Abstract
Using an event-triggered recording system, the quantity of daily song bout production was measured weekly in male zebra finches (Taeniopygia guttata) during sensory-motor learning and at one year of age. Our aim was to ask whether the development of a stereotyped vocal pattern involves a practice-driven component. If so, we hypothesized that juvenile males learning song should sing more often than adults reciting a vocal pattern they had already learned, and that greater levels of juvenile singing should be associated with improvement in the quality of the adult song. Across the period measured (36–365 days of age), subjects showed an inverted U-shaped pattern of daily song bout production. Song bout production was lowest during subsong, with increased production associated with plastic song and song crystallization, although individual differences were large. Daily song bout production decreased in adulthood. Higher levels of song bout production during plastic song correlated with fewer sequencing errors in adult song patterns (r2=0.77). In contrast, quantity of singing during song crystallization showed no relationship to vocal stereotypy (r2=0.002). Our data suggest a sensitive period for vocal practice during zebra finch sensory-motor learning with consequences for the note-sequence fidelity of the adult vocal pattern.
Keywords: Songbird, Learning, Juvenile, Sensitive period, Vocal production, Circadian
1. Introduction
Juvenile songbirds learn to imitate the song of an adult in at least two behaviorally defined stages [16]. In an initial stage of auditory learning, young birds must identify, listen to, and memorize the song of an adult conspecific. This memory (or song template) serves to guide the subsequent motor development of song, termed sensory-motor learning. At the beginning of sensory-motor learning, birds vocalize in a highly variable fashion, but rely on auditory feedback of their own vocal patterns to gradually develop the ability to produce a song that matches the song template acquired during auditory learning.
In terms of neural mechanisms underlying song learning, the zebra finch (Taeniopygia guttata) is perhaps the most intensively studied songbird. Many of the particulars of how juvenile male zebra finches learn song, especially the duration and social modulation of the sensitive period for auditory learning, have been well characterized (for review see [21,27,29,32]). Sensory-motor learning in zebra finches has received somewhat less attention, although classic studies by Immelmann [11] and Arnold [1] revealed that zebra finch vocal patterns, like those of other songbirds, emerge gradually in sequential behavioral phases. Initial vocal patterns, termed subsong, begin as early as 28 days post-hatch and were aptly described by Arnold [1] as ‘quiet bursts of sound of variable structure that are produced at irregular intervals’ (p. 265). Between 40 and 50 days post-hatch birds transition to a phase of plastic song, where individual note types become recognizable but the sequencing of these notes remains variable. As birds reach young adulthood (80–100 days post-hatch) they enter a stage of song crystallization. That is, birds begin to produce their song notes in a characteristic sequence, although note-sequencing errors do occur. In adulthood, the vocal pattern is used to court receptive females (directed song), or is produced in an apparently spontaneous fashion, not addressed to any particular conspecific (undirected song). In fact, adult male zebra finches continue to produce hundreds of bouts of undirected song per day even when housed in complete social isolation [25].
Here, using an event-triggered recording system, we periodically measured the number of daily bouts of undirected song (i.e. no females were present) produced by male zebra finches during vocal development and in adulthood. We then used the stereotypy score of Scharff and Nottebohm [26], which is sensitive to note sequencing errors, to assess relationships between quantity of juvenile vocal production and the note sequence fidelity of the crystallized song pattern. Our theoretical rationale was to ask to what extent the gradual development of stereotyped vocal patterns reflects a behavioral practice-driven process, or a maturational one. If the former, we predicted that birds should produce more song bouts per day as juveniles than as adults. That is, learning a song pattern should involve more vocal rehearsal than maintaining an already-learned pattern. Similarly, greater levels of juvenile vocal production should be associated with fewer note-sequencing errors in the crystallized song pattern.
However, if song development is guided to a greater degree by maturational processes, the emergence and quality of crystallized song should show little relationship to the level of vocal production at earlier stages. Indeed, Pytte and Suthers [24] used 2–3-week periods of vocal paralysis (induced by botulinum toxin injections into the muscles controlling vocalization) during subsong, plastic song, and song crystallization to probe the requirement for vocal practice in song learning. Impaired learning was found only when treatments included the phase of song crystallization, a pattern of findings that led Pytte and Suthers [24] to conclude that ‘maturational processes may have a larger influence on juvenile song development than is generally recognized, and/or that a relatively short period of motor learning is sufficient to match song elements to those of a tutor’ (p. 186). Thus, it seems likely that both practice-driven and maturational processes are involved in zebra finch vocal development [19,20] and the purpose of our study was to further examine how these two processes might interact during song learning.
