Abstract
Immune or brain proinflammatory signaling has been linked to some of the behavioral effects of alcohol. Immune signaling appears to regulate voluntary ethanol intake in rodent models, and ethanol intake activates the immune system in multiple models. This bidirectional link raises the possibility that consumption increases immune signaling, which in turn further increases consumption in a feed-forward cycle. Data from animal and human studies provide overlapping support for the involvement of immune-related genes and proteins in alcohol action, and combining animal and human data is a promising approach to systematically evaluate and nominate relevant pathways. Based on rodent models, neuroimmune pathways may represent unexplored, nontraditional targets for medication development to reduce alcohol consumption and prevent relapse. Peroxisome proliferator-activated receptor agonists are one class of anti-inflammatory medications that demonstrate antiaddictive properties for alcohol and other drugs of abuse. Expression of immune-related genes is altered in animals and humans following chronic alcohol exposure, and the regulatory influences of specific mRNAs, microRNAs, and activated cell types are areas of intense study. Ultimately, the use of multiple datasets combined with behavioral validation will be needed to link specific neuroimmune pathways to addiction vulnerability.
1. INTRODUCTION
Effects of excessive alcohol consumption on innate immune signaling have been appreciated for many years, in part because of the critical role of immune dysregulation in alcoholic liver disease (Szabo, Mandrekar, Petrasek, & Catalano, 2011). More recently, the actions of alcohol on neuroimmune function have attracted attention. This area of research likely originated from gene expression profiles of human alcoholic brain, which found an unexpected abundance of changes in immune-related genes (Lewohl et al., 2000). These results were confirmed and extended in subsequent studies of human alcoholics, and changes in neuroimmune gene expression and signaling were also found in rodent models of excessive alcohol consumption. Recent studies led to the surprising finding that neuroimmune signaling is critical for regulation of neuroplasticity and is required for learning and memory (Williamson & Bilbo, 2013; Yirmiya & Goshen, 2011). These findings support the hypothesis that actions of alcohol on neuroimmune function may be important for the development of hallmarks of dependence such as escalation of consumption, craving, tolerance, and withdrawal. This chapter reviews evidence that immune signaling regulates alcohol consumption and that excessive alcohol consumption changes neuroimmune signaling, allowing a positive feedback increase in consumption, craving, and dependence.
2. IMMUNE REGULATION OF ETHANOL CONSUMPTION AND ETHANOL REGULATION OF IMMUNE SIGNALING
Immune signaling has been shown to regulate alcohol consumption in various mouse models and drinking paradigms. Alcohol consumption was studied in mutant mice with deletions of chemokine (Ccl2, Ccl3) and che-mokine receptor genes (Ccr2, Ccr5) (Blednov et al., 2005). Deletion of Ccr2, Ccl2 (in females), and Ccl3 reduced ethanol preference and consumption in a two-bottle choice test, and ethanol administration (i.p.) produced a stronger conditioned taste aversion in Ccr2-, Ccl2-, and Ccl3-knock-out mice.
Knock-out mice were used to study six other immune-related genes previously linked to alcohol consumption in a gene expression analysis in mouse brain: B2m (beta-2 microglobulin), Cd14 (cluster of differentiation 14), Il 1rn (interleukin-1 receptor antagonist), Il6 (interleukin 6), Ctss (cathepsin S), and Ctsf (cathepsin F) (Blednov et al., 2011). Ethanol consumption and preference were reduced in all of these knock-out mice in a 24-h two-bottle choice test with no changes in saccharin or quinine consumption. In addition, ethanol consumption was reduced in B2m-, Il6-, Cd14-, Il1rn-, and Ctss-knock-out mice in a limited access two-bottle choice test, and in Il1rn and Ctss knockouts in a limited access test to one bottle of ethanol only. In contrast, a transgenic mouse line overexpressing Il6 showed increased alcohol preference in females but not in males (Harris & Blednov, 2013).
Manipulations of the Toll-like receptor 4 (TLR4)-myeloid differentiation primary response gene 88 (MyD88) signaling cascade (Fig. 2.1) also alter ethanol responses. Knockout of TLR4 and MyD88 as well as TLR4 antagonism by (+)-naloxone in mice decreased both the duration of loss of righting reflex and motor impairment recovery time induced by ethanol (Wu et al., 2012). C3H/HeJ mice are naturally TLR4 deficient and show decreased operant self-administration of ethanol compared to the control strain (C3H/HeOuJ) (Harris & Blednov, 2013); however, ethanol consumption is not changed in TLR4-knock-out mice (Pascual, Balino, Alfonso-Loeches, Aragon, & Guerri, 2011). In contrast, injection of TLR4 siRNA into the central amygdala of alcohol preferring rats reduced operant self-administration of ethanol (Liu et al., 2011). These targeted disruptions of TLR4 pathways are important for understanding how discrete immune signaling in the brain may impact consumption. TLRs are key regulators of immune activation in the CNS in response to alcohol and have well-established roles in pathogen detection and initiation of innate and adaptive immunity during infection. Future experiments are needed to determine if other TLRs also regulate behavioral responses to alcohol.
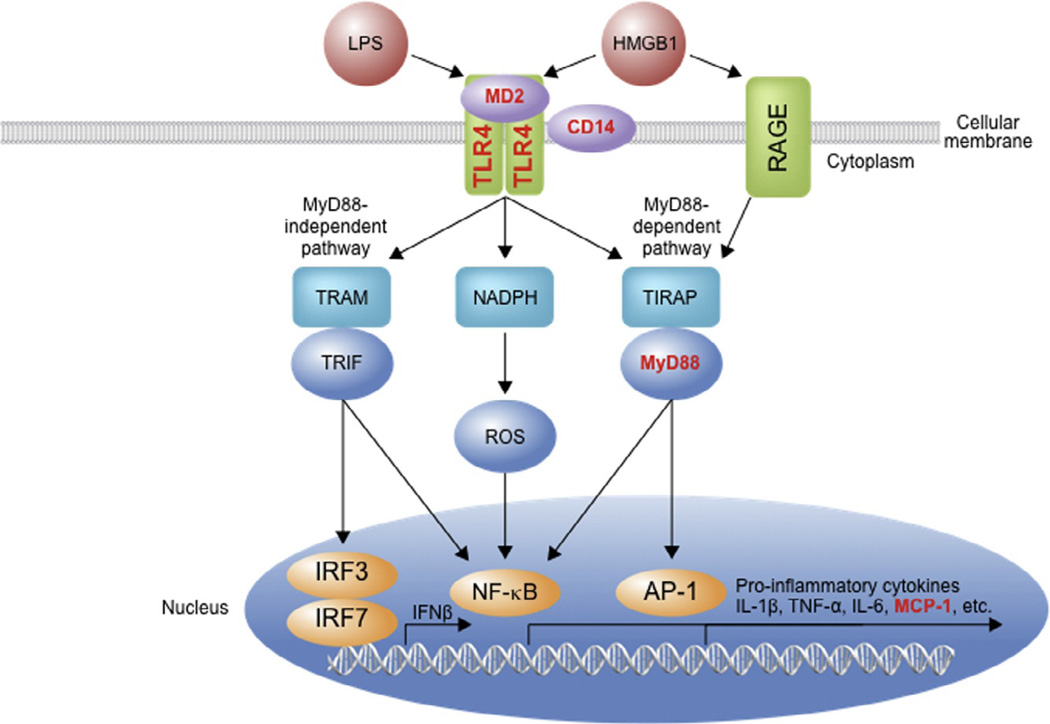
Figure 2.1.
