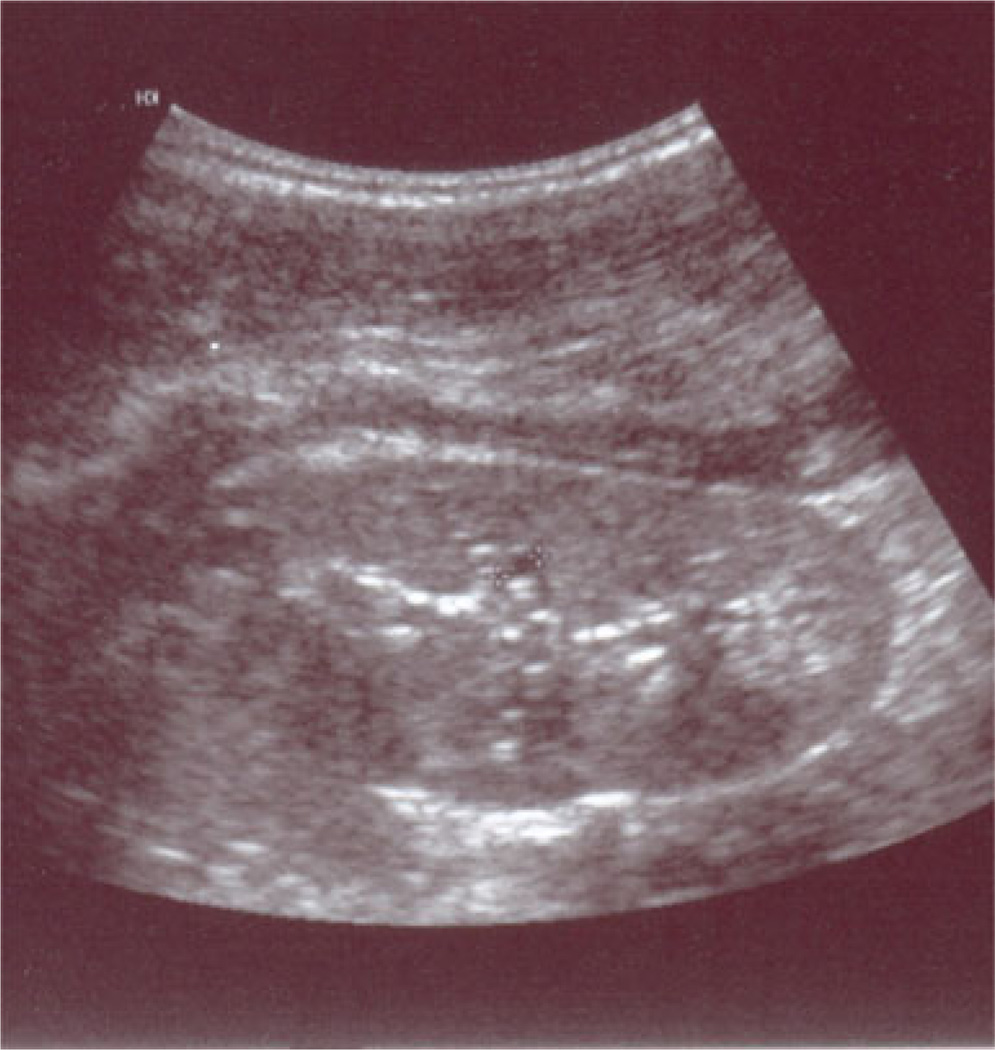
Based on these findings, the sister of the index case (Patient 2) was also evaluated at age 5 years. At this time, the patient’s height and weight were 113 cm (78th percentile) and 18.5 kg (59th percentile), respectively. The blood pressure could not be measured as the child was uncooperative. The patient’s laboratory studies revealed a hemoglobin of 98 g/L, serum creatinine of 66 µmol/L, BUN of 10.2 mmol/L, and serum uric acid level of 615 µmol/L. A 24-h urine collection was performed and revealed a creatinine clearance of 63.7 ml/min/1.73 m2 (Fig. 1b) and a fractional excretion of uric acid of 3.5%. The patient was started on allopurinol, 100 mg/day, which resulted in a prompt decline in her serum uric acid level to 308 umol/l. The initial renal US scan was unremarkable, but subsequent US scans revealed small echodense areas in the renal cortex and decreased parenchymal thickness (see Fig. 4). The patient also had negligible urinary protein throughout the course of the study, with a maximum urinary protein of 98 mg/24 h in one of the seven 24-h collections.
