Abstract
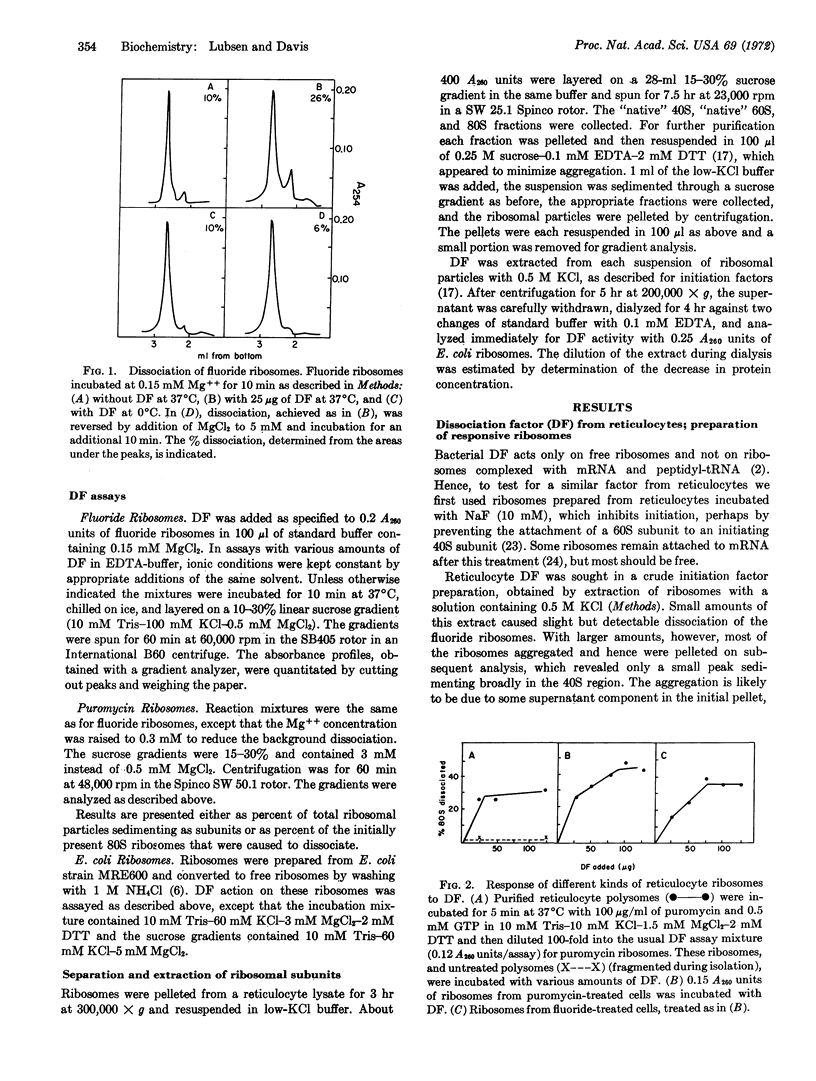
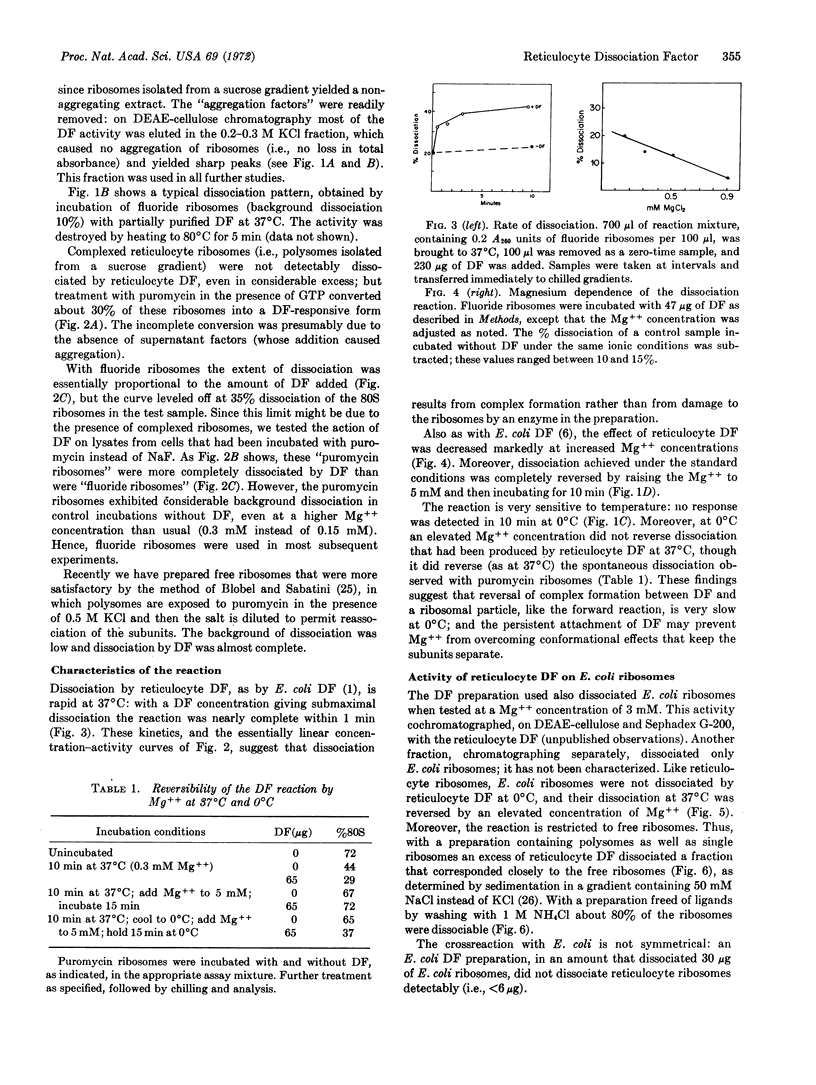
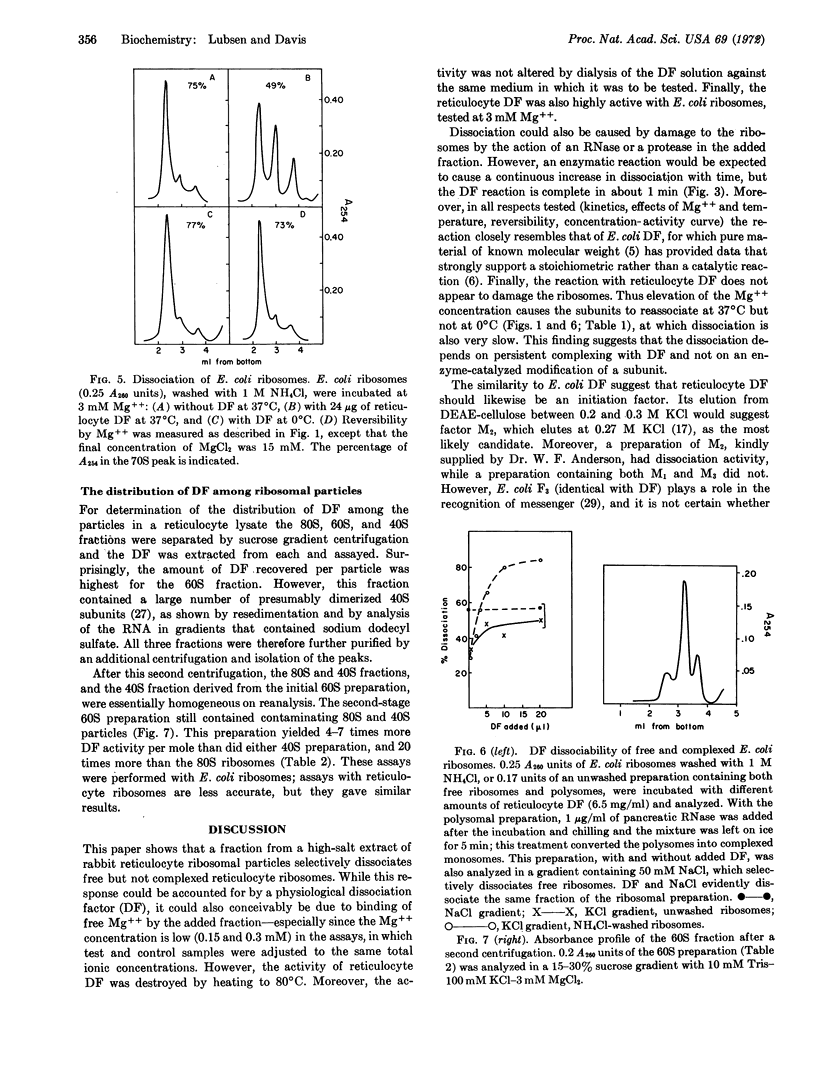
A ribosome dissociation factor has been detected in an extract of ribosomal particles from rabbit reticulocytes. This factor dissociates free ribosomes from reticulocytes and also from Escherichia coli; it does not dissociate ribosomes complexed with peptidyl-tRNA and mRNA. The reaction appears to be stoichiometric rather than catalytic; it reaches completion in one minute at 37°C, but is very slow at 0°C, and it is antagonized and reversed by Mg++. Reticulocyte dissociation factor thus closely resembles that from E. coli. However, the activity has been found primarily associated with the native large subunits rather than the small subunits in lysates.
