Abstract
A comparison of the effectiveness of whole house (point-of-entry) and point-of-use arsenic water treatment systems in reducing arsenic exposure from well water was conducted. The non-randomized observational study recruited 49 subjects having elevated arsenic in their residential home well water in New Jersey. The subjects obtained either point-of-entry or point-of-use arsenic water treatment. Prior ingestion exposure to arsenic in well water was calculated by measuring arsenic concentrations in the well water and obtaining water-use histories for each subject, including years of residence with the current well and amount of water consumed from the well per day. A series of urine samples were collected from the subjects, some starting before water treatment was installed and continuing for at least nine months after treatment had begun. Urine samples were analyzed and speciated for inorganic-related arsenic concentrations. A two-phase clearance of inorganic-related arsenic from urine and the likelihood of a significant body burden from chronic exposure to arsenic in drinking water were identified. After nine months of water treatment the adjusted mean of the urinary inorganic-related arsenic concentrations were significantly lower (p < 0.0005) in the point-of-entry treatment group (2.5 μg/g creatinine) than in the point-of-use treatment group (7.2 μg/g creatinine). The results suggest that whole house arsenic water treatment systems provide a more effective reduction of arsenic exposure from well water than that obtained by point-of-use treatment.
Keywords: arsenic, water treatment, point-of-entry, point-of-use, body burden, New Jersey
1. Introduction
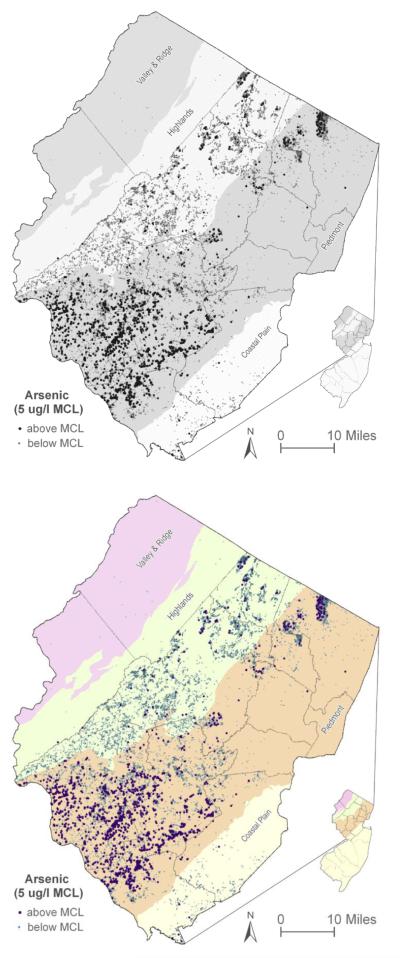
The New Jersey Department of Environmental Protection (NJDEP) has set the New Jersey maximum contaminant level (MCL) for arsenic at 5 μg/L (NJDEP, 2004), which is currently the most protective arsenic drinking water standard in the world. In New Jersey private wells, arsenic exceeds the maximum contaminant level at a higher percentage (11.8%) than all other contaminants with primary drinking water standards (NJDEP, 2008). In some New Jersey communities in the Piedmont Physiographic Province, over 40% of the private wells tested for arsenic under New Jersey’s Private Well Testing Act (PWTA) exceed the MCL (NJDEP, 2012). As shown in Figure 1, between September 2002 and April 2007, New Jersey’s PWTA Program identified 1,445 out of 12,263 private wells tested for arsenic exceeding the New Jersey MCL in the northern counties of the state (NJDEP, 2008). A substantial number of public community and public non community wells also have arsenic exceeding the MCL. Concentrations of arsenic in New Jersey well water can be as high as 400 μg/L. The arsenic in New Jersey well water is predominantly naturally occurring in specific geologic settings and has been found to occur in two inorganic species: arsenate (AsV) and arsenite (AsIII) (Serfes et al., 2005).
Figure 1.
Private Wells Exceeding 5 μg/L Arsenic in Northern NJ (2002-2007)
A variety of special water treatment systems can remove arsenic from drinking water, and can be configured to treat all the water in the home (whole house) or just water at a single tap for drinking and cooking (AWWA Research Foundation, 2005; Spayd, 2007). The available arsenic treatment technologies include adsorption processes such as granular iron, activated alumina, and titanium based medias; anion exchange resins; hybrid media that contains iron impregnated anion resin, and membrane processes such as reverse osmosis (AWWA Research Foundation, 2005; NJDEP, 2012; Spayd, 2007).
The goal of treating arsenic-contaminated water is to reduce arsenic levels in the water below the MCL and as close as possible to the maximum contaminant level goal of zero, and thus reduce the risk of cancer and many other health problems associated with arsenic exposure. The NJDEP is conducting a study of the effectiveness of various arsenic water treatment systems, and is evaluating both whole-house water treatment systems, commonly referred to as point-of-entry (POE) treatment, and single faucet treatment options for treating only drinking and cooking water, commonly referred to as point-of-use (POU) treatment. This study has been very successful with most treatment systems reducing arsenic to levels below 3 μg/L, and many systems reducing the arsenic level to below 1 μg/L.
In homes with POE water treatment, all water taps in the home provide treated water. In homes with POU water treatment, typically only one water tap in the home, usually at the kitchen sink, provides treated water. In the POU homes, the opportunity for family members to ingest water from untreated taps in the home is very high.
Though drinking and cooking with arsenic contaminated water is obviously the main exposure pathway in the home, the lack of data on exposure to arsenic via a household water supply from uses other than drinking and cooking (e.g., bathing, brushing teeth, etc.) is a major data gap. In the final federal arsenic MCL rule, published in January 2001, EPA was not able to assess inhalation or dermal pathways. At the time of adoption of the arsenic MCL rule, EPA stated that exposure by modes other than consumption were not a concern (USEPA, 2001). Hence, the final rule allows POU treatment as an acceptable technology for arsenic exposure reduction.
Arsenic water treatment systems are expensive in New Jersey with the average cost of installing a POE treatment system at $2,740 and a POU treatment system at $365 based on a cost survey conducted in 2003 (Spayd, 2007). Maintenance costs are also higher for POE systems at just under $1.00 per day whereas the POU system maintenance is about $0.33 per day for each tap treated. Due to this cost difference, many families faced with the need to treat their water for arsenic opt for the less expensive POU treatment system.
The NJDEP study is evaluating both POE and POU water treatment systems, and this provided an opportunity to compare overall exposure reduction via the two types of treatment systems. Based on a literature review, it appears this is the first published study to compare the effectiveness of POE and POU water treatment systems in reducing exposure from arsenic or any other contaminant in residential well water.
