Abstract
Translational stories range from straightforward to complex. In this commentary, the story of the rapid and successful translation of rituximab therapy for the treatment of non‐Hodgkin's lymphoma (NHL) is examined. Development of this monoclonal antibody therapy began in the late 1980s. In 1994, rituximab received its first approval for the treatment of NHL by the United States Food and Drug Administration (FDA). Rituximab has since been approved for additional indications and has transformed medical practice. However, the social and political implications of these rapid successes are only beginning to become clear. In this commentary, key events in the rapid translation of rituximab from the bench to bedside are highlighted and placed into this historical framework. To accomplish this, the story of rituximab is divided into the following six topics, which we believe to be widely applicable to case studies of translation: (1) underlying disease, (2) key basic science, (3) key clinical studies in translation, (4) FDA approval process, (5) changes to medical practice, and (6) the social and political influences on translation.
Keywords: oncology, hematology, hematopathology, lymphoma, leukemia, immunotherapy
Introduction
The translational process from bench to beside has been estimated to take 17 years.1 Some of these stories are famous, but others are forgotten or untold. For each story of success in translation, there are many more stories of failure. Even stories of success are filled with barriers to translation, which range from scientific and clinical to social and political. To accelerate this expensive process, the NIH created the Clinical and Translational Science Award (CTSA) program in 2006 and is targeting research funding with the goal of overcoming translational barriers.2, 3 In this context, the story of rituximab for the treatment of NHL represents one example of a contemporary story of rapid and successful translation. This story serves as a model and template for future translational discoveries.
Underlying Disease
Non‐Hodgkin's lymphoma (NHL) accounts for approximately 4% of all cancer cases in the Unites States.4 Since the 1970s, the incidence of NHL in the United States has increased by several percent per year across most age, race, and sex demographics.5 However, interpretation of the causality of this trend is confounded by changes in the diagnostic methods, treatment, and the incidence of HIV/AIDS. Although the initial clinical presentation of lymphoma may be symptomless, classical signs include lymph node enlargement, pancytopenia, and the constellation of B cell symptoms: fever, night sweats, and weight loss.6 Lymphoma staging is based on the Ann Arbor system (I–IV) and includes a modifier based on the presence or absence of B cell symptoms. Grading is based on standard histological criteria and scored from low to high. Treatments for lymphoma range from watchful waiting to chemotherapy, radiation therapy, and transplant (autologous or allogeneic).7 A classic chemotherapeutic approach, which is still commonly used in the treatment of lymphoma, is CHOP therapy, which consists of cyclophosphamide, doxorubicin (aka hydroxydaunorubicin), vincristine (brand name Oncovin), and prednisone. However, using variations of standard CHOP therapy, 5‐year survival rates have historically been poor, particularly in the case of aggressive NHL.8
NHL is further classified based upon growth rate. Slow growing NHL subtypes are classified as indolent, while fast growing subtypes are classified as aggressive. The most common indolent NHL is follicular lymphoma (FL), while the most common aggressive NHL is diffuse large B cell lymphoma (DLBCL). Chronic lymphocytic lymphoma (CLL) or small lymphocytic lymphoma, a related disease, is frequently considered an indolent NHL. However, it can progress to aggressive forms of NHL. The pathogenesis of each of these NHL subtypes is different. From a molecular standpoint, dysfunction in different stages of the B cell maturation process accounts for differences, though all of these B cell‐specific NHL subtypes share a common B cell origin.9 Additionally, nearly all B cell lymphomas are characterized by the presence of the B cell‐specific CD20 cell surface protein.10 This common feature is the basis for which rituximab therapy can be used to treat this wide range of conditions.