Fig. 4.
Renal ultrasound of Patient 2
Due to the findings of disease in both sisters, a third sister (Patient 3) was evaluated at 9 months of age. At this time, her serum creatinine was elevated at 51 µmol/L, with a BUN of 8.5 mmol/L and a serum uric acid level of 362 µmol/L. The patient underwent a 24-h urine collection at age 3 years, revealing a serum uric acid level of 439 µmol/L and a fractional excretion of uric acid of 7.5%. The hemoglobin level was low at 100 g/L, and the serum creatinine was elevated at 44 µmol/L. She was started on allopurinol at age 3 years. The patient had two subsequent measurements of fractional excretion of uric acid which were 4.5 % at age 4 years 9 months and 4.9% at age 5 years. Patient 3 has had relatively stable renal function over time compared to her two sisters (Fig. 1c). Her urinary protein was also negligible over six different collections.
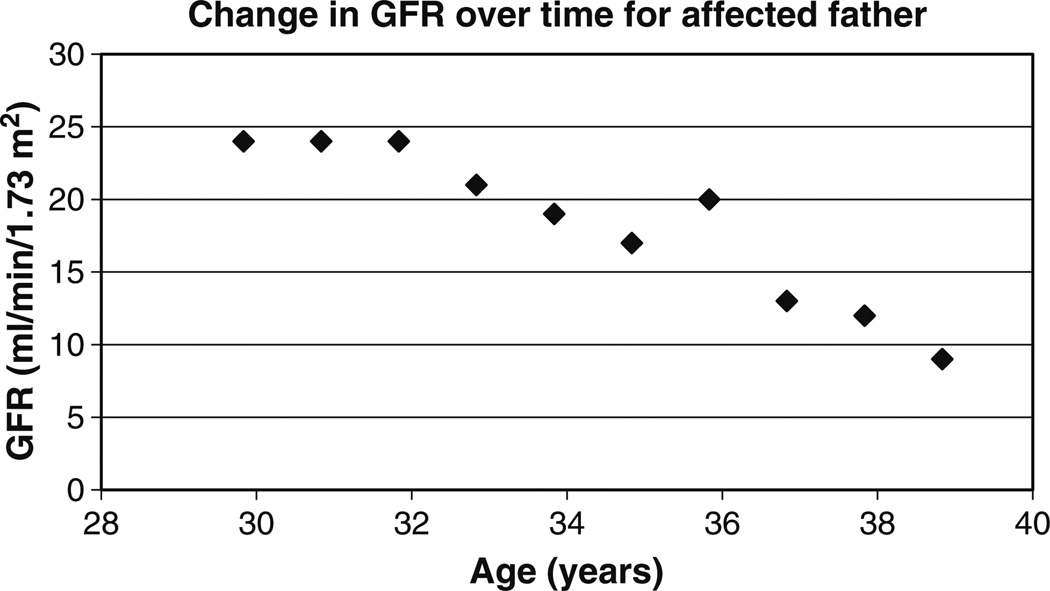
The 36-year-old father has chronic renal failure. Hyperuricemia was first noted at age 17 years when he was treated for gout. Renal US revealed bilateral small kidneys with reduced parenchyma. The father was started on allopurinol at age 25 years. Figure 5 shows the father’s change in renal function over time. A 24-h protein collection revealed 46 mg/day at age 32 years, 800 mg/day at age 34 years, and 390 mg/day at age 35 years. The patient started renal replacement therapy at age 39 years.
Fig. 5.
Change in estimated GFR over time for the father based on the MDRD (modification of diet in renal disease) formula (filled diamonds)
Methods
Human studies were approved by the Ethics Committee, Children’s Hospital, Budapest, Hungary and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. A mutation analysis with directed sequencing of exons 4 and 5 of the UMOD gene was performed as previously described [1].