2. Materials and methods
2.1. Selection of wells and subjects
As the NJDEP study of arsenic in ground water and the effectiveness of various arsenic water treatment systems proceeded, owners of wells with elevated arsenic concentrations were asked to participate in a study of arsenic water treatment and biomonitoring. Subject recruitment and the study protocol were reviewed and approved by the Institutional Review Board of the University of Medicine and Dentistry of New Jersey. All participation was voluntary and written informed consent was obtained.
2.2. Water treatment technologies used
POE water treatment systems were installed in 12 homes where 31 subjects resided. The POE systems were predominantly adsorption media based systems and included seven using Adedge AD33 granular ferric oxide media (also known as Bayoxide E33), one with Apyron Aqua-Bind MP granular metal oxide composite media, and one with Aquatic Treatment System proprietary granular adsorption media. Two strong-base anion exchange systems were also used.
The typical POE adsorption system installation was a lead/lag or worker tank/safety tank system including a minimum of two 10-inch by 44-inch fiberglass tanks with one-cubic foot of adsorption media (Spayd, 2007).
This type of POE system consists of a shut-off valve, raw water sampling tap, 5-micron sediment pre-filter, flow meter, two adsorption tanks, backwash control valves on each tank, a sampling tap between and after the tanks, and a shut-off valve after the system. This is the preferred arsenic water treatment system design in New Jersey (Spayd, 2007). This type of system is thoroughly backwashed before being placed into service, and the backwash valves are set to backwash the media at least once per month, each tank on a separate day. The backwash line is piped to a suitable disposal location according to local plumbing codes; however, the backwash water from an adsorption system runs through the treatment media and therefore contains very low concentrations of arsenic.
The POE anion exchange treatment systems included one cubic foot of strong-base anion exchange resin in 10-inch by 44-inch fiberglass tanks. These tanks were set to regenerate with salt brine based on the sulfate content in the water. AsV in the well water is exchanged for chloride bound to the resin. If the system is not regenerated on the proper schedule or if the salt level is not maintained, the system will allow the accumulated arsenic attached to the resin to dump into the treated water at concentrations higher than in the raw water. The regeneration discharge water from anion exchange systems contains high levels of arsenic and a proper disposal location is critical. In addition, anion exchange systems only remove AsV, an anion. They do not remove AsIII, which is uncharged at normal pH. When problems with anion exchange for arsenic removal became apparent, NJDEP began to discourage the use of anion exchange for arsenic removal in New Jersey. This was based on the critical importance of the maintenance required to be conducted by the homeowner for anion exchange systems to prevent arsenic dumping into the treated water, the limitation of the system in removing only AsV, and the problem of extracted arsenic disposal to the environment near the home (Spayd, 2007).
POU water treatment systems were installed in nine homes where 20 subjects resided. The POU systems were predominantly adsorption media based systems and included two using Adedge AD33 granular ferric oxide media, two using Multi-Pure granular ferric oxide impregnated carbon block media, two using Isolux zirconium media, and one using titanium based media. One home used a POU reverse osmosis system. One final home with two subjects did not install a treatment system, but used bottled water for all drinking and cooking as a surrogate for a POU treatment system.
At a few homes, pre-treatment of the water was required for iron, manganese, and/or hardness.
2.3. Water treatment monitoring and analysis
Throughout the project, arsenic levels were regularly measured in both the raw water entering the home from the well and the treated water. Water samples were routinely collected by NJDEP, at approximately the same schedule as urine sample collection. These water samples were analyzed by an NJDEP lab certified to analyze drinking water for arsenic via EPA Method 200.8 - Inductively Coupled Plasma – Mass Spectrometry (ICP-MS). Water samples were occasionally split from NJDEP as a quality control procedure and analyzed by ICP-MS at the Environmental and Occupational Health Sciences Institute (EOHSI) Chemical Analysis laboratory in Piscataway, New Jersey.
2.4. Biomonitoring protocol, questionnaire, and sample analyses
The biomonitoring protocol was previously described in detail (Spayd et al., 2012). Briefly, a series of first morning void urine samples were collected from the subjects, some starting before water treatment was installed, and continuing for at least nine months. Based on in-person interviews at the time of subject enrollment, a household water use and exposure history questionnaire was completed for each subject by the investigator as previously described (Spayd et al., 2012). Urine samples were analyzed at EOHSI for total arsenic, inorganic-related arsenic (AsV + AsIII + their methylated metabolites), and creatinine as previously described (Spayd et al., 2012; Xie et al., 2007).
2.5. Data analysis
Statistical analyses were run using SPSS Version 15.0 for Windows. Scatter plots and bivariate Pearson correlation coefficients were used to examine relationships between variables. Paired t-tests were used to compare means of initial and final biomonitoring data within groups and independent t-tests were used to compare means between the groups.
In a non-randomized observational study like this one, there was little or no control over the assignment of treatment group. Therefore, the resulting POE and POU treatment groups may have large biases on some of the observed covariates. The propensity score, in this case defined as the conditional probability of being in one treatment group or the other given the covariates, can be used to balance the covariates, reduce bias, and create a quasi-randomized experiment (D’Agostino, 1998). Propensity scores were calculated for each subject using predicted probabilities from the results of a logistic regression model with the binary dependent variable being treatment group and covariates including prior arsenic ingestion dose per body weight, age, and showers per week at home. These covariates were chosen using stepwise selection methods such that prior arsenic ingestion dose per body weight, age, and showers per week at home were significant at the 0.10 level. The propensity scores are entered as adjustment covariates in the analyses examining the effects of treatment group on urinary arsenic.
The analysis must also account for the potential correlation of data within families because some of the subjects were family members sharing the same well and water treatment system. If only a standard statistical analysis was used (e.g., analysis of covariance), which assumes all observations are independent, the results may be misleading (Ghisletta and Spini, 2004; Hanley et al., 2003). Generalized estimating equations (GEE) are an extension of the basic generalized linear model, and were developed to accommodate the analysis of correlated data (Ghisletta and Spini, 2004; Hanley et al., 2003). GEE provide population-averaged estimates of regression coefficients. Therefore, GEE were used to examine the association between urinary arsenic and urinary arsenic reduction, by treatment group, at nine months after the subjects stopped drinking the water or obtained water treatment, while controlling for correlation among family members and including propensity scores as a covariate.