TLR4 signaling cascade. TLRs signal as dimers and heterodimers that recruit adaptor proteins such as CD14 and MD2. Depending on the adaptors recruited by the activated TLR, different pathways are triggered, all of which culminate in activation of the proinflammatory transcription factors. One pathway involves MyD88 and TIRAP and activates NF-κB via IκB kinase and also activates AP-1. Another pathway involving NADPH oxidase activates NF-κB via ROS. TRIF and TRAM signaling proteins (via MyD88-independent pathway) also initiate signal cascades, culminating in activation of NF-κB and other proinflammatory transcription factors. RAGE is another transmembrane receptor operating in innate immune cells that is known to respond to HMGB1, and this pathway also induces proinflammatory gene transcription via NF-κB activation. The release of cytokines such as TNF-α, HMGB1, IL-1β, chemokines, proteases, and ROS activate adjacent cells. These cytokines affect the brain and are thought to contribute to the etiology, progression, and persistence of alcohol addiction. Bold red font (bold grey in the print version) indicates a gene that has been manipulated and shown to affect ethanol-related behavior. NF-κB, nuclear factor-κ-light-chain-enhancer of activated B cells; MyD88, myeloid differentiation primary response gene 88; AP-1, activated protein-1; TIRAP, toll-interleukin-1 receptor (TIR) domain containing adaptor protein; ROS, reactive oxygen species; TRIF, TIR-domain-containing adaptor-inducing IFNβ; IRF, interferon regulatory factor; TRAM, TRIF-related adaptor molecule; RAGE, receptor for advanced glycation end products; TNF-α, tumor necrosis factor-α; IL-1 β, interleukin-1β; IL-6, interleukin-6; MCP-1, monocyte chemoattractant protein-1.
Many studies have shown that alcohol increases expression of immune-related mRNA and protein across species (summarized in Table 2.1). Increased expression of the cytokine MCP-1 (monocyte chemoattractant protein 1, also known as CCL2) and microglial markers was observed in postmortem human alcoholic brains (He & Crews, 2008). In rats, binge ethanol (intragastric injection) exposure increases DNA binding of brain nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), cyclooxygenase 2 (COX-2) expression, and microglial activation, and ethanol also induces NF-κB binding and production of proinflammatory cytokines in rat brain slice cultures (Crews et al., 2006; Zou & Crews, 2010). Chronic ethanol exposure in mice increases levels of the immune proteins CD14, TNF-α (tumor necrosis factor α), IL-1β (interleukin-1 beta), NF-κB p65, iNOS (inducible nitric oxide synthase), and COX-2 in the cortex, but these changes are not observed in TLR4-knock-out mice (Alfonso-Loeches et al., 2010).
Table 2.1.
Immune-related expression changes following ethanol exposure
| Increased mRNA | Increased protein | Cell-type activated |
|
|---|---|---|---|
| Cell cultures | |||
| Macrophages | TNF-α, IL-1β, NO, iNOS, NF-κB p65, COX-2a |
||
| Primary microglial cultures |
TNF-α, IL-1β, NO iNOS, NF-κB p65, COX-2b |
Microglial activationb |
|
| Primary astrocyte cultures |
MyD88, iNOS, COX-2c | ||
| Brain slices | |||
| Rats | TNF-α, IL-1β, MCP- 1/CCL2, iNOSd |
NF-κB (DNA binding)d; HMGB1, IL-1βe |
|
| Chronic ethanol* | |||
| Mice | MCP-1/CCL2, IL-1β, TNF-α, TLR4, HMGB1f; TLR2, TLR9, RAGE, inflammasome componentsg |
CD14, TNF-α, IL-1β, NF-κB p65, iNOS, COX- 2c; MCP-1/CCL2, phospho-HMGB1, acetyl- HMGB1g |
Microglia, GFAP +astrocytec,g |
| Binge ethanol* | |||
| Mice | TLR2, TLR3, TLR4, HMGB1e; MCP-1/CCL2, IL-6h |
TLR2, TLR3, TLR4, HMGB1e |
GFAP +astrocyteh |
| Rats | COX-2i | NF-κB (DNA binding)i | Microglial activationi |
| Postmortem brain* | |||
| Humans | RAGEj | MCP-1/CCL2k; RAGEj; IL-1β, TLR2, TLR3, TLR4, HMGB1e |
Microgliak |
Differences may be brain region specific. Abbreviations are defined in the text.
Because TLR4 deficiency protects against many alcohol-induced immune changes and normal TLR4 signaling is central to immune function, this pathway is the focus of many alcohol-immune studies. There is even evidence from studies in Drosophila showing that ethanol increases expression of genes in the Toll pathway (Kong et al., 2010). Lipopolysaccharide (LPS) is an endotoxin found on Gram-negative bacteria and a ligand for TLR4. In mice, LPS injections increased expression of proinflammatory cytokines (TNF-α, MCP-1, IL-1β, and NF-κB p65) in liver, brain, and serum and increased proliferation of brain microglia (Qin et al., 2008, 2007). Pretreatment with ethanol potentiated LPS-induced increases of TNF-α, MCP-1, and IL-1β (Qin et al., 2008). The cytokine increases observed in mouse brain remain elevated after peripheral levels return to normal, and a single LPS injection can increase alcohol consumption for up to 80 days (Blednov et al., 2011; Qin et al., 2008), suggesting that immune signaling in the brain can be much more persistent compared to peripheral immune activation. It is also noteworthy that chronic alcohol consumption and LPS treatment produce similar changes in mouse brain transcriptomes (Osterndorff-Kahanek, Ponomarev, Blednov, & Harris, 2013).