Key Basic Science
It is difficult to delineate where a story of translation actually begins (Figure 1). The historical foundation for monoclonal antibody therapy is hybridoma technology, which was developed in the 1970s and subsequently awarded the Nobel Prize in 1984.11 The first mouse antithymus antibody was developed and administered experimentally in a mouse model of leukemia by 1980.12 Around this time, early studies characterizing the toxicity of various antibody therapies were simultaneously conducted in humans. In one pilot safety study, a mouse anti‐lymphoma‐associated‐antigen antibody was administered to a single lymphoma patient.13 In another such study, a mouse anti‐B cell antibody was administered to one patient with B cell lymphoma.14 By 1985, an antibody was developed against B cell polypeptide 35kD (Bp35), which would eventually be renamed Cluster of Differentiation 20 (CD20).15 In 1987, the experimental administration of this mouse anti‐CD20 antibody to four patients with refractory, malignant B cell lymphoma was published.16 By this time, a mouse‐human chimeric anti‐CD20 antibody had also been developed by applying recombinant DNA technology to an existing mouse antibody.17 In vitro, this antibody was shown to mediate complement‐dependent cytotoxicity (CDC) and antibody‐dependent cell‐mediated cytotoxicity (ADCC) using human complement factors and effector cells, respectively. The mechanism of this B cell depletion is now understood to include (1) CDC, (2) ADCC, and (3) direct cellular lysis.18 In 1994, IDEC Pharmaceuticals Corporation demonstrated that another chimeric anti‐CD20 antibody, named IDEC‐C2B8, was capable of causing B cell depletion in monkeys.19 In 1997, this antibody, which would eventually be renamed rituximab (trade names Rituxan and MabThera), became the fourth antibody therapy approved by the Food and Drug Administration (FDA) in the United States.20
Figure 1.
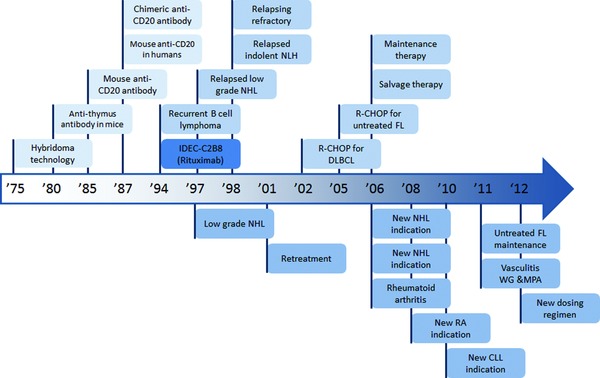
Historical timeline of key events in the translation of rituximab. Top: preclinical and clinical publication dates. Bottom: United States FDA approval dates.
Key Clinical Studies in Translation
Early clinical trials of rituximab focused on the treatment of indolent NHL. In 1994, the first phase I clinical trial of rituximab for patients with recurrent B cell lymphoma was published, demonstrating extremely promising results.21 The first phase II clinical trials of rituximab were reported a few years later for patients with indolent NHL. In one study, patients with relapsed, low grade NHL were treated with rituximab; in another, patients with relapsed, indolent lymphoma.22, 23 In 1998, the first phase II clinical trial was published that explored the use of rituximab in patients with relapsing or refractory aggressive lymphoma, including DLBCL and mantle cell lymphoma.24 In all of these phase II trials, results were extremely promising. By the mid‐2000s, the benefits of increasingly sophisticated dosing regimens of rituximab were investigated for patients with indolent NHL, including prolonged treatment of FL patients with rituximab and maintenance therapy versus retreatment at progression for patients with indolent NHL.25, 26 For both, results were extremely positive. Four years later, adding rituximab to standard CHOP chemotherapy (R‐CHOP) was demonstrated to improve outcomes compared to standard CHOP therapy in elderly patients with DLBCL.27 In 2005, R‐CHOP was demonstrated to significantly improve outcomes in patients with previously untreated FL in a phase III clinical trial.28
In subsequent phase III clinical trials, variations of earlier trials were performed to demonstrate the efficacy of rituximab for additional applications. Improvement in clinical outcome was shown in patients with relapsed/resistant FL who were treated with rituximab maintenance therapy.29 Another application is the addition of rituximab to a modified CHOP therapy in patients with previously untreated, advanced FL.30 Additionally, rituximab has shown promise as a salvage (or rescue) therapy for patients with relapsed NHL who fail standard therapies.31 These rapid and clinically successful applications of rituximab eventually led to its approval by the FDA for additional indications.