3. Results
3.1. Wells and subjects
Characteristics of the overall study population were previously described (Spayd et al., 2012). Briefly, recruitment included 53 subjects in 22 families with elevated arsenic concentrations in their private well water, ranging from 8 to119 μg/L. Five colleagues with drinking water arsenic concentrations below 3 μg/L were also recruited as the Comparison Group. Four subjects were lost to follow-up or provided insufficient samples to be included in the analysis. The remaining 49 subjects (in 19 families) are referred to as the Exposed Group.
A subset of the Exposed Group, called the “Pre-Post Group”, was established for data analyses and includes subjects who provided both pre-treatment and post-treatment urine samples. This was necessary because during the NJDEP study, when arsenic concentrations above the MCL were identified, the well owners were notified by telephone. Many subjects then stopped using the water for drinking and cooking before they were enrolled in the biomonitoring study and collection of their initial urine samples. As a result, only 24 of the Exposed Group subjects (49%) were able to provide urine samples that allowed measurement of arsenic levels while they were still drinking and cooking with the arsenic-contaminated water.
To be included in the Pre-Post Group, the pre-treatment urine samples had to be collected before obtaining water treatment or ceasing to drink the water with elevated arsenic, and a nine-month post-treatment urine sample was also required. The Exposed Group and the Pre-Post Group were not significantly different from each other as previously described (Spayd et al., 2012). The Pre-Post Group subjects were then divided by treatment type (POE or POU) and their characteristics are shown in Table 1.
Table 1.
Characteristics of Subjects in the Pre-Post Group by Treatment Type
| Water Treatment Groups | POE | POU | Comparison |
|---|---|---|---|
| General | |||
| Subjects Per Water Treatment Group (n) | 16 | 8 | 5 |
| Families Per Water Treatment Group (n) | 8 | 4 | 5 |
| Race (% Caucasian) | 100 | 100 | 80 |
| Sex (% Male) | 63 | 50 | 100 |
| Age in Years, (Mean ± SE) | 31 ± 5 *† | 61 ± 3 * | 54 ± 4 |
| Children < 18 Years Old (%) | 38 | 0 | 0 |
| Weight in kg, (Mean ± SD) | 58 ± 8 † | 70 ± 5 | 83 ± 7 |
| Any Tobacco Use During Study (%) | 6 | 0 | 0 |
| Prior Water Ingestion Exposure (Mean ± SE) | |||
| Well Water As (μg/L) | 40 ± 8 † | 45 ± 7 † | 1.1 ± 0.5 |
| As Water Ingestion Reported (L/d) | 0.7 ± 0.1 * | 1.5 ± 0.4 * | 1.3 ± 0.6 |
| Years of Exposure | 6.8 ± 1.2 * | 27.0 ± 6.1 *† | 7 ± 5 |
| Cumulative As Ingestion Dose (mg) | 65 ± 19 *† | 482 ± 122 *† | 3 ± 2 |
| Ingestion Dose per Body Weight (mg/kg) | 1.3 ± 0.5 *† | 7.0 ± 1.8 *† | 0.05 ± 0.03 |
| Dermal Exposure (Mean ± SE) | |||
| Showers per Week at Home | 5.1 ± 0.7 | 6.4 ± 0.6 | 6.6 ± 0.5 |
| Baths in per Week at Home | 0.8 ± 0.4 | 0.03 ± 0.03 | 0 ± 0 |
| Teeth Brushing per Week at Home | 10.8 ± 1.1 † | 14.0 ± 1.3 | 14.4 ± 0.4 |
| Pool Use per Week During Season | 0.4 ± 0.3 | 0.4 ± 0.3 | 1.9 ± 1.4 |
| Dietary Exposure (Mean ± SE) | |||
| Seafood and Fish Meals per Week | 1.1 ± 0.2 | 1.8 ± 0.4 | 1.5 ± 0.4 |
| Mushrooms with Meals per Week | 0.5 ± 0.3 | 0.3 ± 0.2 | 0.3 ± 0.1 |
| Rice with Meals per Week | 1.5 ± 0.2 * | 0.5 ± 0.2 * | 2.0 ± 1.3 |
| Poultry with Meals per Week | 2.6 ± 0.4 | 1.6 ± 0.4 | 2.8 ± 0.6 |
| Urine Biomonitoring (Mean ± SE) | |||
| Creatinine (g/L) | 1.6 ± 0.1 | 1.5 ± 0.2 | 1.8 ± 0.2 |
| Arsenic in Urine (Geometric Mean ± SE) | |||
| Initial Inorganic-Related As (μg/g creatinine) | 9.0 ± 1.9 †# | 14.8 ± 4.8 †# | 1.5 ± 0.4 |
| Inorganic-Related As at Nine-Months (μg/g creatinine) | 3.4 ± 0.5 †# | 2.8 ± 1.4 # | |
| Inorganic-Related As Reduction (Mean μg/g creatinine) | 7.3 ± 2.1 | 13.8 ± 4.9 | |
| ANCOVA Adjusted As in Urinea (Est. Mean ± SE) | |||
| Inorganic-Related As at Nine-Months (μg/g creatinine) | 2.6 ± 0.7 * | 7.0 ± 1.2 * | |
| GEE Adjusted As in Urinea (Estimated Mean ± SE) | |||
| Inorganic-Related As at Nine-Months (μg/g creatinine) | 2.5 ± 0.6 * | 7.2 ± 0.8 * | |
| Inorganic-Related As Reduction (μg/g creatinine) | 10.2 ± 0.4 * | 8.1 ± 0.9 * |
p < 0.05, significant difference between POE and POU.
p < 0.05, significant difference from Control.
p < 0.05, significant difference within group between initial and final concentration.
Inorganic-related arsenic in final urine adjusted for propensity score and family correlation in GEE.
Comparison subjects provided only one urine sample.
In the Pre-Post Group, there are 16 POE subjects in eight families and eight POU subjects in four families. The difference in age between the two groups is significant with a p-value < 0.0005. Although the arsenic well water concentrations are not significantly different between the POE and POU groups, the amount of water ingested per day and years of exposure are both significantly higher in the POU Group and this results in a significantly higher mean arsenic ingestion dose and ingestion dose per body weight in the POU Group.
3.2. Water treatment effectiveness
Regular collection of raw and treated water samples throughout the study confirmed that both the POU and POE arsenic water treatment systems used in this study consistently and effectively reduced the arsenic concentrations in water to below 3 μg/L. Only at one home, a POE arsenic water treatment system had a temporary arsenic breakthrough during the biomonitoring time period that was identified by the NJDEP water treatment system monitoring program. The homeowners were notified by NJDEP to switch to bottled water until the problem with the treatment system was corrected. The urine data collected from the breakthrough time period was excluded from the statistical analyses.