Results
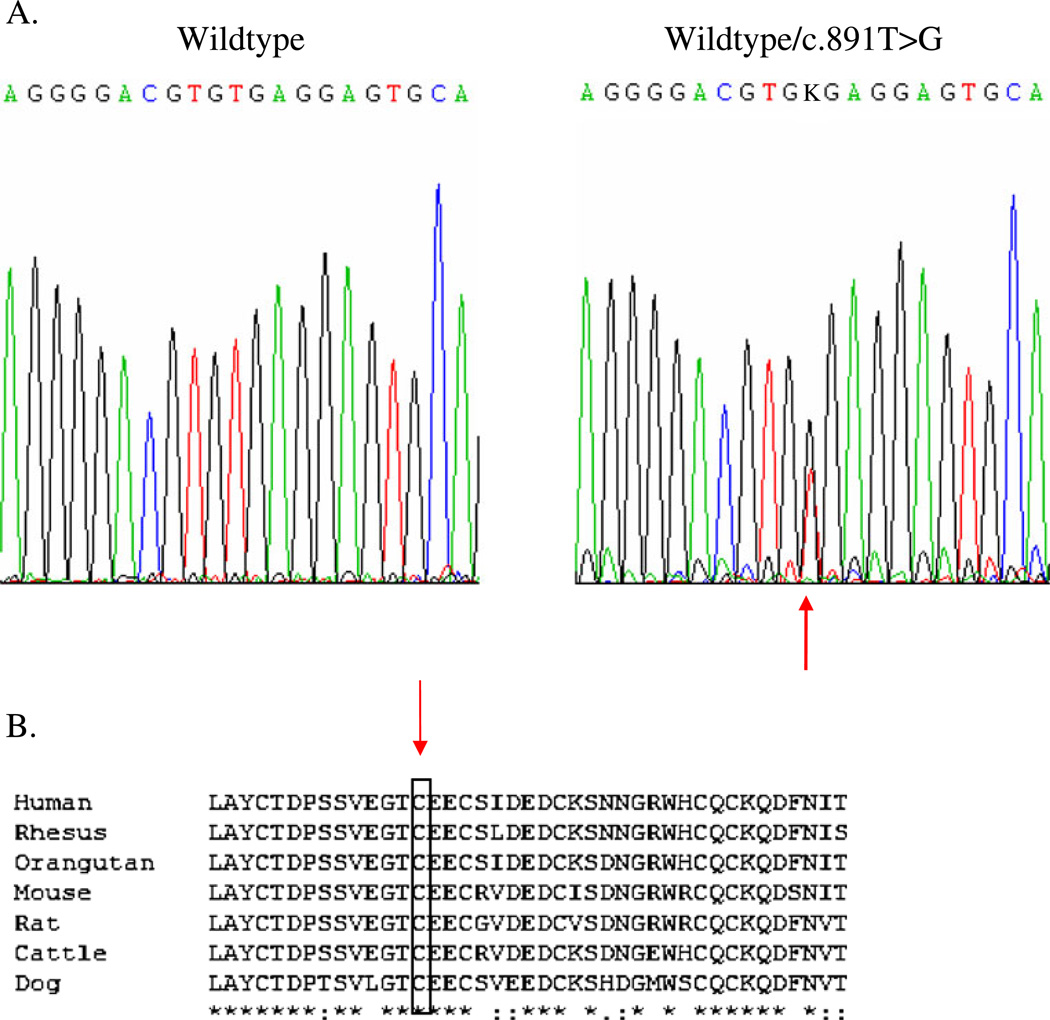
A novel transversion mutation (c.891T>G) was noted in exon 4, resulting in the substitution of a tryptophan for a cysteine at position 297 (p.C297W) (reference sequence NM 00361) in all three children and the father (see Fig. 6a). The mutation was not found in 200 normal European controls, and the cysteine at position 297 is preserved in other animal species (see Fig. 6b).