In the brain, both alcohol and LPS influence GABAergic transmission through immune signaling. Acute ethanol and LPS increased the amplitude of eIPSPs (evoked inhibitory postsynaptic potentials) in the central amygdala of wild-type mouse brain slices, while these increases were attenuated in CD14-knock-out mice (Bajo et al., 2014). Pretreatment with LPS-potentiated ethanol’s effect on eIPSP amplitude in wild-type mice and restored the effect in CD14-knock-out mice, while (+)-naloxone blocked the LPS effect and delayed the ethanol effect on eIPSPs in wild-type mice (Bajo et al., 2014). Injection of LPS also decreased dopamine neuron firing in the ventral tegmental area that persisted for at least 1 week after treatment, which may contribute to the increased alcohol consumption seen at this time point (Blednov et al., 2011).
One hypothesis is that chronic alcohol consumption compromises the tight junctions of gut epithelial cells, allowing gut-derived bacterial toxins (e.g., LPS and peptidoglycan) to be released from the gut into the bloodstream. Once in the bloodstream, LPS or peptidoglycan binds to TLR4 receptors on liver Kupffer cells and peripheral blood mononuclear cells to initiate a signaling cascade that culminates in the activation of NF-κB or AP-1 (Fig. 2.1), which increases the transcription of proinflammatory cytokines and toxic intermediates (such as reactive oxygen species and nitric oxide (NO)). Cytokines cross the blood–brain barrier via diffusion or active transport where they promote increased alcohol consumption, which increases the production of proinflammatory cytokines, which in turn increases alcohol consumption in a positive-feedback loop that leads to excessive alcohol consumption. This hypothesis is supported by studies showing an increase in serum levels of LPS, peptidoglycan, and proinflammatory cytokines in human alcoholics compared to control subjects. Moreover, these studies are the first to reveal a correlation between the levels of bacterial products and proinflammatory molecules and alcohol craving and consumption in human alcoholics (Leclercq et al., 2012; Leclercq, De Saeger, Delzenne, de Timary, & Starkel, 2014).
Although it is unlikely that LPS crosses the blood–brain barrier, it is feasible that peripheral TLR4 activation releases endogenous ligands and proinflammatory cytokines that are able to promote immune signaling in the brain (Mayfield, Ferguson, & Harris, 2013). High-mobility group box 1 (HMGB1) is an endogenous ligand for TLR2, TLR4, TLR9, and RAGE (receptor for advanced glycation end products), which is increased following ethanol consumption in mice and is also increased in postmortem alcoholic brains (Crews et al., 2013; Whitman et al., 2013). The role of HMGB1 in alcohol-induced inflammation is discussed in more detail in Chapter 10 by Crews and Vetreno.
In addition to evidence that ethanol consumption increases HMGB1 release and activates TLR signaling, Guerri and colleagues found that ethanol induces the association of TLR4 with TLR2 in lipid rafts, triggering an inflammatory response. They demonstrated that in vitro treatment of murine macrophages or astrocytes with ethanol or LPS induces translocation and clustering of TLR4 and its signaling molecules in lipid rafts (Blanco, Perez-Arago, Fernandez-Lizarbe, & Guerri, 2008; Fernandez-Lizarbe et al., 2008). In microglial cultures, ethanol also promotes TLR4/TLR2 association in lipid rafts (Fernandez-Lizarbe, Montesinos, & Guerri, 2013), inducing the production of inflammatory mediators. However, in microglial cultures derived from TLR4-knock-out mice, this inflammatory response and activation of NF-κB are not seen (Fernandez-Lizarbe et al., 2013, 2009). TLR4 activation signals through two distinct pathways: the MyD88-dependent and TRIF-dependent pathways (Fig. 2.1). Ethanol and LPS can promote TLR4 endocytosis through clathrin- and caveolae-dependent pathways, and in cortical astrocytes, TRIF-dependent signaling relies on the clathrin pathway, while disruption of rafts/caveolae inhibits both the MyD88- and TRIF-dependent signaling pathways (Pascual-Lucas, Fernandez-Lizarbe, Montesinos, & Guerri, 2014).
Alcohol treatment in mice has also been shown to activate the NLRP3/ASC inflammasome, leading to IL-1β signaling in the brain (Lippai et al., 2013). Mice-consuming ethanol for 5 weeks showed increased protein levels of MCP-1, TNF-α, IL-1β, acetyl and phosphorylated HMGB1 as well as increased TLR2, TLR4, TLR9, RAGE, and HMGB1 mRNA in the cerebellum (Lippai et al., 2013). Increases in inflammasome components (NLRP1, NLRP3, NLRC4, pannexin-1), the inflammasome adaptor molecule ASC, and the effector proteins procaspase-1 and proIL-1β were found in both the cerebellum and cortex of ethanol-consuming mice compared to controls. After ethanol treatment, TLR4-knock-out mice showed no increase in TNF-α and MCP-1 and reduced levels of IL-β and inflammasome components compared to controls (Lippai et al., 2013). Future experiments will be important to determine how alcohol affects the inflammasome cascade operating in the innate immune system and the resulting actions in brain.
Another important area of study is ethanol-induced neuroimmune signaling in adolescent models and how changes in the adolescent brain could impact consumption as adults. Age-related differences in immune signaling in response to alcohol have been demonstrated in several studies. For example, adult mice given ethanol via gavage for 10 days showed increased mRNA levels of MCP-1/CCL2 in the hippocampus, cerebellum, and cortex and increased IL-6 in the cerebellum, while adolescent mice given the same treatment showed no changes in MCP-1 or IL-6 (Kane et al., 2014). Both adult and adolescent mice showed increased GFAP (glial fibrillary acidic protein) immunostaining, suggesting that astrocytes may play a role in the inflammatory response to ethanol (Kane et al., 2014). A bioinformatics analysis of adult mouse brains showed differential expression of TLR, JAK/STAT (Janus kinase/signal transducers and activators of transcription), MAPK (mitogen-activated protein kinase), T-cell, and chemokine signaling following a drinking in the dark paradigm, while these pathways were not overexpressed in adolescent mice (Agrawal et al., 2014). Also, minocycline, which has antiinflammatory properties, reduced drinking in adult but not in adolescent mice (Agrawal et al., 2014). However, other studies have reported opposite results. Binge-like ethanol administration increased expression of TLR4, TLR2, TNF-α, and IL-1β mRNA in the prefrontal cortex of adolescent rats, while these changes were not seen in adult rats after the same treatment (Pascual, Pla, Minarro, & Guerri, 2014). These discrepancies might be attributed to differences in drinking paradigms and brain regions. In human postmortem alcoholic brains, there is an increase in protein and mRNA expression of RAGE in the orbitofrontal cortex, and a younger age of drinking onset correlated with increased expression of RAGE, TLR4, and HMGB1 (Vetreno et al., 2013). In rats, RAGE expression declines in the prefrontal cortex with age, and this decline is reduced by adolescent binge alcohol exposure (Vetreno et al., 2013). Adolescent binge ethanol treatment also led to increases in TLR4 and HMGB1 that persisted into early adulthood as well as increased TNF-α, MCP-1, NOX2 (NADPH oxidase), COX-2, and MyD88 mRNA in the young adult frontal cortex (Vetreno et al., 2013). Thus, adolescent drinking may ultimately impact immune signaling in adult brain, producing persistent alterations that could influence the development of alcohol abuse disorders. Changes in inflammatory responses in peripheral cells of teenage binge drinkers and in peripheral cells and brain microglia in a rat model of binge drinking were recently reported (Ward, Lallemand, & de Witte, 2014). Plasma cytokine levels were altered in the adolescent heavy session drinkers, and these peripheral markers point to activation of the innate immune system, which could impact the neuroimmune system, potentially affecting neurogenesis or other developmental processes that are active during this time period.