3.3. Potential dermal and dietary arsenic exposure pathways
There were no significant differences in potential dermal exposure pathways between the Pre-Post POE and POU water treatment groups, including showers, baths, teeth brushing, and pool use per week (Table 1). There were no significant differences in potential dietary exposure pathways including seafood and fish, mushrooms, and poultry meals per week between the Pre-Post POE and POU water treatment groups. However, meals with rice per week were significantly greater (p = 0.005) in the POE Group (1.5 ± 0.2 meals per week) than in the POU Group (0.5 ± 0.2 meals per week) (Table 1).
3.4. Arsenic reduction in POE and POU Group urine samples
Urine samples were analyzed for both total arsenic and inorganic-related arsenic as previously described (Spayd et al., 2012). The urine results were corrected for hydration status using creatinine and are presented as μg/g creatinine.
The unadjusted geometric mean inorganic-related arsenic concentrations in the initial urine samples for the POE Group (9.0 ± 1.9 μg/g creatinine) and the POU Group (14.8 ± 4.8 μg/g creatinine) were not significantly different from each other, but both were significantly higher than in the Comparison Group at 1.5 ± 0.4 μg/g creatinine (with respective p-values of < 0.0005 and 0.024). The unadjusted geometric mean inorganic-related arsenic concentration in the nine-month urine samples for the POE Group (3.4 ± 0.5 μg/g creatinine) and the POU Group (2.8 ± 1.4 μg/g creatinine) were not significantly different from each other, but the POE Group was significantly higher than in the Comparison Group (p = 0.026). The unadjusted mean reduction in inorganic-related urinary arsenic from the initial sample to the nine-month sample was not significantly different between the POE (7.3 ± 2.1 μg/g creatinine) and POU (13.8 ± 4.9 μg/g creatinine) Groups when analyzed with an independent T-Test.
The within-group differences in the mean initial and mean nine-month urinary inorganic-related arsenic concentrations were significant for both the POE Group (p = 0.003) and the POU Group (p = 0.027).
A one-way between groups analysis of covariance (ANCOVA) was conducted to compare the effectiveness of treatment type (POE vs POU) on the inorganic-related urinary arsenic concentrations after nine months of treatment in the Pre-Post Group. The independent variable was treatment type, and the dependent variable was the inorganic-related urinary arsenic concentrations after nine months of treatment. The subject’s propensity scores, age, and natural log of cumulative arsenic ingestion dose per body weight, were used as covariates in this analysis. Preliminary checks were conducted to ensure that there was no violation of the assumptions of normality, linearity, homogeneity of variances, homogeneity of regression slopes, and reliable measurements of the variables. After adjusting for the covariates, there was a significant difference between the means of the inorganic-related urinary arsenic concentrations after nine months of treatment with the POE Group (2.6 ± 0.7 μg/g creatinine) significantly lower (p = 0.016) than the POU Group (7.0 ± 1.2 μg/g creatinine).
When GEE were used to examine the association between inorganic-related urinary arsenic concentrations after nine months of treatment in the Pre-Post Group, while controlling for correlation among family members, and the propensity score, age, natural log of cumulative arsenic ingestion dose per body weight, and showers per week as covariates, the significant difference between the POE and POU Groups was stronger (p < 0.0005). The GEE estimated means ± the SE of the nine-month inorganic-related urine arsenic concentrations were 2.5 ± 0.6 μg/g creatinine for the POE Group and 7.2 ± 0.8 μg/g creatinine for the POU Group.
The GEE analysis also found the mean reduction in inorganic-related urinary arsenic from the initial sample to the nine-month sample to be significantly different (p = 0.04) between the POE and POU Groups. The GEE estimated means ± the SE of the urinary inorganic-related arsenic reduction were 10.2 ± 0.4 μg/g creatinine for the POE Group and 8.1 ± 0.9 μg/g creatinine for the POU Group.
3.5. Clearance of urinary inorganic-related arsenic
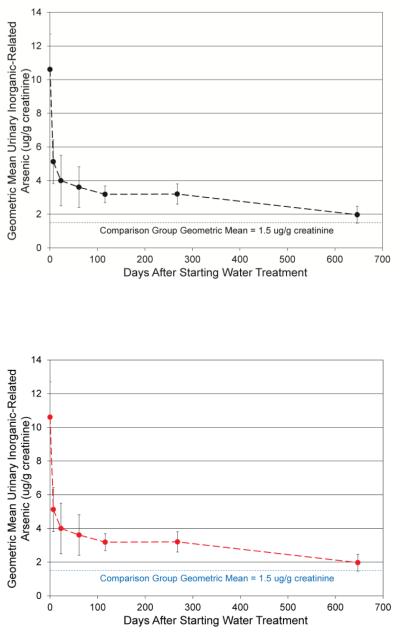
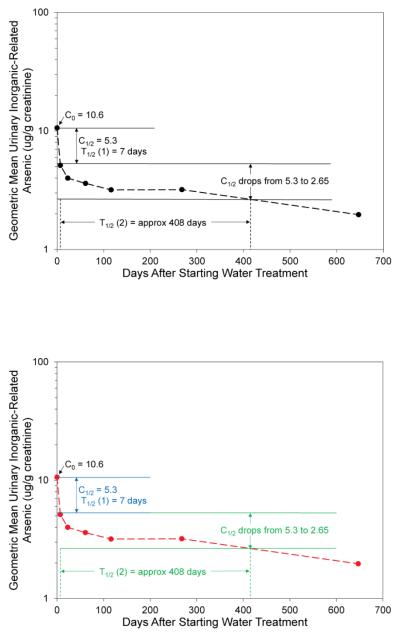
Graphs of the Pre-Post Group’s clearance of inorganic-related arsenic from urine, after these subjects stopped drinking the arsenic contaminated water, are shown in Figures 2a and 2b. A two-phase clearance is apparent. Based on a visual inspection of the log concentration verses time graph (Klaassen, 2001) in Figure 2b, the first clearance phase had a half-life of approximately 7 days. The second clearance phase had a half-life of approximately 408 days or between 245 and 624 days. A range for the half life of the second clearance phase is given as sample collection frequency was limited toward the end of the study. The mean inorganic-related arsenic concentrations in the urine of the exposed Pre-Post Group, although greatly reduced, remained significantly higher than in the Comparison Group, even nine months after ceasing the drinking water exposure.
Figure 2a. Clearance of Urinary Inorganic-Related Arsenic of Pre-Post Group.
Data Points are Geometric Means ± SE for the Group at the Median of Each Time Period
Figure 2b. Clearance and Half Life of Urinary Inorganic-Related Arsenic of Pre-Post Group.