FDA Approval Process
All drugs must obtain approval in the United States through the FDA, which is a branch of the federal, cabinet‐level Department of Health and Human Services (Figure 2). Conventional drugs, such as small molecular inhibitors, must obtain approval through the FDA's Center for Drug Evaluation and Research. The approval process is different for biological products, which the FDA considers to include vaccines, blood, blood components, allergenics, somatic cells, gene therapy, tissues, and recombinant therapeutic proteins.32 Although there are similarities to the process for conventional drugs, approval of biological products must be obtained through the FDA's Center for Biologics Evaluation and Research (CBER).33 In 2009, the concept of biosimilars was introduced in the Biologics Price Competition and Innovation Act.34 According to this definition, a new biological product could be deemed biosimilar by demonstrating it to be “highly similar” to an already‐approved biological product. This Act was passed in 2010 as part of the larger Patient Protection and Affordable Care Act (PPACA). Importantly, for biological products, PPACA established a period of 12 years for data exclusivity, which is the time between original FDA approval and generic filing. This is in contrast to the period for conventional drugs, which is 5 years for a New Drug Application or 3 years following a New Drug Indication.35 As a recombinant therapeutic protein, rituximab antibody therapy received its first FDA approval through CBER in 1997 for relapsed or refractory, CD20+, B cell, low‐grade NHL.
Figure 2.
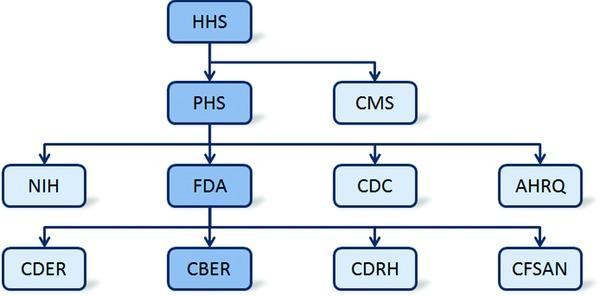
Overview of key administrative structures within the United States Department of Health and Human Services (HHS) and the FDA. Highlighted in dark blue is the Center for Biologics Evaluation and Research (CBER) pathway, which is specific to the approval process for rituximab. CDER = Center for Drug Evaluation and Research, CDRH = Center for Devices and Radiological Health, CFSAN = Center for Food Safety and Applied Nutrition.
Changes to Medical Practice
In 2001, rituximab received a second approval by the FDA for the retreatment of patients who relapsed following initial rituximab therapy.36 Rituximab has since received additional approvals for multiple chemotherapeutic uses. These include indications for DLBCL, CLL, and other advanced NHLs.37 As recently as 2012, rituximab received another approval for a modified dosing regimen for the treatment of NHL. As a therapeutic agent that specifically targets B cells, rituximab has potential for more widespread application as an immunosuppressant therapy. With this in mind, rituximab has recently been investigated extensively for the treatment of rheumatoid arthritis (RA).38, 39, 40 These studies proved extremely positive. As a consequence, in 2006, rituximab was approved by the FDA for the treatment of moderately and severely active RA in combination with methotrexate (a standard RA therapy). Two years later, rituximab received another RA‐related approval by the FDA.41 Although it is beyond the scope of this review, rituximab has been investigated for the treatment of other autoimmune, immune, and/or inflammatory diseases. These include multiple sclerosis,42 systemic lupus erythematosus (SLE),43 transplant rejection,44 Wegener's granulomatosis,45 and microscopic polyangiitis.46, 47 For treatment of the latter two diseases, rituximab received FDA approval in 2011.48 Importantly, with the impending expiration of data exclusivity on rituximab in the United States and Europe, there is now substantial interest from pharmaceutical companies in the development of biosimilars for the treatment of all of these disease.49
It should be noted that rituximab is not the only FDA‐approved, anti‐B cell antibody therapy. In 2009, ofatumumab (human anti‐CD20 antibody) was approved for the treatment of refractory CLL.50 The CD20 epitope it targets is distinct from the target of rituximab. In 2011, belimumab (human anti‐B cell activating factor) was approved for the treatment of SLE.51 Furthermore, ocrelizumab and obinutuzumab (humanized anti‐CD20 antibodies) are currently in Phase III clinical trials for the treatment of multiple sclerosis and NHL, respectively.52 The inevitable introduction of these and other antibody therapies to the biosimilar market is likely to have a substantial impact on the future of medical practice in the United States and around the world. In addition to driving competition and innovation, this market has the potential to increase the availability and decrease the cost of these drugs.