Keywords: fluoride, E. coli ribosomes, initiation factors
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Algranati I. D., Gonzalez N. S., Bade E. G. Physiological role of 70S ribosomes in bacteria. Proc Natl Acad Sci U S A. 1969 Feb;62(2):574–580. doi: 10.1073/pnas.62.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beller R. J., Davis B. D. Selective dissociation of free ribosomes of Escherichia coli by sodium ions. J Mol Biol. 1971 Feb 14;55(3):477–485. doi: 10.1016/0022-2836(71)90331-7. [DOI] [PubMed] [Google Scholar]
- Blobel G., Sabatini D. Dissociation of mammalian polyribosomes into subunits by puromycin. Proc Natl Acad Sci U S A. 1971 Feb;68(2):390–394. doi: 10.1073/pnas.68.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarini C., Campo M. S., Andronico F. Ribosomal subunit exchange in Dictyostelium purpureum. J Cell Biol. 1970 Aug;46(2):428–430. doi: 10.1083/jcb.46.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo B., Vesco C., Baglioni C. Role of ribosomal subunits in protein synthesis in mammalian cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):651–658. doi: 10.1073/pnas.61.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal R. G., Shafritz D. A., Prichard P. M., Anderson W. F. Initial dipeptide formation in hemoglobin biosynthesis. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1810–1814. doi: 10.1073/pnas.68.8.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIBBLE W. E., DINTZIS H. M. The size and hydration of rabbit-reticulocyte ribosomes. Biochim Biophys Acta. 1960 Jan 1;37:152–153. doi: 10.1016/0006-3002(60)90093-7. [DOI] [PubMed] [Google Scholar]
- Davis B. D. Role of subunits in the ribosome cycle. Nature. 1971 May 21;231(5299):153–157. doi: 10.1038/231153a0. [DOI] [PubMed] [Google Scholar]
- Dubnoff J. S., Maitra U. Isolation and properties of polypeptide chain initiation factor FII from Escherichia coli: evidence for a dual function. Proc Natl Acad Sci U S A. 1971 Feb;68(2):318–323. doi: 10.1073/pnas.68.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstadt J. M., Brawerman G. The role of the native subribosomal particles of Escherichia coli in polypeptide chain initiation. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1560–1565. doi: 10.1073/pnas.58.4.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felicetti L., Di Matteo G. F. A supernatant fraction from rat liver capable of replacing G factor in a bacterial cell-free system. J Mol Biol. 1970 Dec 28;54(3):611–614. doi: 10.1016/0022-2836(70)90132-4. [DOI] [PubMed] [Google Scholar]
- Hoerz W., McCarty K. S. Initiation of protein synthesis in a rabbit reticulocyte lysate system. Biochim Biophys Acta. 1971 Jan 28;228(2):526–535. doi: 10.1016/0005-2787(71)90058-x. [DOI] [PubMed] [Google Scholar]
- Housman D., Jacobs-Lorena M., Rajbhandary U. L., Lodish H. F. Initiation of haemoglobin synthesis by methionyl-tRNA. Nature. 1970 Aug 29;227(5261):913–918. doi: 10.1038/227913a0. [DOI] [PubMed] [Google Scholar]
- Howard G. A., Adamson S. D., Herbert E. Subunit recycling during translation in a reticulocyte cell-free system. J Biol Chem. 1970 Nov 25;245(22):6237–6239. [PubMed] [Google Scholar]
- Iwasaki K., Sabol S., Wahba A. J., Ochoa S. Translation of the genetic message. VII. Role of initiation factors in formation of the chain initiation complex with Escherichia coli ribosomes. Arch Biochem Biophys. 1968 May;125(2):542–547. doi: 10.1016/0003-9861(68)90612-7. [DOI] [PubMed] [Google Scholar]