2. Methods
2.1. Subjects and recording of song bouts
The Florida State University Animal Care and Use Committee approved all procedures in this study. Subjects were domestic male zebra finches hatched at Florida State University between March 1999 and September 2000 in flight cages that contained 10 breeding pairs. In the aviary room, food and water were freely available on a 14:00-h light:10:00-h dark cycle with an ambient temperature of 26 °C. Subjects were removed from their home flight cage at 35 days of age and housed singly in a separate room under the same feeding, lighting, and temperature conditions as the aviary room. Note that while juvenile zebra finches clearly retain the ability to learn from an adult tutor after 35 days of age [5,14], song exposure up to 35 days of age is sufficient for establishment of a song template [2,3]. From 35 days of age to the end of the study, subjects were housed in cages that were in visual, but not auditory, isolation from other birds in the study. Consistent with previous reports of birds housed under similar conditions [23,5], there was no evidence that the subjects in the present study copied notes from one another (as determined by analysis of audiospectrograms of the crystallized song pattern of each bird).
Each subject’s cage was fitted with a microphone that was attached to a computer running software designed for real-time monitoring of programmed frequency and duration criteria (Avisoft-Recorder, www.avisoft.de). Adult zebra finch song behavior consists of a specific note pattern (motif) that is repeated several times in song bouts lasting 2–7 s. The majority of zebra finch notes contain fundamental frequencies below 2 kHz, but most of these notes also tend to be spectrally complex, with harmonic frequencies often spanning the 2–10 kHz range [23]. Pilot studies with juvenile birds revealed that subsong and plastic song vocal patterns span similar ranges of time and frequency, although subsong birds lack a motif since they have no recognizable note types. Avisoft-Recorder was configured to capture sound events that contained frequencies between 0 and 10 kHz and lasted 2 s, including a 1-s hold time. That is, once triggered by a sound event, Avisoft-Recorder continued recording until at least 1 s of silence elapsed. Thus, the shortest sound events captured were 1 s in duration. Captured events were saved as time-stamped uncompressed digital audio files (sampling rate=44 kHz) to the computer’s hard disk. During all recordings of all birds, a human observer periodically checked the computer against a real-time spectrographic display to insure that Avisoft-Recorder was accurately capturing song bouts for each subject.
2.2. Quantifying song bout production and the circadian organization of singing
All recordings were of undirected song. One group of subjects (n=5) was recorded for 24 h at 36, 42, 49, 56, 63, 70, 77, 84, 91, 112, and 365 days of age (n=11, 24 h recordings per subject; all ages ± 2 days). Data from these subjects were used to analyze quantitative features of vocal behavior during subsong, plastic song, crystallized song, and in adulthood. However, in order to increase the sample size for analysis of correlations between the note-sequence fidelity of crystallized vocal patterns and the quantity of vocal production during plastic song (recordings from 49 to 70 days of age) and during song crystallization (recordings from 77 to 112 days of age), a second group of subjects (n=3), raised and housed as described above, were recorded for 24 h at 49, 56, 63, 70, 77, 84, 91, and 112 days of age (n=8, 24 h recordings per subject; all ages ± 2 days).
The ‘once weekly’ schedule of 24 h recording sessions was selected based on a pilot study of birds (n=1 juvenile and n=2 adults) that were recorded daily for two weeks. Since these birds showed only 10–20% day-to-day variation in total song bout production, we concluded that one 24-h recording per week should provide a reasonable estimate of daily song bout production. During each 24 h recording day, Avisoft-Recorder was used to capture the total number of song bouts produced. Since each subject generated a number of false-positive triggering events (due to wing flutters, a series of calls, or a combination of the two), after each 24 h recording all audio files for each subject were manually checked using spectrographic sound analysis software (Avisoft-SASLab Pro). Files that did not contain a song bout were deleted. In this way, the total number of song bouts produced during each 24 h recording was determined for each subject. The average duration of song bouts was determined by randomly selecting and measuring the duration of ten song bouts from each 24 h recording for each subject.
We also described the within-day organization of singing, since there are presently no data in the literature on circadian influences over zebra finch song behavior. To do this, the number of song-bouts made by each subject was calculated across the entire daily cycle in 2 h bins for each 24 h recording.