Fig. 6.
a The wildtype sequence of a portion of exon 4 of UMOD is shown on the left. The panel on the right shows the sequence from one of the affected individuals, with the arrow indicating the heterozygous change (c.891T>G) which is predicted to cause substitution of a conserved cysteine residue by tryptophan. The father and all three daughters were heterozygous for the change. b Alignment of a portion of the amino acid sequence of human uromodulin (GenBank accession NP_003352) with the uromodulin of rhesus monkey (XP_001087334), orangutan (NP_001126801), murine (NP_033496), rat (NP_058778), bovine (NP_776638), and dog (NP_001003003). Asterisk Residues completely conserved, rectangle cysteine residue affected by the missense change in this family, colon conserved substitutions, period semi-conserved substitutions
The web-based program PolyPhen (Polymorphism Phenotyping; http://genetics.bwh.harvard.edu/pph/) predicted that the amino acid substitution would result in a significant conformational change that could affect function, based on a PSIC score difference of 3.289.
Discussion
Identification of the UMOD gene encoding uromodulin as a cause of hereditary tubulo-interstitial kidney disease [1, 4, 5] now allows us to better characterize changes in kidney function early in life. Characterization of these children will allow us to better recognize this condition in childhood. While other reports have provided single measurements of kidney function in individuals with known mutations, this is the first report to carefully study longitudinal kidney function in individuals with a known UMOD mutation.
The UMOD gene produces the glycoprotein uromodulin, also known as Tamm-Horsfall glycoprotein. Uromodulin is a large glycoprotein containing many cysteine residues [6]. These residues are very important in the cross-linking of the uromodulin molecule and increase the transit time through the endoplasmic reticulum [7]. Approximately two-thirds of the mutations found in families with this disorder have resulted in the addition or deletion of a cysteine residue [8], similar to the family described here. Uromodulin has a number of potential functions, including maintenance of the water-tight integrity of the thick ascending limb of Henle [9] and prevention of urinary tract infection [10] and nephrolithiasis [11]. A recent genome-wide association study identified an association between a polymorphism in the UMOD gene and chronic kidney disease in the general population [12].
The MCKD–nephronophthisis complex includes disorders resulting from UMOD mutations as well as other causes of MCKD and autosomal recessive nephronophthisis [13]. These disorders are characterized by tubulo-interstitial kidney disease with chronic progression of kidney disease. MCKD is characterized by autosomal dominant inheritance. A form of MCKD (MCKD 1) has been linked to chromosome 1 [14], and the search for the gene is ongoing. Nephronophthisis is characterized by autosomal recessive inheritance [15], and most mutations resulting in this disorder have been found in genes encoding proteins in the renal cilia [16]. Both disorders pathologically show tubulo-interstitial fibrosis. Anemia also occurs in both conditions, although usually at an older age and with more advanced kidney failure in patients with UMOD mutations. While MCKD more commonly presents in adulthood, presentation in childhood does occur [5, 17], as in this family, and the diagnosis could be potentially confused with nephronophthisis. The presence of autosomal dominant inheritance and subsequent genetic analysis allows the differentiation of these disorders.
The hyperuricemia that is found in individuals with UMOD mutations is believed to be due to an increased proximal tubular reabsorption of uric acid, possibly as a result of decreased sodium and water conservation occurring in the thick ascending limb of Henle [1]. The results from our study affirm that hyperuricemia occurs extremely early in this condition, with hyperuricemia (serum uric acid level 362 µmol/L) occurring at the age of 9 months in one child in this family. The level of hyperuricemia can vary in individuals as well as in families, as illustrated by patient 1 in this study, who had a markedly elevated serum uric acid level (690 µmol/L). Hyperuricemia is always found in families with UMOD mutations even though individual family members may not have hyperuricemia [18].
Renal insufficiency in individuals with this condition is believed to occur due to the accumulation of mutant uromodulin in thick ascending limb cells, resulting in increased apoptosis [19]. The mutated uromodulin may also generate an inflammatory response. Our study demonstrates that renal insufficiency in patient 3 was already beginning at 9 months of age. These findings demonstrate that pathophysiologic changes leading to renal insufficiency do not take years to develop but are present in infancy. While the progression of kidney disease is slow, changes occur early in life.