Overall, there is an ample behavioral evidence to suggest that activation of immune pathways regulates alcohol consumption and other responses in rodent models and that alcohol consumption also activates the immune system in multiple experimental models. We propose an interaction in activated cells where consumption increases neuroimmune signaling, which in turn further increases consumption in a positive-feedback fashion.
3. PEROXISOME PROLIFERATOR-ACTIVATED RECEPTORS: ANTI-INFLAMMATORY ACTION AND ROLE IN ALCOHOL CONSUMPTION
Peroxisome proliferator-activated receptors (PPARs) have an established role in regulating inflammatory responses across many tissues (Clark, 2002). This suggests that PPAR agonists could be promising for treating certain aspects of addiction, where neuroimmune and inflammatory processes play an etiopathogenic role. PPARs are intracellular receptors, belonging to the nuclear hormone receptor family of transcription factors (Issemann & Green, 1990). PPARs are widely distributed, but are most abundant in tissues with a high metabolic rate, e.g., liver, heart, and kidney (Issemann & Green, 1990). There are three isotypes of PPARs coded for by different genes on separate chromosomes: PPARα, PPARγ, and PPARβ/δ, which vary in their tissue distribution and physiological function, and all are present in brain. Most physiological functions of PPARs fall under the umbrella category of lipid homeostasis. In addition to inflammation, PPARs have defined roles in fatty acid metabolism (Aoyama et al., 1998; Schoonjans, Staels, & Auwerx, 1996), insulin sensitization (Moller & Berger, 2003), adipogenesis (Tontonoz, Hu, & Spiegelman, 1994), cell differentiation, and apoptosis (Roberts, James, Woodyatt, Macdonald, & Tugwood, 1998).
Endogenous ligands for PPARs include fatty acids and fatty acid derivatives (Gottlicher, Widmark, Li, & Gustafsson, 1992), such as endo-cannabinoids, eicosanoids (e.g., prostaglandins and leukotrienes), pho-sphoinositides, and sphingolipids (Krey et al., 1997; Sun & Bennett, 2007). PPARs can also be activated by synthetic ligands which fall into two major classes: fibrates and thiazolidinediones (TZDs). Fibrates, which include fenofibrate, ciprofibrate, clofibrate, and gemfibrozil, predominantly target PPARα and are used to treat dyslipidemia. TZDs, which include rosiglitazone and pioglitazone, predominately target PPARγ and are used to treat Type 2 diabetes. When activated by a ligand, be it endogenous or synthetic, PPARs translocate to the nucleus where they form a heterodimer with another nuclear hormone receptor, the retinoid × receptor (RXR). The PPAR–RXR complex binds to PPAR response elements in the DNA to regulate the transcription of many target genes. PPARs can also have nongenomic effects through modifying the phosphorylation status of proteins or inhibiting the activities of other transcription factors (the latter is called transrepression).
The ability of PPARs to transrepress other transcription factors, such as NF-κB, NFAT, and AP-1, is thought to be the main mechanism of action for their anti-inflammatory properties. There are three main ways in which ligand-activated PPAR–RXR complexes can negatively regulate the activities of other transcription factors (Fig. 2.2; Daynes & Jones, 2002): they can (1) compete for limited amounts of coactivators, (2) physically associate with transcription factors to inhibit their ability to induce transcription of their target genes, and (3) inhibit protein kinases, e.g., MAPK, from phosphorylating (i.e., activating) other transcription factors.
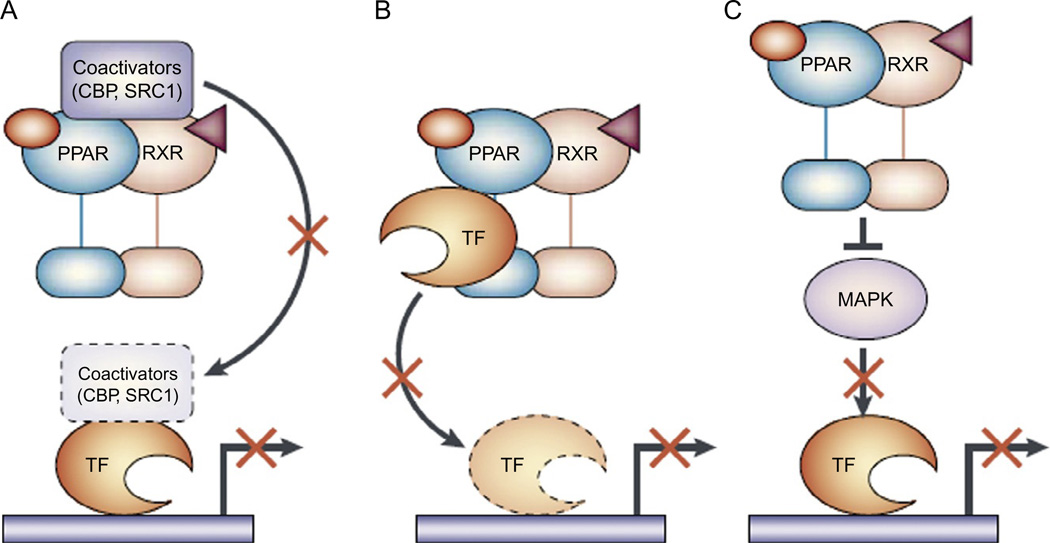
Figure 2.2.