Data Points are Geometric Means for the Group at the Median of Each Time Period
3.6. Treatment system breakthrough increased urine arsenic levels
At the home where the arsenic water treatment system had a temporary arsenic breakthrough (Family 25), the raw water averaged 99 μg/L arsenic, and was 96% AsIII. During the breakthrough period, the arsenic concentration in the treated water at the kitchen sink reached as high as 41 μg/L. The cause of the arsenic breakthrough at this home was determined to be iron bacteria fouling the granular ferric oxide adsorption media. In addition to the high concentration of arsenic, the well water at this home also contained iron at 0.8 mg/l, manganese at 0.25 mg/l, a pH of 7.1, a strongly negative oxidation-reduction potential (−200 millivolts), and a strong sulfur odor. This type of water often contains iron-reducing bacteria which can thrive in a granular ferric oxide media environment. Backwashing the media with a strong chlorine solution removed the fouling. Later, a pulse-feed chlorinator was installed as pre-treatment and prevented any further fouling of the media.
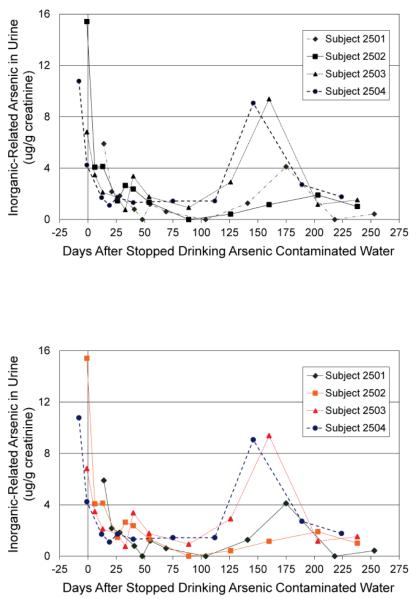
A rebound in the urinary arsenic concentrations was seen during the time period between treatment system breakthrough, its detection, and the families return to use of bottled water until the system was again working effectively (Figure 3).
Figure 3.
Inorganic-related Urinary Arsenic Rebound during Treatment System Breakthrough for Family 25 at approximately Day 104 through Day 167
4. Discussion
In this study, both the POU and POE water treatment technologies performed very well. They consistently reduced water arsenic concentrations from as high as 120 μg/L to concentrations less than 3 μg/L. However, the reduction of inorganic-related arsenic in the urine of study subjects was not the same in the POE and POU water treatment groups. The ANCOVA and GEE-adjusted results from this study demonstrate whole-house POE arsenic water treatment reduced urinary inorganic-related arsenic to significantly lower concentrations than single-tap POU arsenic water treatment.
4.1. Comparison to Reference Ranges and NHANES Data
An evaluation of arsenic biomonitoring data should include a comparison of the observed data to “normal levels”. These normal levels, also known as “Reference Range” or “Reference Interval” are typically determined by using the endpoints of the 95% confidence interval of a sufficiently large sample of appropriate (having the same age, sex, and ethnicity) healthy or non-exposed people (Marshall and Bangert, 2008). Similar sampling protocols and analytical methods must be used to provide the reference range data and the data that is to be compared to the reference range (Marshall and Bangert, 2008). Because very few non-exposed people are tested for arsenic, and sampled populations and analytical methods vary widely, it is very difficult to calculate a typical reference range for arsenic. Rather than using a calculated reference range, many labs use a “text book” range. For example, LabCorp, a commercial laboratory, uses ranges of 0-50 μg/L for total arsenic in urine, and < 20 μg/L for inorganic arsenic in urine (LabCorp, 2008). These ranges are reportedly based on a 1990 publication (Tietz, 1990) which was based on (Iyengar and Woittiez, 1988) who gathered older data from all over the world and likely included many people who were exposed to both drinking water and dietary sources of arsenic. LabCorp also uses < 35 μg/g creatinine of inorganic arsenic plus methylated metabolites in urine, for end of week occupational exposure based on the recommendations of the American Conference of Governmental Industrial Hygienists (ACGIH), Biological Exposure Indices (ACGIH, 2001; LabCorp, 2008). Quest Diagnostics, another commercial laboratory, uses ≤ 80 μg/L total arsenic in urine for their reference range. Due to the currently large variation in published reference range values for urinary arsenic, there is a need to look at the available data that may contribute to more appropriate reference ranges.
The National Health and Nutrition Examination Survey (NHANES) 2003-2004 survey has provided urinary arsenic data for a representative sample of the US population (Caldwell et al., 2008). A comparison of the NHANES data with the urinary arsenic data from the present study is presented in Tables 2a and 2b with geometric means and the 25th, 50th, 75th, and 95th percentiles. In this NHANES survey, the 95th percentiles for total urinary arsenic were 65.4 μg/L and 50.2 μg/g creatinine. For inorganic-related urinary arsenic the 95th percentile was 18.9 μg/L (Table 2b). These values are not much different from the upper limit of the LabCorp reference ranges. However, when evaluating the NHANES data, it must be remembered that this is a representative sample, not an unexposed sample. Some unknown percentage of the NHANES sample population was no doubt exposed to elevated arsenic concentrations in drinking water from both residential and public well water as the samples were collected in 2003-2004, which was before the January 2006 effective date of the new US drinking water standard for arsenic being reduced from 50 μg/L to 10 μg/L.
Table 2a.
Comparison of Treatment Group Data to NHANES 2003-2004
| Total Arsenic Data (μg/g creatinine) | Selected Percentiles | |||||
|---|---|---|---|---|---|---|
| Groups | Sample Size | Geometric Mean | 25 | 50 | 75 | 95 |
| NHANES | 2557 | 8.2 | 4.2 | 7.0 | 14.1 | 50.2 |
| Comparison | 5 | 13.8 | 9.3 | 13.7 | 21.4 | 22.2 |
| Pre-Post POE Time Period 0 | 16 | 18.4 | 9.3 | 18.9 | 32.8 | |
| Pre-Post POE Nine-Months | 16 | 17.2 | 10.1 | 14.9 | 23.6 | |
| Pre-Post POU Time Period 0 | 8 | 38.0 | 21.9 | 31.0 | 90.2 | |
| Pre-Post POU Nine-Months | 8 | 19.4 | 13.3 | 14.7 | 31.0 | |
Table 2b.