The children in this family have a much faster rate of kidney disease progression than their affected father. There is significant variation in the rate of progression of kidney disease both between families and within families with UMOD mutations [5, 18, 20], as has been noted by other authors [5, 20], and its cause is unclear. Understanding the reason for these differences could hold the key to preventing kidney disease in this condition. Increased protein intake in succeeding generations could result in increased uric acid production [21], with subsequent deleterious effects on the kidney, in addition to the general negative effects of protein intake on kidney disease progression [22]. However, this would seem unlikely to have a significant effect at very young ages, as seen in this family. The presence of modifying genes that may slow the progression of kidney disease or decrease production of the abnormal uromodulin (for example, angiotensin converting enzyme polymorphisms that decrease angiotensin production [23]) could alter the development of kidney disease. In addition, modifications in genes that result in increased effectiveness in removing the mutant uromodulin could be responsible for differences in the rate of progression.
This investigation provides the best longitudinal data reported to date on children with UMOD mutations. Other investigators have also described renal findings in individuals with UMOD mutations [8, 20, 24], but these studies have usually only included one or two measurements per child. Renal insufficiency of a similar degree has been noted in these families, although there is significant variation, with some families having only mildly decreased kidney function [18], while in one family the onset of end-stage kidney failure was noted in one child at age 6 years [20].
The use of allopurinol was not found to prevent the progression of kidney disease in this family. While the use of allopurinol in patients with UMOD mutations is well accepted for patients with gout, there is a significant debate as to whether allopurinol slows the progression of kidney disease. Some investigators believe that the initiation of allopurinol early in life will absolutely prevent progression [25], while others do not believe that allopurinol prevents progression at all [26, 27]. However, due to the small number of patients with disease, it is impossible to say at the present time whether allopurinol slows progression, or not. Compliance with medication, especially in childhood, is a factor that is difficult to determine in these families. Allopurinol itself is associated with interstitial nephritis [28], and its administration could lead to the progression of kidney disease. Our study shows progression of kidney failure in this family despite the use of allopurinol. However, it is not possible to determine if allopurinol slowed progression or if a higher dose of allopurinol and lower serum uric acid levels may have stopped progression.
The characteristics of this family are typical of those found in individuals with UMOD mutations. Renal insufficiency, hyperuricemia, and the autosomal dominant inheritance of kidney disease are primary features. Mutational analysis allows one to make the definitive diagnosis without requiring kidney biopsy.
Acknowledgments
The authors thank Victoria Robins, R.N. for her valuable help.
Contributor Information
Péter Schäffer, Department of Nephrology and Gastroenterology, Heim Pál Children’s Hospital, Budapest, Hungary.
Éva Gombos, Department of Nephrology and Gastroenterology, Heim Pál Children’s Hospital, Budapest, Hungary.
Krisztina Meichelbeck, Department of Nephrology and Gastroenterology, Heim Pál Children’s Hospital, Budapest, Hungary.
András Kiss, Department of Urology, Heim Pál Children’s Hospital, Budapest, Hungary.
P. Suzanne Hart, National Human Genome Research Institute, NIH Human Genome Project, Bethesda, MD, USA.
Anthony J. Bleyer, Email: ableyer@wfubmc.edu, Section on Nephrology, Wake Forest University School of Medicine, Medical Center Blvd, Winston-Salem, NC 27157, USA.