Negative regulation of transcription factors by PPARs. The inflammatory and immunomodulatory properties of peroxisome proliferator-activated receptors (PPARs) do not arise primarily through their transcription-factor transactivation abilities, but rather through their ability to antagonize several important signaling cascades. Although different transrepression mechanisms exist, three are mediated by PPARs in cells of the immune system. (A) The first mechanism is the ability of PPARs to compete for limiting amounts of coactivator proteins in a cell, such as cAMP response element binding (CREB)-binding protein (CBP) and steroid receptor coactivator 1 (SRC1), making these coactivators unavailable to other transcription factors (TFs). (B) The second mechanism is known as “cross coupling” or “mutual receptor antagonism” and is facilitated by the ability of PPARs to associate physically with various transcription factors, preventing the transcription factor from binding to its response element and thereby inhibiting its ability to induce gene transcription. (C) Another transrepression mechanism relies on the ability of the PPAR to inhibit activation of a mitogen-activated protein kinase (MAPK). This inhibits the MAPK from phosphorylating and activating downstream transcription factors. RXR, 9-cis-retinoic acid receptor. Figure and legend are from Daynes and Jones (2002).
PPAR agonists have been shown to modulate alcohol consumption. The PPARγ agonists, pioglitazone and rosiglitazone, reduced voluntary alcohol consumption in rats (Stopponi et al., 2013, 2011) and decreased stress-induced (but not cue-induced) relapse and alcohol withdrawal symptoms in alcohol-dependent rats (Stopponi et al., 2011). PPARα agonists also decrease alcohol consumption. The PPARα agonist gemfibrozil decreased voluntary alcohol consumption in male Sprague-Dawley rats (Barson et al., 2009). One study, however, showed that clofibrate increased voluntary alcohol consumption in male spontaneous hypertensive rats, although a very high ethanol concentration was used (30% ethanol, v/v) (Schlicht, 1987). The PPARα agonist, fenofibrate, and the dual PPARα/γ agonist, tesaglitazar, selectively decreased voluntary alcohol consumption and preference in mice (Blednov et al., submitted; Ferguson et al., 2014). Gene expression profiling revealed that fenofibrate and tesaglitazar changed the transcriptome of mouse amygdala and prefrontal cortex (Ferguson et al., 2014), two important brain areas for reward and dependence. The list of PPAR agonist-regulated genes was dominated by known neuronal genes, which was unexpected given the importance of glial cells (especially microglia) in regulating neuroimmune and inflammatory signaling in brain (Ferguson et al., 2014). Genes coding for proteins involved in synaptic plasticity and nerve impulse transmission were prominent. In the amygdala, genes involved in GABAergic, dopaminergic, and neuropeptide signaling were regulated in a coordinated manner (coexpressed) by the PPAR agonists, including many already known to alter alcohol consumption based on mutant mouse data (Ferguson et al., 2014).
In addition to alcohol consumption, PPAR agonists effectively reduced intake of other drugs of abuse. PPARα agonists blocked acquisition of nicotine self-administration in naïve rats and nonhuman primates and decreased nicotine intake and cue- and drug-induced relapse in nicotine-dependent rats and nonhuman primates (Mascia et al., 2011; Panlilio et al., 2012). Also, PPAR agonists modulated sensitization to morphine and methamphetamine (but not cocaine) in mice (Fernandez-Espejo, Ramiro-Fuentes, & Rodriguez de Fonseca, 2009; Maeda et al., 2007).
Thus, PPAR agonists represent a class of anti-inflammatory medications that demonstrate antiaddictive properties for several drugs of abuse. Specifically, PPARα- and PPARγ-selective agonists (fibrates and TZDs, respectively) and PPARα/γ dual agonists have been shown to decrease alcohol consumption in rodent models of alcohol dependence. Whether these properties are mediated via their anti-inflammatory actions remains to be proven. Nevertheless, this class of drugs offers a promising new avenue for treating aspects of alcohol dependence. The favorable safety profile and FDA-approval status of some of the PPAR agonists that are effective for reducing alcohol consumption warrant further investigation into their potential as therapeutics for alcohol dependence.
4. ALCOHOL CONSUMPTION AND NEUROIMMUNE-RELATED GENE EXPRESSION
Chronic alcohol consumption and abuse induce long-term changes in brain gene and protein expression, which likely contribute to the neuropathologies associated with alcohol dependence (Kauer & Malenka, 2007; Nestler, 2001). Alcohol-induced transcriptional reprogramming in the brain may account for some of the effects of repeated ethanol exposure (Mayfield, Harris, & Schuckit, 2008; Ron & Messing, 2013). Neuroadaptations induced by alcohol are ultimately controlled by the regulation of many genes expressed within individual neurons or glial cells (Farris & Miles, 2012), causing brain cells to adapt via alterations involving many biological pathways, including growth factors (Ron & Messing, 2013), serine–threonine kinases (Lesscher et al., 2009; Sanna, Simpson, Lutjens, & Koob, 2002), glutathione pathway enzymes, protein translation (Barak et al., 2013; Neasta, Ben Hamida, Yowell, Carnicella, & Ron, 2010), transcription (Edenberg et al., 2008; Okvist et al., 2007), and inflammatory pathways (Blednov et al., 2011; Gorini, Harris, & Mayfield, 2014).
The role of immune-related genes in alcohol dependence is also supported by genetic association studies and transcriptome meta-analysis in peripheral human cells (Boukli et al., 2010), postmortem brains of alcoholics (Liu et al., 2006; Okvist et al., 2007; Ponomarev, Wang, Zhang, Harris, & Mayfield, 2012), drinking models in mice (Gorini, Nunez, & Mayfield, 2013; Gorini, Roberts, & Mayfield, 2013; Nunez et al., 2013; Osterndorff-Kahanek et al., 2013), and vapor models for alcohol dependence in mice (Gorini, Nunez, et al., 2013; Gorini, Roberts, et al., 2013). Many immune-associated genes that are differentially expressed in brains of ethanol-treated mice are also differentially expressed in brains of human alcoholics. Deletion of several immune-related genes that emerged as candidates from the genomic analyses was validated in behavioral studies and found to reduce ethanol consumption in mice, as discussed previously (Blednov et al., 2012).
Upregulation of genes encoding different classes of chaperones has been reported after ethanol treatment (Miles, Diaz, & DeGuzman, 1991; Nunez et al., 2013; Pignataro, Varodayan, Tannenholz, & Harrison, 2009; Varodayan, Pignataro, & Harrison, 2011). Examples include stress-induced chaperones (Hsp 70 and Hsp 90 family members; Nunez et al., 2013), which are implicated in proteostasis (Hutt & Balch, 2010), endotoxin-like effects mediated through TLR4 (Gupta et al., 2013; Lee, Jeong, & Yoo, 2013), and may cocluster with the LPS complex (Triantafilou & Triantafilou, 2002). Alcohol-responsive gene modules from frontal cortex of alcohol-treated mice were also enriched in cytokine pathways and synaptic transmission processes that involve Toll-like and IL-1 receptor signaling (Nunez et al., 2013).