Social and Political Influences on Translation
Remarkably, rituximab faced no major social or political barriers in its initial or subsequent approval processes in the United States. Rituximab has also been approved by the European Commission and is currently recommended by the National Institute for Health and Clinical Excellence (NICE) in the United Kingdom as first‐line treatment for FL.53 Additionally, NICE has recommended rituximab for the treatment of RA after the failure of tumor necrosis factor antagonists.54 However, new challenges are beginning to arise. One is related to the cost of antibody therapy in general. Roche, the current and global patent holder of rituximab, has shown this therapy to be cost‐effective in combination with chemotherapy for the first‐line treatment of FL in the United Kingdom;55 despite this demonstration, the absolute cost of antibody therapy remains substantial. In the United States, this cost is on the order of tens of thousands of dollars per treatment.56 With this financial expense in mind, another challenge—more specific to rituximab therapy—is its increasingly widespread reappropriation for generalized immunosuppression therapy. In 2009, the first preliminary case series exploring the use of rituximab for the treatment of chronic fatigue syndrome (CFS) was published.57 Although the etiology of CFS is unclear, interest in this subject has continued to increase.58, 59 Further research will be necessary to clearly establish the efficacy of rituximab therapy for the treatment of CFS.
Finally, a new challenge is beginning to arise regarding the long‐term risks associated with rituximab therapy. In general, the toxicity and side effect profiles of rituximab have proven to be minimal. However, long‐term data on the growing number of survivors of rituximab therapy is only now becoming available. As a result, it is apparent that some small number of survivors develops progressive multifocal leukoencephalopathy in the months following rituximab therapy.60 The onset of this rare neuro‐inflammatory disease is usually rapid and fatal. Thus, for all of these reasons, there is likely to be growing social and political awareness of rituximab therapy in the near future.
Conclusion
NHL is a devastating disease, for which treatment was historically limited to CHOP therapy. However, key basic science discoveries in the 1980s allowed for the targeted depletion of B cells. As a result, rituximab was developed and key clinical studies in translational were performed over the course of the 1990s. As a biological product, the review and approval of rituximab in 1994 were handled by the FDA's CBER. Rituximab has transformed medical practice, receiving approval for additional NHL indications, as well as the treatment of several immune‐mediated diseases. However, the social and political implications of the cost of antibody therapy, as well as increasingly widespread applications of rituximab therapy, are only beginning to emerge in the United States and around the world. In spite of these challenges, the story of the rapid translational of rituximab serves as a model and template for future translational discoveries. In particular, these lessons can be applied to the rapidly expanding field of new and/or developing biological products.
Financial Support
This publication was supported by Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Conflicts of Interest
None.
Acknowledgements
We wish to thank Dr. John A. Lust (Mayo Clinic hematologist) for a helpful discussion with Andrew Harrison and Nassir Thalji on the history of rituximab therapy prior to the preparation of this manuscript. Andrew Harrison thanks the Medical Scientist Training Program (MSTP) at Mayo Clinic for fostering an outstanding physician–scientist training environment.