References
- 1.Hart TC, Gorry MC, Hart PS, Woodard AS, Shihabi Z, Sandhu J, Shirts B, Xu L, Zhu H, Barmada MM, Bleyer AJ. Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J Med Genet. 2002;39:882–892. doi: 10.1136/jmg.39.12.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gusmano R, Caridi G, Marini M, Perfumo F, Ghiggeri G, Piaggio G, Ceccherini I, Seri M. Glomerulocystic kidney disease in a family. Nephrol Dial Transplant. 2002;17:813–818. doi: 10.1093/ndt/17.5.813. [DOI] [PubMed] [Google Scholar]
- 3.Lens XM, Banet JF, Outeda P, Barrio-Lucia V. A novel pattern of mutation in uromodulin disorders: Autosomal dominant medullary cystic kidney disease type 2, familial juvenile hyperuricemic nephropathy, and autosomal dominant glomerulocystic kidney disease. Am J Kidney Dis. 2005;46:52–57. doi: 10.1053/j.ajkd.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Turner J, Stacey J, Harding B, Kotanko K, Lhotta J, Puig J, Roberts I, Torres R, Thakker R. Uromodulin mutations cause familial juvenile hyperuricemic nephropathy. J Clin Endocrinol Metab. 2003;88:1398–1401. doi: 10.1210/jc.2002-021973. [DOI] [PubMed] [Google Scholar]
- 5.Dahan K, Devuyst O, Smaers M, Vertommen D, Loute G, Poux J-M, Viron B, Jacquot C, Gagnadoux M-F, Chauveau D, Buchler M, Cochat P, Cosyns J-P, Mougenot B, Rider MH, Antignac C, Verellen-Dumoulin C, Pirson Y. A cluster of mutations in the UMOD gene causes familial juvenile hyperuricemic nephropathy with abnormal expression of uromodulin. J Am Soc Nephrol. 2003;14:2883–2893. doi: 10.1097/01.asn.0000092147.83480.b5. [DOI] [PubMed] [Google Scholar]
- 6.Serafini-Cessi F, Malagolini N, Cavallone D. Tamm-Horsfall glycoprotein biology and clinical relevance. Am J Kidney Dis. 2003;42:658–676. doi: 10.1016/s0272-6386(03)00829-1. [DOI] [PubMed] [Google Scholar]
- 7.Malagolini N, Cavallone D, Serafini-Cessi F. Intracellular transport, cell-surface exposure and release of recombinant Tamm-Horsfall glycoprotein. Kidney Int. 1997;52:1340–1350. doi: 10.1038/ki.1997.459. [DOI] [PubMed] [Google Scholar]
- 8.Scolari F, Caridi G, Rampoldi L, Tardanico R, Izzi C, Pirulli D, Amoroso A, Casari G, Ghiggeri GM. Uromodulin storage diseases: clinical aspects and mechanisms. Am J Kidney Dis. 2004;44:987–999. doi: 10.1053/j.ajkd.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Hoyer JR, Sisson SP, Vernier RL. Tamm-Horsfall glycoprotein ultrastructural immunoperoxidase localization in rat kidney. Lab Invest. 1979;41:168–173. [PubMed] [Google Scholar]
- 10.Raffi HS, Bates JM, Laszik Z, Kumar S. Tamm-Horsfall protein protects against urinary tract infection by proteus mirabilis. J Vasc Surg. 2009;181:2332–2338. doi: 10.1016/j.juro.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hess B. Tamm-Horsfall glycoprotein and calcium nephrolithiasis. Miner Electrolyte Metab. 2004;20:393–398. [PubMed] [Google Scholar]
- 12.Kottgen A, Glazer NL, Dehghan A, Hwang SJ, Katz R, Li M, Yang Q, Gudnason V, Launer LJ, Harris TB, Smith AV, Arking DE, Astor BC, Boerwinkle E, Ehret GB, Ruczinski I, Scharpf RB, Chen I, deBoer IH, Haritunians T, Lumley T. Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet. 2009;41:712–717. doi: 10.1038/ng.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hildebrandt F, Omram H. New insights: nephronophthisis-medullary cystic kidney disease. Pediatr Nephrol. 2001;16:168–176. doi: 10.1007/s004670000518. [DOI] [PubMed] [Google Scholar]
- 14.Wolf MTF, Karle SM, Schwarz S, Anlauf M, Anlauf M, Glaeser L, Kroiss S, Burton C, Feest T, Otto E, Fuchshuber A, Hilderbrant F. Refinement of the critical region for MCKD1 by detection of transcontinental haplotype sharing. Kidney Int. 2003;64:788–792. doi: 10.1046/j.1523-1755.2003.00161.x. [DOI] [PubMed] [Google Scholar]