Studies in human alcoholics identified differential expression of genes related to the NF-κB pathway. Genes with NF-κB elements were generally upregulated in alcoholics (Okvist et al., 2007), and NFKB1 has been associated with alcohol dependence in alcoholics (Edenberg et al., 2008). Furthermore, the NF-κB pathway may undergo a compensatory, adaptive tolerance to alcohol, which could serve to diminish excessive stimulation of this system (Yakovleva, Bazov, Watanabe, Hauser, & Bakalkin, 2011). NF-κB and the signaling pathways that regulate its activity have become a focal point for intense drug discovery efforts (Gupta, Sundaram, Reuter, & Aggarwal, 2010; Karin, Yamamoto, & Wang, 2004) and may also be promising therapeutic targets for alcohol dependence.
A large gene associated study of human alcoholics and healthy controls found a susceptibility locus in neurokinin-1 receptor (NK-1R) that was associated with alcohol dependence (Seneviratne et al., 2009). Furthermore, NK-1R antagonism-altered alcohol consumption in mice (Thorsell, Schank, Singley, Hunt, & Heilig, 2010) and humans (George et al., 2008), and gene therapy in mice using an inhibitory RNA against NK-1R reduced alcohol consumption (Baek et al., 2010). Modulation of the NK-1R system can exacerbate inflammatory immune responses within the CNS, and an NK-1R antagonist attenuates increases in CNS inflammatory cytokine levels and decreases immunosuppressive cytokine production associated with infection (Chauhan, Kluttz, Bost, & Marriott, 2011). NK-1R antagonists are safe clinical drugs that are known to have anti-inflammatory, analgesic, anxiolytic, antidepressant, and antiemetic effects (Rosso, Munoz, & Berger, 2012), and the findings above indicate that these antagonists may warrant further study for the treatment of alcoholism.
5. ALCOHOL CONSUMPTION AND NEUROIMMUNE-RELATED MICRORNAS
microRNAs (miRNAs) comprise a specific class of small noncoding RNAs that bind to complementary sequences on target mRNAs to repress translation and silence gene expression (Ambros, 2001; Lee & Ambros, 2001) and are key regulators of cellular gene expression. They are highly abundant in brain and mediate multiple biological processes, including brain development (Krichevsky, King, Donahue, Khrapko, & Kosik, 2003), synapse formation (Schratt et al., 2006), synaptic plasticity (Cohen, Lee, Chen, Li, & Fields, 2011; Smalheiser & Lugli, 2009), and neuroimmune signaling (Soreq & Wolf, 2011). miRNAs are capable of eliciting targeted actions in innate immunity- and epigenetic-related functions in glial cells (Nunez & Mayfield, 2012) and have been associated with development of the immune system and regulation of multiple immune functions (O’Neill, Sheedy, & McCoy, 2011). Also, TLR signaling can modulate miRNA expression via NF-κB regulation (Taganov, Boldin, Chang, & Baltimore, 2006).
The ability of miRNAs to act as central regulators of multiple cellular pathways may influence the patterns of gene expression produced by alcohol in rodents (Gorini, Nunez, et al., 2013; Pietrzykowski et al., 2008; Tapocik et al., 2013), humans (Lewohl et al., 2011) and human-derived neuroblastoma cells (Yadav et al., 2011). miRNAs are also implicated in the adaptations induced by exposure to other drugs of abuse, including nicotine (Huang & Li, 2009), cocaine (Chandrasekar & Dreyer, 2009, 2011; Im, Hollander, Bali, & Kenny, 2010), and opioids (He, Yang, Kirkmire, & Wang, 2010). Many miRNAs are altered in brains from human alcoholics (Lewohl et al., 2011) and ethanol-treated mice (Nunez et al., 2013), and many are predicted to target neuroimmune processes. Figure 2.3 depicts a model of neuroinflammatory miRNA signaling based on evidence from human alcoholics (Nunez & Mayfield, 2012).
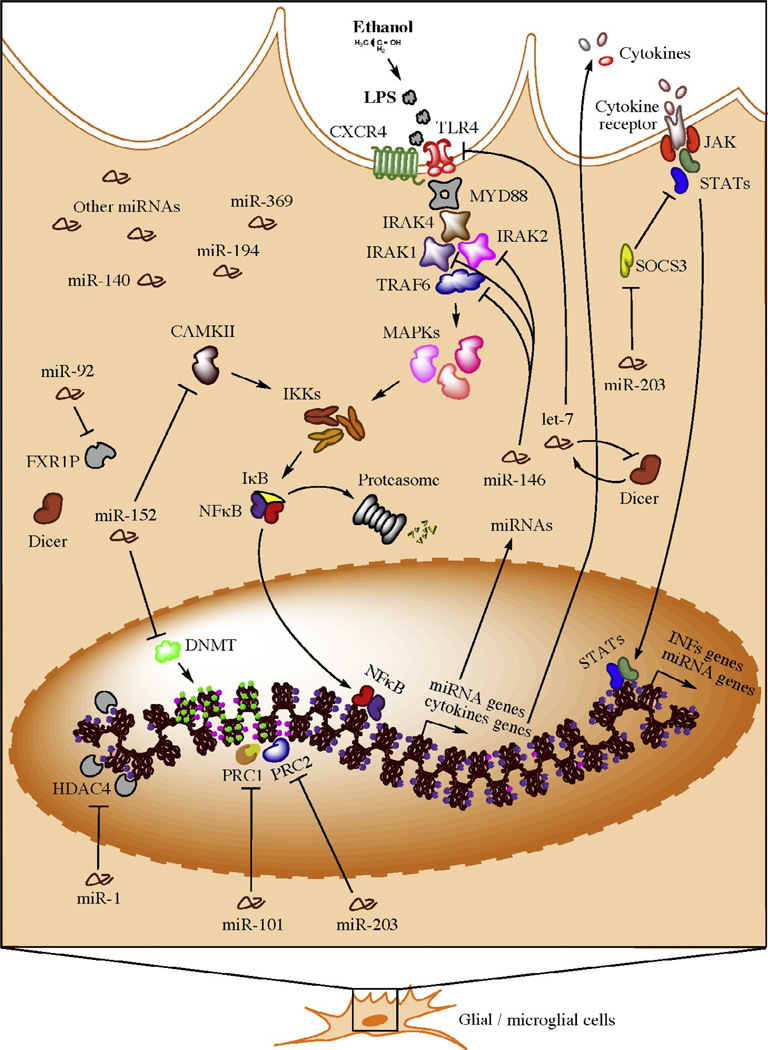
Figure 2.3.