References
- 1. Balas EA, Boren SA. Managing clinical knowledge for health care improvement. Yearb Med Inform. 2000; 2000: 65–70. [PubMed] [Google Scholar]
- 2. Westfall JM, Mold J, Fagnan L. Practice‐based research–“Blue Highways” on the NIH roadmap. JAMA 2007; 297(4): 403–406. [DOI] [PubMed] [Google Scholar]
- 3. Woolf SH. The meaning of translational research and why it matters. JAMA 2008; 299(2): 211–213. [DOI] [PubMed] [Google Scholar]
- 4. Muller AM, Ihorst G, Mertelsmann R, Engelhardt M. Epidemiology of non‐Hodgkin's lymphoma (NHL): trends, geographic distribution, and etiology. Ann Hematol. 2005; 84(1): 1–12. [DOI] [PubMed] [Google Scholar]
- 5. Clarke CA, Glaser SL. Changing incidence of non‐Hodgkin lymphomas in the United States. Cancer. 2002; 94(7): 2015–2023. [DOI] [PubMed] [Google Scholar]
- 6. Shankland KR, Armitage JO, Hancock BW. Non‐Hodgkin lymphoma. Lancet. 2012; 380(9844): 848–857. [DOI] [PubMed] [Google Scholar]
- 7. Ansell SM, Armitage J. Non‐Hodgkin lymphoma: diagnosis and treatment. Mayo Clinic Proceedings. Mayo Clinic. 2005; 80(8): 1087–1097. [DOI] [PubMed] [Google Scholar]
- 8. Shipp M. A predictive model for aggressive non‐Hodgkin's lymphoma. The International Non‐Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med. 1993; 329(14): 987–994. [DOI] [PubMed] [Google Scholar]
- 9. Nogai H, Dorken B, Lenz G. Pathogenesis of non‐Hodgkin's lymphoma. J Clin Oncol. 2011; 29(14): 1803–1811. [DOI] [PubMed] [Google Scholar]
- 10. Hennessy BT, Hanrahan EO, Daly PA. Non‐Hodgkin lymphoma: an update. Lancet Oncol. 2004; 5(6): 341–353. [DOI] [PubMed] [Google Scholar]
- 11. Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975; 256(5517): 495–497. [DOI] [PubMed] [Google Scholar]
- 12. Bernstein ID, Tam MR, Nowinski RC. Mouse leukemia: therapy with monoclonal antibodies against a thymus differentiation antigen. Science. 1980; 207(4426): 68–71. [DOI] [PubMed] [Google Scholar]
- 13. Nadler LM, Stashenko P, Hardy R, Kaplan WD, Button LN, Kufe DW, Antman KH, Schlossman SF. Serotherapy of a patient with a monoclonal antibody directed against a human lymphoma‐associated antigen. Cancer Res. 1980; 40(9): 3147–3154. [PubMed] [Google Scholar]
- 14. Miller RA, Maloney DG, Warnke R, Levy R. Treatment of B‐cell lymphoma with monoclonal anti‐idiotype antibody. N Engl J Med. 1982; 306(9): 517–522. [DOI] [PubMed] [Google Scholar]
- 15. Clark EA, Shu G, Ledbetter JA. Role of the Bp35 cell surface polypeptide in human B‐cell activation. Proc Natl Acad Sci USA. 1985; 82(6): 1766–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Press OW, Appelbaum F, Ledbetter JA, Martin PJ, Zarling J, Kidd P, Thomas ED. Monoclonal antibody 1F5 (anti‐CD20) serotherapy of human B cell lymphomas. Blood. 1987; 69(2): 584–591. [PubMed] [Google Scholar]
- 17. Liu AY, Robinson RR, Murray ED, Jr. , Ledbetter JA, Hellstrom I, Hellstrom KE. Production of a mouse‐human chimeric monoclonal antibody to CD20 with potent Fc‐dependent biologic activity. J Immunol. 1987; 139(10): 3521–3526. [PubMed] [Google Scholar]
- 18. Taylor RP, Lindorfer MA. Drug insight: the mechanism of action of rituximab in autoimmune disease–the immune complex decoy hypothesis. Nat Clin Pract Rheumatol. 2007; 3(2): 86–95. [DOI] [PubMed] [Google Scholar]
- 19. Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, Newman RA, Hanna N, Anderson DR. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994; 83(2): 435–445. [PubMed] [Google Scholar]