- 15.Giselson N, Heinegard N, Holmberg C-G, Lindberg L-G, Lindstedt E, Lindstedt G, Schersten B. Renal medullary cystic disease or familial juvenile nephronophthisis: a renal tubular disease. Am J Med. 1970;48:174–184. doi: 10.1016/0002-9343(70)90113-0. [DOI] [PubMed] [Google Scholar]
- 16.Hildebrandt F, Otto E. Cilia and centrosomes: a unifying pathogenic concept for cystic kidney disease? Nat Rev Genet. 2006;6:928–940. doi: 10.1038/nrg1727. [DOI] [PubMed] [Google Scholar]
- 17.Wolf MTF, Mucha BE, Attanasio M, Zalewski I, Karle SM, Neumann HPH, Rahman N, Bader B, Baldamus CA, Otto E, Witzgall R, Fuchshuber A, Hildebrandt F. Mutations of the Uromodulin gene in MCKD type 2 patients cluster in exon 4, which encodes three EGF-like domains. Kidney Int. 2003;64:1580–1587. doi: 10.1046/j.1523-1755.2003.00269.x. [DOI] [PubMed] [Google Scholar]
- 18.Bleyer AJ, Woodard AS, Shihabi Z, Sandhu J, Zhu H, Satko SG, Weller N, Deterding E, McBride D, Gorry MC, Xu L, Ganier D, Hart TC. Clinical characterization of a family with a mutation in the uromodulin (Tamm-Horsfall glycoprotein) gene. Kidney Int. 2003;64:36–42. doi: 10.1046/j.1523-1755.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- 19.Choi SW, Ryu OH, Choi SJ, Song IS, Bleyer AJ, Hart TC. Mutant Tamm-Horsfall glycoprotein accumulation in the endoplasmic reticulum induces apoptosis that is reversed by colchicine and sodium 4-phenylbutyrate. J Am Soc Nephrol. 2005;16:3006–3014. doi: 10.1681/ASN.2005050461. [DOI] [PubMed] [Google Scholar]
- 20.Wolf MTE, Beck BB, Zaucke F, Kunze A, Misselwitz J, Ruley J, Ronda T, Fischer A, Eifinger F, Licht C, Otto E, Hoppe B, Hildebrandt F. The uromodulin C744G mutation causes MCKD2 and FJHN in children and adults and may be due to a possible founder effect. Kidney Int. 2007;71:574–581. doi: 10.1038/sj.ki.5002089. [DOI] [PubMed] [Google Scholar]
- 21.Yu KH, See LC, Huang YC, Yang CH, Sun JH. Dietary factors associated with hyperuricemia in adults. Semin Arthritis Rheum. 2007;37:243–250. doi: 10.1016/j.semarthrit.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, Striker G. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med. 1994;330:877–884. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- 23.Ruggenenti P, Bettinaglio P, Pinares F, Remuzzi G. Angiotensin converting enzyme insertion/deletion polymorphism and renoprotection in diabetic and nondiabetic nephropathies. Clin J Am Soc Nephrol. 2008;3:1511–1525. doi: 10.2215/CJN.04140907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bleyer AJ, Trachtman H, Sandhu J, Gorry MC, Hart TC. Renal manifestations of a mutation in the uromodulin (Tamm-Horsfall protein) gene. Am J Kidney Dis. 2003;42:1–7. doi: 10.1016/s0272-6386(03)00670-x. [DOI] [PubMed] [Google Scholar]
- 25.Fairbanks L, Cameron J, Venkata-Raman G, Rigden S, Rees L, van’t Hoff W, Mansell M, Pattison J, Goldsmith D, Simmonds H. Early treatment with allopurinol in familial juvenile hyerpuricaemic nephropathy (FJHN) ameliorates the long-term progression of renal disease. Q J Med. 2002;95:597–607. doi: 10.1093/qjmed/95.9.597. [DOI] [PubMed] [Google Scholar]
- 26.Moro F, Simmonds HA, Cameron JS, Ogg CS, Williams GD, McBride MB, Davis PM. Does allopurinol affect the progression of familial juvenile gouty nephropathy? Adv Exp Med Biol. 1991;309A:199–202. doi: 10.1007/978-1-4899-2638-8_45. [DOI] [PubMed] [Google Scholar]
- 27.Bleyer AJ, Hart TC. Familial juvenile hyperuricaemic nephropathy. Q J Med. 2003;96:867–868. doi: 10.1093/qjmed/hcg141. [DOI] [PubMed] [Google Scholar]
- 28.Parra E, Gota R, Gamen A, Moros M, Azuara M. Granulomatous interstitial nephritis secondary to allopurinol treatment. Clin Nephrol. 1995;43:350. [PubMed] [Google Scholar]