Hypothetical model for neuroimmune-related actions of microRNAs in brain of human alcoholics (e.g., in microglia). Bacterial lipopolysaccharides (LPSs) from commensal bacteria may leak into the bloodstream resulting from gut leakiness induced by alcohol consumption. Activation of NF-κB induces the transcription of a variety of proinflammatory and miRNA genes. Newly synthesized proinflammatory factors induce a systemic neuroinflammatory response and a positive-feedback loop in the same activated cell. Subsequent generation of proinflammatory factors results from expression of alternative cytokine receptors in the activated cell, which signal back to the nucleus to induce additional proinflammatory factors (e.g., interferons) and miRNA genes. To avoid overamplification of these signals and excessive inflammation, specific miRNAs (e.g., members of the miR-146, miR-152, and let-7 families) are consequently upregulated, suppressing TLR4/CXCR4 signaling through inhibition of various transducers, such as IL1 receptor-associated kinases (IRAKs), TNF receptor-associated factor 6 (TRAF6), and TLR4/CXCR4 itself. As a compensatory reaction, miRNAs (e.g., miR-203) may also be upregulated to maintain the immune-activated state of the specific cell subtype while promoting a benign, contained inflammatory response. microRNAs that target epige-netic factors are also activated to control and/or fine tune the ongoing remodeling of the cellular epigenome, allowing for long-term homeostatic and cellular adaptations. Figure and legend are from Nunez and Mayfield (2012).
Chemokine receptor 4 (CXCR4) appears to be important in alcohol action and has been reported to interact with the LPS-sensing complex (Triantafilou & Triantafilou, 2002). The CXCR4 gene was significantly over targeted by miRNAs in prefrontal cortex of human alcoholics (Lewohl et al., 2011). Moreover, in mouse frontal cortex, CXCR4 was one of the most highly enriched mRNA pathways following alcohol exposure, and the miRNAs associated with it (let-7g-5p and miR-34c-5p) were highly correlated with the alcohol-responsive miRNAs (Nunez et al., 2013). Examples of alcohol-sensitive, immune-related miRNAs identified in both rodent and human brain are shown in Table 2.2, and some regulate TLR signaling. For example, let-7 family members directly target expression of TLR4 and regulate responsiveness to LPS (Chen, Splinter, O’Hara, & LaRusso, 2007) and form an epigenetic switch with NF-κB and IL-6 that causes persistent inflammation-induced cell transformation (Iliopoulos, Hirsch, & Struhl, 2009). miR-9 may also control the activity of TLR signaling pathway through direct targeting of the NFKB1 gene in human monocytes and neutrophils (O’Neill et al., 2011). Interestingly, miR-9 also targets the BK channel (large conductance calcium- and voltage-activated potassium channel) and selectively destabilizes splice variants, resulting in downregulation of the alcohol-specific splice variant (Pietrzykowski et al., 2008). This mechanism is proposed to mediate development of cellular tolerance to alcohol. miR-92 and miR-140 are other alcohol-sensitive miRNAs found in both mouse and human brain (Table 2.2), which are associated with immune signaling. Differential expression of miR-92 is characteristic of stages of T lymphocyte development (Sonkoly, Stahle, & Pivarcsi, 2008), and increased plasma levels of miR-92a in patients with traumatic brain injury is a good biomarker of the severity of the disease (Redell, Moore, Ward, Hergenroeder, & Dash, 2010). miR-140 is upregulated after LPS treatment in mice (Moschos et al., 2007) and targets the chemokine Cxcl12 (Nicolas et al., 2008). Another member of this chemokine family, Cxcl14, is a predicted target of miR-34b in synaptic preparations from mice treated with ethanol (Most et. al., unpublished results). Ethanol also altered miR-369-3p (miR-369*) in both mouse and human brain (Table 2.2), and miR-369* is known to associate directly with TNF-α mRNA to mediate activation of TNF-α under conditions of arrested growth (Vasudevan & Steitz, 2007). This effect is dependent on the recruitment of the RNA-binding proteins, fragile-X mental retardation-related protein 1 (FXR1) and argonaute 2 (Ago2), and can be reversed when cells are actively proliferating, in which case miR-369* represses TNF-α (Vasudevan, Tong, & Steitz, 2007). Interestingly, miR-369*, TNF-α, FXR1, and Ago2 were all altered in mouse brain synaptoneurosomes from chronic ethanol-treated mice (Most et al., unpublished results).
Table 2.2.
Alcohol-sensitive immune-related microRNAs in rodent and human brain.
| microRNAs | Alcohol studies |
|---|---|
| miR-92 | Human braina; mouse brain synaptoneurosomesb |
| miR-140 | Human and mouse braina,c; mouse brain synaptoneurosomesb |
| miR-369* | Human braina; mouse brain synaptoneurosomesb |
| let-7 | Human and mouse braina,c; mouse brain synaptoneurosomesb |
| miR-9 | Mouse and rat brainb,d |
| miR-34 | Human and mouse braina,c; mouse brain synaptoneurosomesb |
Human prefrontal cortex (Lewohl et al., 2011).
Mouse amygdala synaptoneurosomes (unpublished results).
Mouse frontal cortex (Nunez et al., 2013).
Rat striatum (Pietrzykowski et al., 2008).
Synaptoneurosomes (Hollingsworth et al., 1985; Quinlan, Philpot, Huganir, & Bear, 1999) contain pre- and postsynaptic compartments of neurons, astrocytes, oligodendrocytes, and microglia and have been used to study local translation of mRNAs in the synapse (Raab-Graham, Haddick, Jan, & Jan, 2006; Sosanya et al., 2013). Discrete alcohol-mediated changes within the transcriptome can be measured by comparing synaptoneurosome versus total homogenate preparations in the same samples from control and chronic ethanol-treated mice, and miRNAs that may target immune pathways were specifically identified in synaptoneurosomes from ethanol-treated mice (Table 2.2).
Furthermore, comparison of ethanol-sensitive mRNAs from synaptoneurosomes (Most et al., 2014) with immune (LPS)-induced mRNAs (Osterndorff-Kahanek et al., 2013) identified 55 mRNAs from mouse brain common to both groups. Astrocyte and microglial transcripts belonging to immune/inflammatory categories (e.g., chemokine- and complement-related transcripts) were upregulated by both treatments, suggesting that some of the ethanol-induced changes in the synaptic transcriptome may be mediated by immune signaling.
Alcohol consumption alters individual or families of miRNAs in human and rodent brain, some of which may regulate the expression of neuroimmune-related mRNAs. The findings in synaptoneurosomes also indicate the importance of the cellular microenvironment in identifying discrete effects of alcohol and suggest that the mouse synaptic transcriptome may be a useful model for predicting expression changes in humans.