- 20. Waldmann TA. Immunotherapy: past, present and future. Nat Med. 2003; 9(3): 269–277. [DOI] [PubMed] [Google Scholar]
- 21. Maloney DG, Liles TM, Czerwinski DK, Waldichuk C, Rosenberg J, Grillo‐Lopez A, Levy R. Phase I clinical trial using escalating single‐dose infusion of chimeric anti‐CD20 monoclonal antibody (IDEC‐C2B8) in patients with recurrent B‐cell lymphoma. Blood. 1994; 84(8): 2457–2466. [PubMed] [Google Scholar]
- 22. Maloney DG, Grillo‐Lopez AJ, White CA, Bodkin D, Schilder RJ, Neidhart JA, Janakiraman N, Foon KA, Liles TM, Dallaire BK, et al. IDEC‐C2B8 (Rituximab) anti‐CD20 monoclonal antibody therapy in patients with relapsed low‐grade non‐Hodgkin's lymphoma. Blood. 1997; 90(6): 2188–2195. [PubMed] [Google Scholar]
- 23. McLaughlin P, Grillo‐Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, Heyman MR, Bence‐Bruckler I, White CA, Cabanillas F, et al. Rituximab chimeric anti‐CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four‐dose treatment program. J Clin Oncol. 1998; 16(8): 2825–2833. [DOI] [PubMed] [Google Scholar]
- 24. Coiffier B, Haioun C, Ketterer N, Engert A, Tilly H, Ma D, Johnson P, Lister A, Feuring‐Buske M, Radford JA, et al. Rituximab (anti‐CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: a multicenter phase II study. Blood. 1998; 92(6): 1927–1932. [PubMed] [Google Scholar]
- 25. Ghielmini M, Schmitz SF, Cogliatti SB, Pichert G, Hummerjohann J, Waltzer U, Fey MF, Betticher DC, Martinelli G, Peccatori F, et al. Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event‐free survival and response duration compared with the standard weekly x 4 schedule. Blood. 2004; 103(12): 4416–4423. [DOI] [PubMed] [Google Scholar]
- 26. Hainsworth JD, Litchy S, Shaffer DW, Lackey VL, Grimaldi M, Greco FA. Maximizing therapeutic benefit of rituximab: maintenance therapy versus re‐treatment at progression in patients with indolent non‐Hodgkin's lymphoma–a randomized phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol. 2005; 23(6): 1088–1095. [DOI] [PubMed] [Google Scholar]
- 27. Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large‐B‐cell lymphoma. N Engl J Med. 2002; 346(4): 235–242. [DOI] [PubMed] [Google Scholar]
- 28. Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, Reiser M, Metzner B, Harder H, Hegewisch‐Becker S, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced‐stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low‐Grade Lymphoma Study Group. Blood. 2005; 106(12): 3725–3732. [DOI] [PubMed] [Google Scholar]
- 29. van Oers MH, Klasa R, Marcus RE, Wolf M, Kimby E, Gascoyne RD, Jack A, Van't Veer M, Vranovsky A, Holte H, et al. Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non‐Hodgkin lymphoma in patients both with and without rituximab during induction: results of a prospective randomized phase 3 intergroup trial. Blood. 2006; 108(10): 3295–3301. [DOI] [PubMed] [Google Scholar]
- 30. Marcus R, Imrie K, Solal‐Celigny P, Catalano JV, Dmoszynska A, Raposo JC, Offner FC, Gomez‐Codina J, Belch A, Cunningham D, et al. Phase III study of R‐CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol. 2008; 26(28): 4579–4586. [DOI] [PubMed] [Google Scholar]
- 31. Hess G, Flohr T, Kolbe K, Bonn S, Schuler M, Derigs HG, Huber C. Effect of rituximab on the long‐term outcome after high‐dose therapy for relapsed B‐cell non‐Hodgkin's lymphoma. Ann Hematol. 2006; 85(11): 769–779. [DOI] [PubMed] [Google Scholar]
- 32. What is a biological product? 01/04/2010 ed: U.S. Food and Drug Administration; 2010.