2.3. Quantifying note-sequence fidelity of crystallized vocal patterns
Crystallized vocal patterns were quantified using ten song bouts randomly selected from each subjects’ 24 h recording at 112 days of age. Avisoft-SASLab Pro was used to generate amplitude waveforms and audiospectrograms and two observers independently identified individual note types and the characteristic motif produced by each subject as an adult. Notes were identified on audiospectrograms as discrete traces or by sharp changes in frequency modulation. The observers further distinguished individual note types based on five acoustic features: fundamental frequency, shape of frequency modulation, spectral complexity, duration, and shape of the amplitude waveform. Individual notes were assigned letters and the adult motif of each subject was described by a sequence of letters (e.g. ABCDE). Introductory notes were given the designation ‘i’. The two observers reached complete agreement on the characteristic adult motif for all subjects.
Following the method described by Scharff and Nottebohm [26] and using the ten song bouts randomly selected from each subject at 112 days of age, an average stereotypy score was generated for each subject. The stereotypy score is a function of song linearity and song consistency and is defined by the following equations:
Stereotypy scores are sensitive to note sequencing errors made by birds as they recite their vocal pattern. That is, vocal patterns with perfect linearity and consistency generate stereotypy scores of 1, whereas variation in note sequencing leads to stereotypy scores <1 (see [26] for additional details). Male birds taken from our aviaries as adults have stereotypy scores of 0.8 and above [31] and the birds in the present study showed a similar range of scores.
2.4. Statistical analyses
All statistics were completed using Excel 2000 (Microsoft, Redmond, WA), Prism 3.0 (GraphPad, San Diego, CA), and SigmaStat 2.03 (SPSS Science, Chicago, IL). Data from birds recorded between 36 and 365 days of age were analyzed using one-way repeated measures ANOVAs (song bout production × age, song bout duration ×age) followed by Student–Newman–Keuls (SNK) pairwise comparisons. Linear regressions were used to test relationships between the note-sequence fidelity of crystallized vocal patterns (stereotypy scores from song bouts recorded at 112 days of age) and cumulative song bouts recorded during plastic song (49–70 days of age) and during song crystallization (77–112 days of age).
3. Results
3.1. Progression through subsong, plastic song, and crystallized song
All subjects recorded at 36 days of age were producing subsong. As such, their song bouts could be described as highly variable, with no discernable structure in terms of note morphology or note sequence. However, these song bouts also showed uniformity in that they were comprised of spectrally complex notes with fundamental frequencies near or below 2 kHz. Song bouts captured at 42 days of age revealed subjects transitioning from subsong to plastic song; audiospectrograms contained combinations of subsong-like notes and emerging note types. By 49 days of age, all subjects were producing plastic song; audiospectrograms contained only note types that bore clear resemblance to note types found in the adult song, but note sequencing was variable. By 77 days of age, song patterns began to crystallize, with each subject producing a motif that resembled or was identical to the motifs recorded at the end of song crystallization (112 days of age) and at 365 days of age. These observations are consistent with the reports of Immelmann [11] and Arnold [1].
3.2. Influence of the light:dark cycle on song bout production
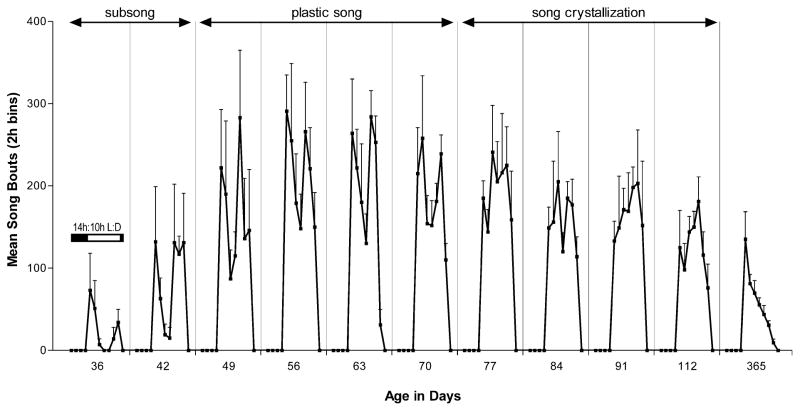
Fig. 1 shows the diurnal pattern of undirected song bout production on each day that birds were recorded. While no singing was recorded during the 10-h dark phase of any recording day, the light-phase pattern of singing showed distinct morning and evening peaks when birds were producing subsong and plastic song (36–70 days of age), but tended to show a single diurnal peak of singing during song crystallization and adult song (70–365 days of age). The large peak of morning singing that we measured at 365 days of age is identical to what we have observed in other studies of undirected song bout production by adult birds [25].