Comparison of Treatment Group Data to NHANES 2003-2004
| Inorganic-Related Arsenic Data (μg/L) | Selected Percentiles | |||||
|---|---|---|---|---|---|---|
| Groups | Sample Size | Geometric Mean | 25 | 50 | 75 | 95 |
| NHANES | 2557 | N/A | <LOD | 6.0 | N/A | 18.9 |
| Comparison | 5 | 3.9 | 2.5 | 3.6 | 6.6 | 7.6 |
| Pre-Post POE Time Period 0 | 16 | 13.5 | 10.7 | 14.1 | 16.7 | |
| Pre-Post POE Nine-Months | 16 | 7.0 | 4.3 | 7.6 | 12.3 | |
| Pre-Post POU Time Period 0 | 8 | 30.0 | 14.2 | 26.3 | 50.8 | |
| Pre-Post POU Nine-Months | 8 | 6.2 | 2.3 | 5.8 | 16.5 | |
Due to the presence of organic arsenic in many foods, especially in fish and seafood, inorganic-related arsenic is the better measure of exposure to arsenic in drinking water (Spayd et al., 2012). However, some inorganic arsenic (AsIII and AsV) is present in food and this inorganic arsenic and its metabolites, upon reaching the urine, would be included in the component labeled “inorganic-related” arsenic in urine. There is no analytical method capable of separating the inorganic arsenic originating in food from the inorganic arsenic originating in ingested water. The average total inorganic arsenic intake from food in the United States, based on the FDA Total Diet Study, is estimated at 9 μg/d for adults, aged 25 and over, 5 μg/d for children aged 2-16 years, and 1.3 μg/d for children aged 6-11 months (National Research Council, 1999; Tao and Bolger, 1999). These intake amounts can have an effect on the final “inorganic-related” arsenic concentrations in urine. However, the effect should be similar in the POE, POU, and Comparison Groups in the present study because there were little to no significant differences found between these groups in their potential arsenic exposure from dietary sources (Table 1).
The comparison of the NHANES data with the present study shows that compared to a representative sample of the US population, the Pre-Post Group initial arsenic concentrations were quite elevated (between the 75th and 95th percentiles of the NHANES sample), and after having effective arsenic water treatment for approximately nine months, the concentrations had dropped to near but still greater than the 50th percentile of the NHANES sample inorganic-related arsenic concentrations (Table 2b). When comparing the GEE-adjusted inorganic-related urinary arsenic data after nine months of water treatment for POE subjects from the Pre-Post Group (2.5 μg/g creatinine) (Table 1), the POE subjects are well below the 50th percentile of the NHANES sample, while the POU subjects at 7.2 μg/g creatinine (Table 1) are near but above the 50th percentile of the NHANES sample.
Based on this review of the data it appears that more study is needed to establish adequate reference ranges for urinary inorganic arsenic.
4.2. Controlling for Study Design
The non-randomized nature of this observational study resulted in the need for propensity score and GEE analysis. The POE and POU water treatment groups were significantly different on several covariates including age, weight, amount of water consumed at home per day, years of exposure to the arsenic contaminated well water, and prior cumulative arsenic ingestion dose from the well water (Table 1). The POE, POU, and Comparison Groups were similar on potential dermal and dietary arsenic exposure variables. Propensity scores were calculated for each subject to balance the covariates, reduce the bias presented by the covariates, and convert the study into a quasi-randomized experiment (D’Agostino, 1998). Furthermore, because some of the subjects were family members sharing the same well and water treatment system, GEE were needed to allow for analysis of the correlated observations within families.
4.3. Arsenic body burden
Both the two-phase clearance of arsenic from urine and the failure of the exposed subjects to reach the geometric mean of the urinary inorganic-related arsenic concentrations found in the Comparison Group (1.5 ± 0.4 μg/g creatinine) indicate that a body burden of arsenic was present in the exposed subjects (Figure 2a and 2b).
4.4. Arsenic Reduction in POE and POU Group Urine Samples
After an average of nine months of water treatment, the adjusted inorganic-related urinary arsenic concentrations were significantly lower in the POE Group than in the POU Group. In addition, the reduction of inorganic-related urinary arsenic from the initial arsenic concentration to the nine-month concentration was greater in the POE Group than in the POU Group. Potential reasons for these POE and POU differences could be non-compliance with drinking and cooking with only treated water, other exposure pathways in the POU homes, and body burden. However, body burden was accounted for in the computation of adjusted inorganic-related urinary arsenic concentrations by controlling for prior exposure using cumulative arsenic ingestion dose as described in Sections 3.4 and 4.2. Even with body burden differences accounted for, there are still unexplained differences between the POE and POU groups’ reduction of inorganic urinary arsenic concentrations.
A population in northern Chile, chronically exposed to 600 μg/L arsenic in their drinking water, was provided an alternate drinking water supply (with 45 μg/L arsenic) for two months (Hopenhayn-Rich et al., 1996). A substantial decrease in total urine arsenic was observed; however, the final urinary levels of arsenic were higher than what would be expected from consumption of drinking water at 45 μg/L. Inorganic-related urine arsenic levels determined by hydride generation atomic absorption spectroscopy dropped from 696 μg/L to only 185 μg/L at the end of the two-month study. In addition to the possibility that these subjects may still have been mobilizing arsenic from their body burden after the two month period, other explanations include non-compliance with drinking the lower arsenic water that was provided by the investigators, and the potential exposure to arsenic via water uses other than drinking and cooking.
In a home with single-tap POU water treatment, one likely cause of continued exposure to arsenic in water is from subject non-compliance by ingesting water from untreated taps in the home (e.g., from a bathroom sink). In this study, subjects with POU water treatment were encouraged by the investigators to only ingest water from the POU treatment system. However, we did not attempt to assess compliance with this request.
In the present study, another potential cause of higher inorganic-related urinary arsenic concentrations in the POU Group are secondary routes of exposure which could arise from inhalation of aerosols during showering or cooking, and dermal absorption during showering, bathing, or brushing of teeth. The contribution of these secondary routes in household exposure is uncertain (National Research Council, 1999). Other contaminants in drinking water have, in addition to the ingestion pathway, been found to be absorbed through the skin and inhaled while showering (Weisel and Jo, 1996).