- 33. Mullard A. 2010 FDA drug approvals. Nat Rev Drug Discov. 2011; 10(2): 82–85. [DOI] [PubMed] [Google Scholar]
- 34. Carver KH, Elikan J, Lietzan E. Unofficial Legislative History of the Biologics Price Competition and Innovation Act 2009, Food Drug Law J. 2010; 65: 671–818. [PubMed] [Google Scholar]
- 35. Grabowski H, Long G, Mortimer R. Data exclusivity for biologics. Nat Rev Drug Discov. 2011; 10(1): 15–16. [DOI] [PubMed] [Google Scholar]
- 36. Gurcan HM, Keskin DB, Stern JN, Nitzberg MA, Shekhani H, Ahmed AR. A review of the current use of rituximab in autoimmune diseases. Int Immunopharmacol. 2009; 9(1): 10–25. [DOI] [PubMed] [Google Scholar]
- 37.FDA Approval for Rituximab. 10/22/2012 ed: National Cancer Institute at the National Institutes of Health; 2012.
- 38. Edwards JC, Szczepanski L, Szechinski J, Filipowicz‐Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T. Efficacy of B‐cell‐targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004; 350(25): 2572–2581. [DOI] [PubMed] [Google Scholar]
- 39. Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, Keystone EC, Loveless JE, Burmester GR, Cravets MW, et al. Rituximab for rheumatoid arthritis refractory to anti‐tumor necrosis factor therapy: results of a multicenter, randomized, double‐blind, placebo‐controlled, phase III trial evaluating primary efficacy and safety at twenty‐four weeks. Arthritis Rheum. 2006; 54(9): 2793–2806. [DOI] [PubMed] [Google Scholar]
- 40. Emery P, Fleischmann R, Filipowicz‐Sosnowska A, Schechtman J, Szczepanski L, Kavanaugh A, Racewicz AJ, van Vollenhoven RF, Li NF, Agarwal S, et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double‐blind, placebo‐controlled, dose‐ranging trial. Arthritis Rheum. 2006; 54(5): 1390–1400. [DOI] [PubMed] [Google Scholar]
- 41. Furst DE, Keystone EC, So AK, Braun J, Breedveld FC, Burmester GR, De Benedetti F, Dörner T, Emery P, Fleischmann R, et al. Updated consensus statement on biological agents for the treatment of rheumatic diseases, 2012. Ann Rheum Dis. 2013; 72 (Suppl 2): ii2–ii34. [DOI] [PubMed] [Google Scholar]
- 42. Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar‐Or A, Panzara M, Sarkar N, Agarwal S, et al. B‐cell depletion with rituximab in relapsing‐remitting multiple sclerosis. N Engl J Med. 2008; 358(7): 676–688. [DOI] [PubMed] [Google Scholar]
- 43. Looney RJ, Anolik JH, Campbell D, Felgar RE, Young F, Arend LJ, Sloand JA, Rosenblatt J, Sanz I. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose‐escalation trial of rituximab. Arthritis Rheum. 2004; 50(8): 2580–2589. [DOI] [PubMed] [Google Scholar]
- 44. Vo AA, Lukovsky M, Toyoda M, Wang J, Reinsmoen NL, Lai CH, Peng A, Villicana R, Jordan SC. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med. 2008; 359(3): 242–251. [DOI] [PubMed] [Google Scholar]