Fig. 1.
The within-day pattern of undirected song bout production during sensory-motor learning and in adulthood (14:00-h light:10:00-h dark cycle). Data are the mean number of song bouts produced every 2 h (+S.E.) by a group of birds (n=5) recorded for 24 h at 36, 42, 49, 56, 63, 70, 77, 84, 91, 112, and 365 days of age. During subsong and plastic song, birds showed distinct morning and evening peaks of song bout production, whereas singing during song crystallization and in adulthood tended to be organized in a single diurnal peak.
3.3. Song bout production is highest during plastic song and song crystallization
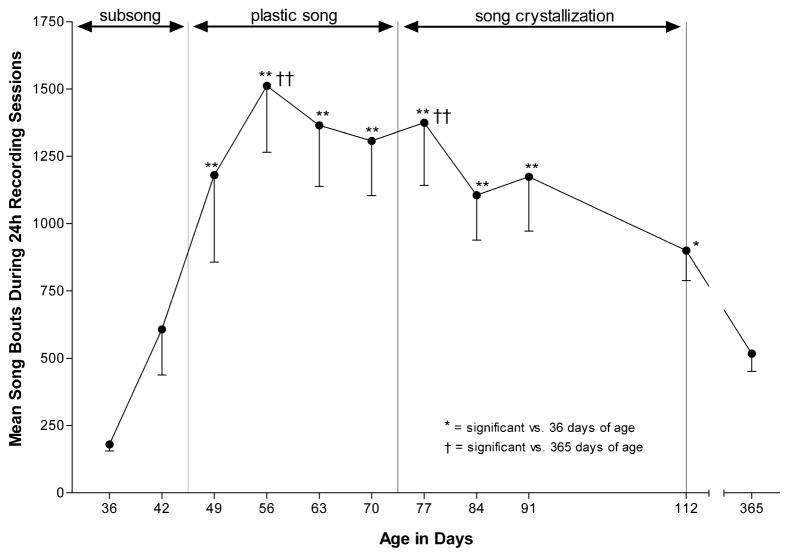
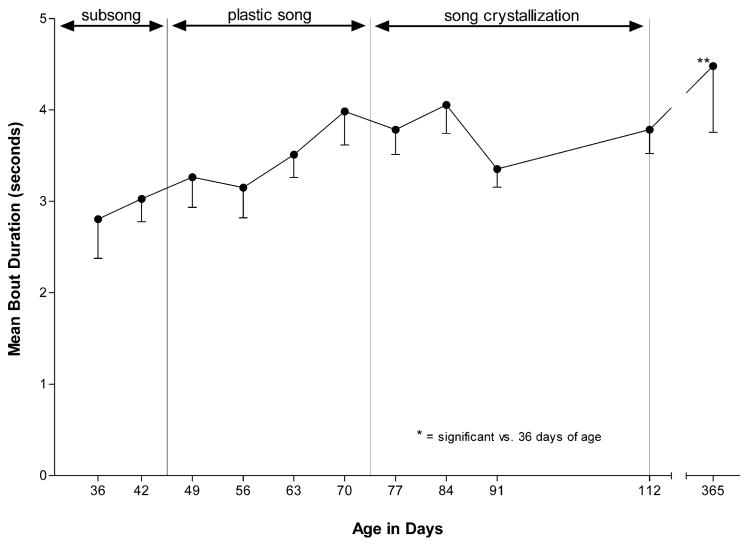
Between 36 and 365 days of age, birds showed an inverted U-shaped pattern of undirected song bout production (F4,10 =5.44, P < 0.001), with the greatest song bout production occurring during plastic song and song crystallization. SNK comparisons revealed that birds sang significantly fewer song bouts at 36 days of age than at any age between 49 and 112 days of age, and that birds sang significantly fewer song bouts at 365 days of age than at 56 and 77 days of age (see Fig. 2). The song bout production that we measured at 365 days of age fell within the range of variation we have observed in other studies of daily undirected song bout production by adult birds [25]. Although song bout duration tended to increase as a function of age (F4,10 = 2.84, P < 0.01), the only significant age-group difference was that birds sang longer bouts at 365 days of age versus 36 days of age (see Fig. 3).
Fig. 2.
The number of undirected song bouts produced per day shows an inverted U-shaped pattern across vocal development. Data are the mean number of song bouts produced per day (−S.E.) by a group of birds recorded for 24 h at 36, 42, 49, 56, 63, 70, 77, 84, 91, 112, and 365 days of age. Daily song bout production was significant higher during plastic song and song crystallization than during subsong or in adulthood. An extrapolation of these data suggests that on average, male zebra finches produce well over 50 000 bouts of undirected song during vocal development.