There is a high likelihood that skin contact with waters containing arsenic above drinking water standards will result in some arsenic absorption. The highly keratinized epidermis provides ample sulfhydryl binding sites for arsenic. Rahman found up to 62% absorption when applying arsenic concentrations as low as 50 μg/L to the skin of mice in vitro for 24 hours (Rahman et al., 1994). About half of the absorbed arsenic passed through the skin and half remained in the skin. Wester identified arsenic absorption through the skin of live monkeys and demonstrated that up to 6.4% of an applied dose of 4.8 μg/L arsenic was absorbed through skin and excreted via urine after 24 hours of exposure (Wester et al., 1993). In a follow-up study, they found up to 4.4% of an arsenic dose was absorbed through skin and excreted via urine after eight hours of exposure (Wester et al., 2004). Another study, using artificial skin, found absorption of both AsV and AsIII at up to 8% of the applied dose after 6 hours (Bernstam et al., 2002; Nriagu and Bernstam, 2004). Assuming the thickness of water film on the body during a shower is 100 μm, their data indicate that a 15 minute shower with water containing 100 μg/L arsenic would result in dermal absorption up to 1.9 μg of arsenic in an 8-year old child, and 3.8 μg of arsenic in a 70 kg adult (Nriagu and Bernstam, 2004). These dermal absorption studies had single exposure events lasting from only 6 to 24 hours. A long-term chronic dermal exposure based on daily showering or bathing, hand washing, and teeth brushing may increase the long-term absorption of arsenic via the dermal route. Therefore, humans with elevated arsenic in their well water and a chronic daily exposure via showering or bathing could potentially incur a substantial arsenic exposure without POE water treatment.
4.5. Treatment system breakthrough increased urine arsenic levels
The rebound in the inorganic-related urinary arsenic concentrations in the subjects at the home with the arsenic water treatment system breakthrough (Figure 3) demonstrates how quickly a failure in a treatment system can result in the return of elevated urinary inorganic-related arsenic concentrations. The children in this family reported drinking home water at a rate of 0.56 L/d whereas the parents reported drinking home water at a rate of 0.35 L/d, and this difference may have contributed to the rebound in the children (Subjects 2503 and 2504) being greater than in the adults (Subjects 2501 and 2502). Arsenic water treatment systems require maintenance and routine sampling to confirm adequate performance. The rapid increase of urinary inorganic-related arsenic concentrations of the subjects at this home after treatment system breakthrough demonstrates the importance of the maintenance and sampling requirements.
4.6. Implications of the Study Findings
There is a significant cost difference between installing and maintaining a POE or POU arsenic water treatment system, with POE treatment costing about eight times more than a single POU treatment device. Considering the fact that arsenic is a known human carcinogen, that the maximum contaminant level goal for arsenic is zero μg/L, and the present study indicates that POE water treatment provides a greater urinary arsenic reduction than POU water treatment, the additional cost of a POE treatment system may be warranted. Considering the higher costs associated with POE arsenic water treatment, a larger randomized study should be conducted to confirm the present findings and quantify the contribution of the dermal and inhalation exposure pathways, as well as the exposure from drinking at untreated taps in homes with POU arsenic water treatment.
Some regulatory agencies in New Jersey are requiring POE arsenic water treatment when and where they have jurisdiction and recommending it elsewhere. The Hunterdon County Health Department requires POE arsenic water treatment be installed before issuing a certificate of occupancy for new homes with arsenic in well water exceeding the MCL. The Hopewell Township Health Department in Mercer County requires POE arsenic water treatment be installed before signing off on property transfers when the drinking water well is found to exceed the arsenic MCL. Both of these programs were enabled by New Jersey’s PWTA Program that requires well-water testing when new wells are installed for drinking water or a property supplied by a well for drinking water is sold. POE water treatment is especially important when a home with an arsenic contaminated well is sold to a new owner. Typically, the new owner will be unfamiliar with the water problem and may not even know why the treatment system is in the home. They are often told that the well has a problem, but the problem has been remediated by a water treatment system. Even if a POU water treatment system is installed at the kitchen sink, the new owner may not realize that other taps in the home do not have treated water. If POE water treatment is installed, all water taps in the home will be treated, and the only remaining problem for a new homeowner will be to educate them about the need to occasionally monitor the quality of the treated water and have maintenance conducted to ensure the continued effectiveness of the system.
4.7. Limitations
One limitation of this study was the comparison of small and uneven numbers of subjects. There were only 24 subjects in the Pre-Post Group, with 16 subjects in the POE treatment Group and 8 subjects in the POU treatment Group. The questionnaires depended on subject recall rather than measurement to determine each subject’s exposure history. Subject characteristics such as rate of drinking water from the home water supply, the years of exposure to the home water supply, the weight of the subject, as well as the subject’s bathing and dietary habits all depended on recall which has its limitations.
5. Conclusions
This study demonstrated that effective POU and POE arsenic water treatment is available for residential well remediation. Arsenic water treatment reduces arsenic exposure as evidenced by statistically significant reductions in urinary inorganic-related arsenic concentrations after initiation of water treatment.
Arsenic body burden is present after chronic exposure to arsenic in drinking water as demonstrated by the two-phase clearance of inorganic-related arsenic from urine identified for the Pre-Post subset of subjects. After nine months of water treatment, the previously exposed subject’s inorganic-related arsenic concentrations were greatly reduced, but remained significantly higher than in the Comparison Group subjects. The two-phase clearance combined with the failure of the previously exposed subjects’ urine concentrations to decline to the level in the Comparison Group subjects is an important contribution to the weight of evidence that arsenic bioaccumulates in humans during chronic exposure to arsenic-contaminated water and that its toxicity may linger well beyond the point at which remediation is instituted.
The results suggest that POE arsenic water treatment systems provide a more effective reduction of arsenic exposure from well water than that obtained by POU treatment. After nine months of water treatment in the Pre-Post subset, the adjusted mean of the inorganic-related arsenic concentrations were significantly lower in the POE treatment Group than in the POU treatment Group. This difference in exposure outcome between the two treatment methods may be attributed to subjects occasionally drinking water from untreated taps in the POU water treatment homes and/or related to dermal absorption of arsenic which has been reported in studies of monkeys, other mammals, and artificial human skin. There is a significant cost difference between installing and maintaining a POE or POU arsenic water treatment system. Considering the fact that arsenic is a known human carcinogen and that the maximum contaminant level goal for arsenic is zero μg/L, the additional cost of a POE treatment system may be warranted.
A larger randomized study should be conducted to confirm the present findings, especially the presence of an arsenic body burden, a two-phase clearance after chronic exposure to arsenic contaminated water has ended, and the apparent better protection provided by POE arsenic water treatment systems. In addition, further study should be conducted to quantify the contribution of the dermal and inhalation exposure pathways, and to establish appropriate reference ranges for arsenic in urine. If further study confirms POE treatment provides more effective arsenic exposure reduction than POU treatment, then regulatory agencies should consider requiring POE arsenic water treatment when and where they have jurisdiction.
Highlights.
Effective arsenic water treatment is available for residential well water.
Arsenic water treatment results in significantly reduced urinary inorganic arsenic.
Arsenic body burden is present after chronic exposure to arsenic in drinking water.