- 45. Keogh KA, Ytterberg SR, Fervenza FC, Carlson KA, Schroeder DR, Specks U. Rituximab for refractory Wegener's granulomatosis: report of a prospective, open‐label pilot trial. Am J Respir. Crit. Care Med. 2006; 173(2): 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, Kallenberg CG, St Clair EW, Turkiewicz A, Tchao NK, et al. Rituximab versus cyclophosphamide for ANCA‐associated vasculitis. N Engl J Med. 2010; 363(3): 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jones RB, Tervaert JW, Hauser T, Luqmani R, Morgan MD, Peh CA, Savage CO, Segelmark M, Tesar V, van Paassen P, et al. Rituximab versus cyclophosphamide in ANCA‐associated renal vasculitis. N Engl J Med. 2010; 363(3): 211–220. [DOI] [PubMed] [Google Scholar]
- 48. FDA approves Rituxan to treat two rare disorders. 04/19/2011 ed: U.S Food and Drug Administration; 2011.
- 49. Kresge N. Roche Faces New Foe as Boehringer Plans a Rituxan Copy. 10/05/2012 ed: Bloomberg; 2012.
- 50. Nelson AL, Dhimolea E, Reichert JM. Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov. 2010; 9(10): 767–774. [DOI] [PubMed] [Google Scholar]
- 51. Landmark lupus approval opens door for next wave of drugs. Nat Rev Drug Discov. 2011; 10(4): 243–245. [DOI] [PubMed] [Google Scholar]
- 52. Reichert JM. Which are the antibodies to watch in 2013? MAbs. 2013; 5(1): 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Doss S, Garrett Z, Sutcliffe F, Stevens A. NICE guidance on rituximab for first‐line treatment of symptomatic stage III‐IV follicular lymphoma in previously untreated patients. Lancet Oncol. 2012; 13(2): 128–130. [DOI] [PubMed] [Google Scholar]
- 54. Kiely PD, Deighton C, Dixey J, Ostor AJ. Biologic agents for rheumatoid arthritis–negotiating the NICE technology appraisals. Rheumatology (Oxford). 2012; 51(1): 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ray JA, Carr E, Lewis G, Marcus R. An evaluation of the cost‐effectiveness of rituximab in combination with chemotherapy for the first‐line treatment of follicular non‐Hodgkin's lymphoma in the UK. Value Health. 2010; 13(4): 346–357. [DOI] [PubMed] [Google Scholar]
- 56. Shaughnessy AF. Monoclonal antibodies: magic bullets with a hefty price tag. BMJ 2012; 345: e8346. [DOI] [PubMed] [Google Scholar]
- 57. Fluge O, Mella O. Clinical impact of B‐cell depletion with the anti‐CD20 antibody rituximab in chronic fatigue syndrome: a preliminary case series. BMC Neurol. 2009; 9: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fluge O, Bruland O, Risa K, et al. Benefit from B‐lymphocyte depletion using the anti‐CD20 antibody rituximab in chronic fatigue syndrome. A double‐blind and placebo‐controlled study. PLoS One. 2011; 6(10): e26358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bradley AS, Ford B, Bansal AS. Altered functional B cell subset populations in patients with chronic fatigue syndrome compared to healthy controls. Clin Exp Immunol. 2013; 172(1): 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Carson KR, Evens AM, Richey EA, Habermann TM, Focosi D, Seymour JF, Laubach J, Bawn SD, Gordon LI, Winter JN, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV‐negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood 2009; 113(20): 4834–4840. [DOI] [PMC free article] [PubMed] [Google Scholar]