- Kaempfer R. Ribosomal subunit exchange in the cytoplasm of a eukaryote. Nature. 1969 Jun 7;222(5197):950–953. doi: 10.1038/222950a0. [DOI] [PubMed] [Google Scholar]
- Kohler R. E., Ron E. Z., Davis B. D. Significance of the free 70 s ribosomes in Escherichia coli extracts. J Mol Biol. 1968 Aug 28;36(1):71–82. doi: 10.1016/0022-2836(68)90220-9. [DOI] [PubMed] [Google Scholar]
- Krisko I., Gordon J., Lipmann F. Studies on the interchangeability of one of the mammalian and bacterial supernatant factors in protein biosynthesis. J Biol Chem. 1969 Nov 25;244(22):6117–6123. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lebleu B., Huez G., Burny A., Marbaix G. Evidence for a naF-resistant association between mRNA and ribosomes in rabbit reticulocytes. Biochim Biophys Acta. 1967 Mar 29;138(1):186–188. doi: 10.1016/0005-2787(67)90599-0. [DOI] [PubMed] [Google Scholar]
- Prichard P. M., Gilbert J. M., Shafritz D. A., Anderson W. F. Factors for the initiation of haemoglobin synthesis by rabbit reticulocyte ribosomes. Nature. 1970 May 9;226(5245):511–514. doi: 10.1038/226511a0. [DOI] [PubMed] [Google Scholar]
- Pétre J. Specific dissociation of ribosomes from Saccharomyces cerevisiae by a protein factor. Eur J Biochem. 1970 Jul;14(3):399–405. doi: 10.1111/j.1432-1033.1970.tb00303.x. [DOI] [PubMed] [Google Scholar]
- Rabinovitz M., Freedman M. L., Fisher J. M., Maxwell C. R. Translational control in hemoglobin syntheskis. Cold Spring Harb Symp Quant Biol. 1969;34:567–578. doi: 10.1101/sqb.1969.034.01.064. [DOI] [PubMed] [Google Scholar]
- Sabol S., Sillero M. A., Iwasaki K., Ochoa S. Purification and properties of initiation factor F3. Nature. 1970 Dec 26;228(5278):1269–1273. doi: 10.1038/2281269a0. [DOI] [PubMed] [Google Scholar]
- Shafritz D. A., Anderson W. F. Isolation and partial characterization of reticulocyte factors M1 and M2. J Biol Chem. 1970 Nov 10;245(21):5553–5559. [PubMed] [Google Scholar]
- Shafritz D. A., Prichard P. M., Gilbert J. M., Anderson W. F. Separation of two factors, M1 and M2, required for poly U dependent polypeptide synthesis by rabbit reticulocyte ribosomes at low magnesium ion concentration. Biochem Biophys Res Commun. 1970 Feb 20;38(4):721–727. doi: 10.1016/0006-291x(70)90641-8. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Marcker K. A. Cytoplasmic methionine transfer RNAs from eukaryotes. Nature. 1970 May 16;226(5246):607–610. doi: 10.1038/226607a0. [DOI] [PubMed] [Google Scholar]
- Subramanian A. R., Davis B. D. Activity of initiation factor F3 in dissociating Escherichia coli ribosomes. Nature. 1970 Dec 26;228(5278):1273–1275. doi: 10.1038/2281273a0. [DOI] [PubMed] [Google Scholar]
- Subramanian A. R., Davis B. D., Beller R. J. The ribosome dissociation factor and the ribosome-polysome cycle. Cold Spring Harb Symp Quant Biol. 1969;34:223–230. doi: 10.1101/sqb.1969.034.01.028. [DOI] [PubMed] [Google Scholar]
- Subramanian A. R., Ron E. Z., Davis B. D. A factor required for ribosome dissociation in Escherichia coli. Proc Natl Acad Sci U S A. 1968 Oct;61(2):761–767. doi: 10.1073/pnas.61.2.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro Y., Morimoto T. Sedimentation studies on the interaction of ribosomal subunits from liver. Biochim Biophys Acta. 1966 Sep;123(3):523–533. doi: 10.1016/0005-2787(66)90220-6. [DOI] [PubMed] [Google Scholar]