Fig. 3.
Song bout duration is relatively stable across vocal development. Data are the mean song bout duration (−S.E.) produced by a group of birds recorded for 24 h at 36, 42, 49, 56, 63, 70, 77, 84, 91, 112, and 365 days of age. Ten song bouts were randomly selected and measured from each subject at each age to generate means. The song bout duration of birds at 365 days of age was significantly longer than at 36 days of age.
3.4. Note-sequence fidelity of crystallized song patterns is related to quantity of plastic song
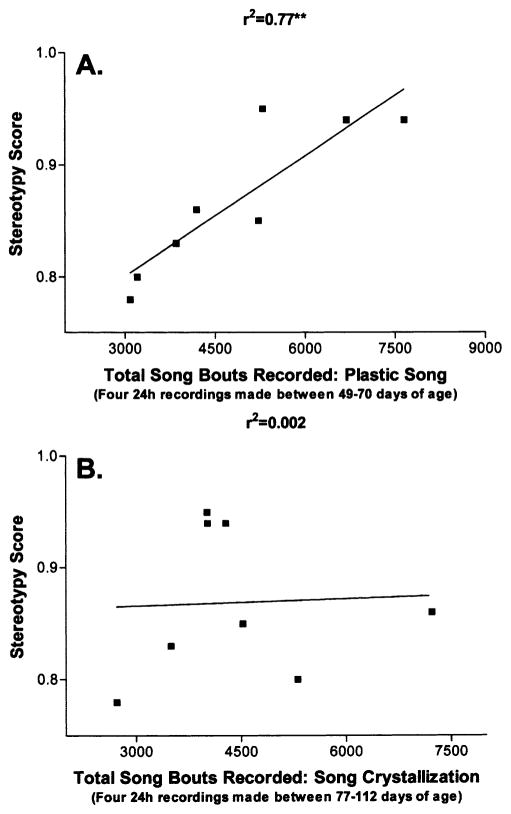
The birds sang at high levels when they were in plastic song and during song crystallization, although individual differences were large (Fig. 2). These individual differences appear to be meaningful in that greater vocal production during plastic song was associated with fewer note-sequencing errors in the crystallized vocal pattern. That is, the cumulative number of song bouts recorded when birds were in plastic song (four 24 h recordings between 49 and 70 days of age) showed a significant positive correlation to the stereotypy scores generated at 112 days of age (r2 = 0.77, P < 0.01, see Fig. 4A). In contrast, there was no relationship between the cumulative number of song bouts recorded during song crystallization (four 24 h recordings between 77 and 112 days of age) and the stereotypy scores (r2 = 0.002, n.s., Fig. 4B).
Fig. 4.
Cumulative undirected song bout production recorded during plastic song (A), but not during song crystallization (B), is related to the note-sequence fidelity of the crystallized vocal pattern. Data are the total number of song bouts recorded from individual birds (n=8) during plastic song (A, four 24 h recordings), or during song crystallization (B, four 24 h recordings), as a function of the stereotypy score of the vocal patterns recorded at the end of song crystallization (112 days of age). See text for details on how stereotypy scores were measured.
4. Discussion
In part, our findings replicate the classic studies of Immelman [11] and Arnold [1] in showing that during sensory-motor learning, male zebra finches progress through distinct behavioral stages of subsong, plastic song, and song crystallization. We also present new data that reveal a strong diurnal pattern of undirected song bout production during vocal development and in adulthood. However, the major finding reported here is our observation that birds sing more frequently during plastic song and song crystallization than they do as adults. Moreover, individual variation in the amount of plastic song produced appears to have consequences for the note-sequence fidelity of the crystallized vocal pattern. We found that birds with higher levels of song bout production during plastic song (49–70 days of age) had higher stereotypy scores at 112 days of age. Since we found no correlation between the amount of singing during song crystallization (77–112 days of age) and song stereotypy, our data suggest a developmental sensitive period for vocal practice during plastic song with consequences for the note-sequence fidelity of the crystallized song pattern.