POE water treatment provided more effective arsenic exposure reduction than POU.
Acknowledgements
The authors thank Pamela Ohman-Strickland for assistance with the statistical analyses and Dr. Michael Gochfeld for inspiration and guidance, and acknowledge partial support from NIEHS ES05022.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ACGIH Biological Exposure Indices. (7th Edition) 2001 [Google Scholar]
- AWWA Research Foundation [accessed 2014];POU/POE implementation feasibility study for arsenic treatment. 2005 Available: http://www.waterrf.org/PublicReportLibrary/91083F.pdf.
- Bernstam L, Lan CH, Lee J, Nriagu JO. Effects of arsenic on human keratinocytes: morphological, physiological, and precursor incorporation studies. Environ Res. 2002;89:220–35. doi: 10.1006/enrs.2002.4367. [DOI] [PubMed] [Google Scholar]
- Caldwell KL, Jones RL, Verdon CP, Jarrett JM, Caudill SP, Osterloh JD. Levels of urinary total and speciated arsenic in the US population: National Health and Nutrition Examination Survey 2003-2004. J Expos Sci Environ Epidemiol. 2008;19:59. doi: 10.1038/jes.2008.32. [DOI] [PubMed] [Google Scholar]
- D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–81. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Ghisletta P, Spini D. An Introduction to Generalized Estimating Equations and an Application to Assess Selectivity Effects in a Longitudinal Study on Very Old Individuals. Journal of Educational and Behavioral Statistics. 2004;29:421. [Google Scholar]
- Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157:364–75. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- Hopenhayn-Rich C, Biggs ML, Kalman DA, Moore LE, Smith AH. Arsenic methylation patterns before and after changing from high to lower concentrations of arsenic in drinking water. Environ Health Perspect. 1996;104:1200–7. doi: 10.1289/ehp.961041200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar V, Woittiez J. Trace Elements in Human Clinical Specimens: Evaluation of Literature Data to Identify Reference Values. Clinical Chemistry. 1988;34:474–481. [PubMed] [Google Scholar]
- Klaassen CD. Casarett and Doull’s Toxicology. 6th Edition McGraw Hill; 2001. pp. 226–229. [Google Scholar]
- LabCorp [accessed 2013];Arsenic Exposure Profile, Urine. 2008 Available: https://www.labcorp.com/wps/portal/provider/testmenu/
- Marshall WJ, Bangert SK. Clinical Biochemistry: Metabolic and Clinical Aspects. Elsevier Health Sciences; 2008. [Google Scholar]
- National Research Council . Arsenic in Drinking Water. National Academy Press; Washington, D.C.: 1999. [Google Scholar]
- NJDEP, Division of Water Supply and Geoscience [accessed 2014];Private Well Testing Act Frequently Asked Questions. 2012 Available: http://www.nj.gov/dep/watersupply/pwta/pwta_faq.htm#3q13.
- NJDEP, Office of Science [accessed 2013];Private Well Testing Act Web Site. 2012 Available: http://www.nj.gov/dep/dsr/pwta/
- NJDEP [accessed 2013];Private Well Testing Act Program Well Test Results for September 2002 - April 2007. 2008 Available: http://www.state.nj.us/dep/pwta/pwta_report_final.pdf.
- NJDEP Safe Drinking Water Act Rules Readoption with amendments: N.J.A.C. 7:10. 2004 Dec 6; New Jersey Register, December 6, 2004, 2004. [Google Scholar]
- Nriagu J, Bernstam L. Biomarkers of arsenic effects on gene expression in human skin. AWWA Research Foundation; 2004. [Google Scholar]
- Rahman MS, Hall LL, Hughes MF. In vitro percutaneous absorption of monosodium methanearsonate and disodium methanearsonate in female B6C3F1 mice. Toxicol In Vitro. 1994;8:441–448. doi: 10.1080/15287399409531854. [DOI] [PubMed] [Google Scholar]
- Serfes ME, Spayd SE, Herman GC. Chapter 13 - Arsenic Occurrence, Sources, Mobilization, and Transport in Groundwater in the Newark Basin of New Jersey. In: O’Day PA, Vlassopoulos D, Meng X, Benning LG, editors. Advances in Arsenic Research. Integration of Experimental and Observational Studies and Implications for Mitigation. American Chemical Society; Washington, DC: 2005. pp. 175–190. [Google Scholar]
- Spayd SE. [accessed 2013];Arsenic Water Treatment for Residential Wells in New Jersey. 2007 Available: http://www.state.nj.us/dep/pwta/Arsenic_Treatment.pdf.
- Spayd SE, Robson MG, Xie R, Buckley BT. Importance of Arsenic Speciation in Populations Exposed to Arsenic in Drinking Water. Human and Ecological Risk Assessment: An International Journal. 2012;18:6, 1271–1291. [Google Scholar]
- Tao SSH, Bolger PM. Dietary arsenic intakes in the United States: FDA Total Diet Study, September 1991-December 1996. Food Additives & Contaminants. 1999;16:465–472. doi: 10.1080/026520399283759. [DOI] [PubMed] [Google Scholar]
- Tietz NW. Clinical Guide to Laboratory Tests. 2nd Edition W B Saunders Co; 1990. [Google Scholar]
- USEPA [accessed 2013];National Primary Drinking Water Regulations: Arsenic and Clarifications to Compliance and New Source Contaminants Monitoring. 2001 Available: http://www.epa.gov/fedrgstr/EPA-WATER/2001/January/Day-22/w1668.htm.
- Weisel CP, Jo WK. Ingestion, inhalation, and dermal exposures to chloroform and trichloroethene from tap water. Environ Health Perspect. 1996;104:48–51. doi: 10.1289/ehp.9610448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wester RC, Hui X, Barbadillo S, Maibach HI, Lowney YW, Schoof RA, et al. In vivo percutaneous absorption of arsenic from water and CCA-treated wood residue. Toxicol Sci. 2004;79:287–95. doi: 10.1093/toxsci/kfh114. [DOI] [PubMed] [Google Scholar]
- Wester RC, Maibach HI, Sedik L, Melendres J, Wade M. In vivo and in vitro percutaneous absorption and skin decontamination of arsenic from water and soil. Fundam Appl Toxicol. 1993;20:336–40. doi: 10.1006/faat.1993.1043. [DOI] [PubMed] [Google Scholar]
- Xie R, Johnson W, Spayd S, Hall G, Buckley B. Determination of total toxic arsenic species in human urine using hydride generation inductively coupled plasma mass spectrometry. J Anal At Spectrom. 2007;22:553–560. [Google Scholar]