Important for the interpretation of our results is the distinction that zebra finch sensory-motor learning involves at least two motor processes: vocal imitation (learning to produce notes) and vocal stereotypy (learning to recite a stable sequence of notes). The development of these two processes may involve separate mechanisms. For example, Morrison and Nottebohm [18] reported that birds raised from 28 days of age in visual isolation produced stereotyped vocal patterns as adults (as judged by visual inspection of sonagrams), yet these birds were subsequently able to learn new notes from a tutor. These data suggest that vocal stereotypy develops independent of the ability to learn a new vocal gesture. However, Morrison and Nottebohm [18] did not quantify vocal stereotypy, so it is possible that the ability of their birds to learn new notes as adults may have been related to the degree of stereotypy of their vocal pattern at the time of tutoring. It is also important to emphasize that our findings do not address the issue of practice in vocal imitation, since we do not have access to the songs of our subjects’ tutors (an annual replacement of breeding stock in our aviaries prevents us from making this comparison post-hoc). However, subjects in the present study were exposed to their tutor’s song up to 35 days of age, which Bohner reports as sufficient for good copying [2,3], and some of them are now being successfully bred in the laboratory, suggesting that female zebra finches find their songs to be an acceptable courtship display. In any case, while the relationship between vocal production during plastic song and note-sequence fidelity seems clear, we cannot say whether this influenced the fidelity of note copying, which we view as a related, but separate question. Readers should see a recent report by Tchernichovski et al. [30] where note learning during plastic song is described in detail.
4.1. A sensitive period for vocal practice
To our knowledge, Pytte and Suthers [24] were the first to identify a sensitive period for vocal practice during sensory-motor learning, and their data are an important point of reference for the present findings. These investigators induced 2–3-week periods of vocal paralysis during subsong, plastic song, or song crystallization; in addition, combined treatments were given during subsong and plastic song, subsong and song crystallization, or plastic song and song crystallization. Surprisingly, vocal paralysis during subsong and/or plastic song had no effect on note learning. However, vocal paralysis during song crystallization impaired note learning, and vocal paralysis during subsong and again during song crystallization resulted in abnormal note structure. Vocal paralysis during plastic song and again during song crystallization produced the greatest disruption of note learning, with song patterns containing frank abnormalities of note structure and temporal patterning.
Although we measured a different behavioral outcome (vocal stereotypy), a theoretical model where the effects of vocal rehearsal during plastic song are consolidated during song crystallization is consistent with our data and the findings of Pytte and Suthers [24]. That is, vocal production during song crystallization may help to consolidate a vocal pattern rehearsed during subsong and plastic song. This would explain why disruption of the storage mechanism alone (vocal paralysis during song crystallization) is sufficient to produce a deficit in note learning. However, if the quality of the vocal pattern to be stored depends to a greater degree on vocal rehearsal during plastic song than subsong, combined disruption of plastic song and song crystallization should produce the greatest deficit, which is the result observed by Pytte and Suthers [24]. Why then should a period of vocal paralysis during subsong and/or plastic song produce no deficit in note learning? One possibility suggested by our data is that the high level of vocal production during plastic song and song crystallization (see Fig. 2) allows birds to recover from a temporary vocal paralysis. It would be of interest to determine whether birds subject to periods of vocal paralysis during song development show unusually high levels of singing as the capacity for vocal production returns.
A sensitive period for vocal practice during plastic song requires developmental processes that open and close this period and neural mechanisms to store the effects of vocal rehearsal. A candidate mechanism, suggested by Bottjer and Hewer [4], is that gonadal steroids may regulate the phase of plastic song. These investigators treated juvenile male zebra finches with an estrogen synthesis inhibitor and/or an androgen receptor antagonist and found that the adult songs of these birds contained poorly modulated notes and lacked note-sequence stereotypy. This pattern of results suggests that the hormonal manipulations had broad disruptive effects on song development that may have included a delayed onset of song crystallization, impaired storage of the effects of vocal rehearsal, and impaired note learning. It may be useful in future studies to manipulate levels of gonadal steroids during song development to detect relationships between steroid levels, the quantity of vocal rehearsal during plastic song and song crystallization, and the quality of note learning and vocal stereotypy in adulthood.
An unexpected observation in the present study was the change in the diurnal pattern of song bout production during vocal development. A morning/evening diurnal rhythm of vocal production was evident when birds were producing subsong and plastic song, but as birds reached the stage of song crystallization, vocal production tended to be produced in a single diurnal peak (Fig. 1). One possible explanation for this rhythm is that vocal production may be particularly energetically inefficient during subsong and plastic song, such that birds must break from singing to replenish their reserve of energy. Efforts to measure the energy cost of singing suggest that passerine song may introduce an energy cost that, while substantially less than flight, may be greater than for other common behaviors [6,7,9]. Alternatively, perhaps a period of sustained singing leads to the saturation of molecular mechanisms that store the effects of song rehearsal, and a period of relative quiescence is required before further singing can reactivate such mechanisms. For example, because singing drives expression of the transcription factor ZENK in several vocal-control regions of the songbird telencephalon [12,13,31], a saturation of ZENK transcriptional activity could potentially underlie the strong diurnal rhythm of vocal production in juveniles. Whether the diurnal pattern of singing reflects energetic constraints or an optimization of the molecular benefits of vocal rehearsal, it is interesting to note a remarkable similarity in the diurnal pattern of singing by juvenile zebra finches and practice by human students who are mastering a fine perceptual/motor skill (learning to play a musical instrument at an expert level, see [8]).
A final relationship is worth mentioning in the context of practice and maturation in vocal development. In mammalian systems, it is well established that size of functionally defined brain regions (e.g. digit representations in primary sensory or primary motor cortex) shows expansion/contraction as a consequence of use/disuse [22,15]. Although no brain morphological measurements were made in the present study, previous morphological studies of the song-control system [17,10] indicate that the telencephalic song region RA (the robust nucleus of the archistriatum) undergoes a developmental pattern of volumetric growth and regression that is similar to the inverted U-shaped pattern of daily song bout production that we have observed. Since RA forms the primary motor output of forebrain song processing, one possibility is that the morphology of this structure is a reflection of its use. However, it might also be the case that developmental growth and regression of RA volume are driven by maturational processes that in turn lead to changes in song bout production. To determine which hypothesis is correct, experiments must be conducted that manipulate motivational mechanisms and stimulus conditions to influence the amount of daily song production. As a complement to the paralytic technique of Pytte and Suthers [24], we have developed two methods to manipulate daily song production (cannabinoid exposure [28] and food restriction [25]) that we plan to use in tests of these and other hypotheses in future studies.
The authors are indebted to Elizabeth Foster, Tony Conigliaro, and Clayton Jones for assistance in collecting and analyzing the over 15 GB of digitally recorded zebra finch vocal patterns presented here. This research was supported by a National Institute of Health grants (DC02035) to Frank Johnson and Ken Soderstrom (DA05986-01). Osceola Whitney is an American Psychological Association MFP Fellow in Neuroscience.
References
- 1.Arnold AP. The effects of castration on song development in zebra finches (Poephila guttata) J Exp Zool. 1975;191:261–78. doi: 10.1002/jez.1401910212. [DOI] [PubMed] [Google Scholar]
- 2.Bohner J. Song learning in the zebra finch (Taeniopygia guttata): selectivity in the choice of a tutor and accuracy of song copies. Anim Behav. 1983;31:231–7. [Google Scholar]
- 3.Bohner J. Early acquisition of song in the Zebra Finch, Taeniopygia guttata. Anim Behav. 1990;39:369–74. [Google Scholar]
- 4.Bottjer SW, Hewer SJ. Castration and antisteroid treatment impair vocal learning in male zebra finches. J Neurobiol. 1992;23:337–53. doi: 10.1002/neu.480230402. [DOI] [PubMed] [Google Scholar]
- 5.Eales LA. The influences of visual and vocal interaction on song learning in zebra finches. Anim Behav. 1989;37:507–8. [Google Scholar]
- 6.Eberhardt LS. Oxygen consumption during singing by male carolina wrens (Thryothorus ludovicianus) Auk. 1994;111:124–30. [Google Scholar]
- 7.Eberhardt LS. Energy expenditure during singing: a reply to Gaunt et al. Auk. 1996;113:721–3. [Google Scholar]
- 8.Ericsson KA, Krampe RTh, Tesch-Romer C. The role of deliberate practice in the acquisition of expert performance. Psychol Rev. 1993;100:363–406. [Google Scholar]
- 9.Gaunt AS, Bucher TL, Gaunt SLL, Baptista LF. Is singing costly? Auk. 1996;113:718–21. [Google Scholar]
- 10.Herrmann K, Bischof HJ. Delayed development of song control nuclei in the zebra finch is related to behavioral development. J Comp Neurol. 1986;245:167–75. doi: 10.1002/cne.902450204. [DOI] [PubMed] [Google Scholar]
- 11.Immelmann K. Song development in the zebra finch and other estrildid finches. In: Hinde RA, editor. Bird Vocalizations. London and New York: Cambridge University Press; 1969. pp. 61–77. [Google Scholar]
- 12.Jarvis ED, Nottebohm F. Motor-driven gene expression. Proc Natl Acad Sci USA. 1997;94:4097–102. doi: 10.1073/pnas.94.8.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin H, Clayton DF. Localized changes in immediate-early gene regulation during sensory and motor learning in zebra finches. Neuron. 1997;19:1049–59. doi: 10.1016/s0896-6273(00)80396-7. [DOI] [PubMed] [Google Scholar]
- 14.Jones AE, Ten-Cate C, Slater PJB. Early experience and plasticity of song in adult male zebra finches. J Comp Psychol. 1996;110:354–69. [Google Scholar]
- 15.Kaas JH. The reorganization of somatosensory and motor cortex after peripheral nerve or spinal cord injury in primates. Prog Brain Res. 2000;128:173–9. doi: 10.1016/S0079-6123(00)28015-1. [DOI] [PubMed] [Google Scholar]
- 16.Konishi M. The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Z Tiepsychol. 1965;22:770–83. [PubMed] [Google Scholar]
- 17.Konishi M, Akutagawa A. Neuronal growth, atrophy and death in a sexually dimorphic song nucleus in the zebra finch brain. Nature. 1985;315:145–7. doi: 10.1038/315145a0. [DOI] [PubMed] [Google Scholar]
- 18.Morrison RG, Nottebohm F. Role of a telencephalic nucleus in the delayed song learning of socially isolated zebra finches. J Neurobiol. 1993;24:1045–64. doi: 10.1002/neu.480240805. [DOI] [PubMed] [Google Scholar]
- 19.Marler P. On innateness: are sparrow songs “learned” or “innate”? In: Hauser MD, Konishi M, editors. The Design of Animal Communication. Cambridge and London: MIT Press; 1999. pp. 293–318. [Google Scholar]
- 20.Marler P, Peters S. Subsong and plastic song: their role in the vocal learning process. In: Kroodsma DE, Miller EH, editors. Acoustic Communication in Birds. New York: Academic Press; 1982b. pp. 25–50. [Google Scholar]
- 21.Nottebohm F. Anatomy and timing of vocal learning in birds. In: Hauser MD, Konishi M, editors. The Design of Animal Communication. Cambridge and London: MIT Press; 1999. pp. 63–110. [Google Scholar]
- 22.Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price PH. Developmental determinants of structure in zebra finch song. J Comp Physiol Psychol. 1979;93:268–77. [Google Scholar]
- 24.Pytte CL, Suthers RA. Sensitive period for sensorimotor integration during vocal motor learning. J Neurobiol. 2000;42:172–89. doi: 10.1002/(sici)1097-4695(20000205)42:2<172::aid-neu2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 25.Rashotte ME, Sedunova EV, Johnson F, Pastukhov IF. Influence of food and water availability on undirected singing and energetic status in adult male zebra finches (Taeniopygia guttata) Physiol Behav. 2001 doi: 10.1016/s0031-9384(01)00600-x. in press. [DOI] [PubMed] [Google Scholar]
- 26.Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J Neurosci. 1991;11:2896–913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slater PJB, Eales LA, Clayton NS. Song learning in zebra finches (Taeniopygia guttata): progress and prospects. In: Rosenblatt J, Beer C, Busnel MC, Slater PJB, editors. Advances in the Study of Behaviour. Vol. 18. San Diego: Academic; 1988. pp. 1–32. [Google Scholar]
- 28.Soderstrom K, Johnson F. Zebra finch CB1 cannabinoid receptor: pharmacology and in vivo and in vitro effects of activation. J Pharm Exp Ther. 2001;279:189–97. [PubMed] [Google Scholar]
- 29.Tchernichovski O, Nottebohm F. Social inhibition of song imitation among sibling male zebra finches. Proc Natl Acad Sci USA. 1998;95:8951–6. doi: 10.1073/pnas.95.15.8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tchernichovski O, Mitra PP, Lints T, Nottebohm F. Dynamics of the vocal imitation process: how a zebra finch learns its song. Science. 2001;291:2564–9. doi: 10.1126/science.1058522. [DOI] [PubMed] [Google Scholar]
- 31.Whitney O, Soderstrom K, Johnson F. Post-transcriptional regulation of zenk expression associated with zebra finch vocal development. Mol Brain Res. 2000;80:279–90. doi: 10.1016/s0169-328x(00)00178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zann R. The Zebra Finch: A Synthesis of Field and Laboratory Studies. New York: Oxford University Press; 1996. [Google Scholar]