6. CONCLUSIONS
The evidence is now quite strong that excessive alcohol consumption not only alters peripheral innate immune signaling but also perturbs neuroimmune signaling in both humans and rodent models of chronic alcohol exposure. The behavioral consequences of these changes in peripheral and central immune signaling are only beginning to emerge, but manipulation of immune genes and administration of LPS to activate innate immune pathways in mice indicate that alcohol consumption is regulated by these signaling systems. In addition, measurement of levels of LPS and proinflammatory molecules in human alcoholics suggests that alcohol craving is correlated with the level of activation of innate immunity (Leclercq et al., 2012, 2014). Overall, there is support for the idea that excessive alcohol consumption in humans and rodents regulates not only peripheral but also central immune signaling, by altering gene expression. These changes in mRNA expression are driven, at least in part, by changes in miRNAs. Some of the diverse changes in immune function appear to be important for regulation of alcohol consumption and craving, indicating a vicious cycle of increased alcohol consumption, leading to increased perturbation of neuroimmune function, which further increases alcohol craving, consumption, and dependence. Despite recent progress, there are many unanswered questions. Most of the approaches (e.g., global gene deletion, measurement of brain gene expression) do not define whether the site of alcohol action is the peripheral immune system (which then affects the brain) or direct action on the brain. Gene expression studies have identified changes in many different mRNAs potentially important for immune signaling, but do not define which are most important. In addition, we have little insight as to how alcohol-mediated changes in neuroimmune signaling might affect circuits important for consumption, reward, or craving. In particular, there are neuroimmune components not only on neurons but also on astrocytes and microglia, and which cell types are critical for the behavioral effects are unknown. Lastly, the recognition of the importance of these signaling systems in alcohol consumption and dependence provides new opportunities for therapeutic interventions (e.g., PPARα- and PPARγ-selective agonists). Indeed, there are many existing and emerging treatments for inflammatory diseases, and interest in the neuroimmune basis of many mental illnesses and repurposing of immune therapies for alcoholism as well as other addictions and mental illnesses is an attractive possibility, but will require better knowledge of the mechanisms of neuroimmune function.
ACKNOWLEDGMENTS
This work was supported by NIAAA grants AA006399, AA012404, and AA013520 (INIA West Consortium).
REFERENCES
- Agrawal RG, Owen JA, Levin PS, Hewetson A, Berman AE, Franklin SR, et al. Bioinformatics analyses reveal age-specific neuroimmune modulation as a target for treatment of high ethanol drinking. Alcoholism, Clinical and Experimental Research. 2014;38(2):428–437. doi: 10.1111/acer.12288. http://dx.doi.org/10.1111/acer.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. The Journal of Neuroscience. 2010;30(24):8285–8295. doi: 10.1523/JNEUROSCI.0976-10.2010. http://dx.doi.org/10.1523/JNEUROSCI.0976-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambros V. microRNAs: Tiny regulators with great potential. Cell. 2001;107(7):823–826. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- Aoyama T, Peters JM, Iritani N, Nakajima T, Furihata K, Hashimoto T, et al. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha) The Journal of Biological Chemistry. 1998;273(10):5678–5684. doi: 10.1074/jbc.273.10.5678. [DOI] [PubMed] [Google Scholar]
- Baek MN, Jung KH, Halder D, Choi MR, Lee BH, Lee BC, et al. Artificial microRNA-based neurokinin-1 receptor gene silencing reduces alcohol consumption in mice. Neuroscience Letters. 2010;475(3):124–128. doi: 10.1016/j.neulet.2010.03.051. http://dx.doi.org/10.1016/j.neulet.2010.03.051. [DOI] [PubMed] [Google Scholar]
- Bajo M, Madamba SG, Roberto M, Blednov YA, Sagi VN, Roberts E, et al. Innate immune factors modulate ethanol interactions with GABAergic transmission in mouse central amygdala. Brain, Behavior, and Immunity. 2014;40:191–202. doi: 10.1016/j.bbi.2014.03.007. http://dx.doi.org/10.1016/j.bbi.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak S, Liu F, Ben Hamida S, Yowell QV, Neasta J, Kharazia V, et al. Disruption of alcohol-related memories by mTORC1 inhibition prevents relapse. Nature Neuroscience. 2013;16(8):1111–1117. doi: 10.1038/nn.3439. http://dx.doi.org/10.1038/nn.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Karatayev O, Chang GQ, Johnson DF, Bocarsly ME, Hoebel BG, et al. Positive relationship between dietary fat, ethanol intake, triglycerides, and hypothalamic peptides: Counteraction by lipid-lowering drugs. Alcohol. 2009;43(6):433–441. doi: 10.1016/j.alcohol.2009.07.003. http://dx.doi.org/10.1016/j.alcohol.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco AM, Perez-Arago A, Fernandez-Lizarbe S, Guerri C. Ethanol mimics ligand-mediated activation and endocytosis of IL-1RI/TLR4 receptors via lipid rafts caveolae in astroglial cells. Journal of Neurochemistry. 2008;106(2):625–639. doi: 10.1111/j.1471-4159.2008.05425.x. http://dx.doi.org/10.1111/j.1471-4159.2008.05425.x. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Black M, Goate AM, Edenberg HJ, Wetherill L, et al. Peroxisome proliferator-activated receptors α and γ are linked with alcohol consumption in mice and withdrawal and dependence in humans. Alcoholism: Clinical and Experimental Research. doi: 10.1111/acer.12610. (submitted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Geil C, Perra S, Morikawa H, Harris RA. Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain, Behavior, and Immunity. 2011;25(Suppl. 1):S92–S105. doi: 10.1016/j.bbi.2011.01.008. http://dx.doi.org/10.1016/j.bbi.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Bergeson SE, Walker D, Ferreira VM, Kuziel WA, Harris RA. Perturbation of chemokine networks by gene deletion alters the reinforcing actions of ethanol. Behavioural Brain Research. 2005;165(1):110–125. doi: 10.1016/j.bbr.2005.06.026. http://dx.doi.org/10.1016/j.bbr.2005.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Ponomarev I, Geil C, Bergeson S, Koob GF, Harris RA. Neuroimmune regulation of alcohol consumption: Behavioral validation of genes obtained from genomic studies. Addiction Biology. 2012;17(1):108–120. doi: 10.1111/j.1369-1600.2010.00284.x. http://dx.doi.org/10.1111/j.1369-1600.2010.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukli NM, Saiyed ZM, Ricaurte M, Rodriguez JW, Rios Olivares E, Cubano LA, et al. Implications of ER stress, the unfolded protein response, and pro- and anti-apoptotic protein fingerprints in human monocyte-derived dendritic cells treated with alcohol. Alcoholism, Clinical and Experimental Research. 2010;34(12):2081–2088. doi: 10.1111/j.1530-0277.2010.01304.x. http://dx.doi.org/10.1111/j.1530-0277.2010.01304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekar V, Dreyer JL. microRNAs miR-124, let-7d and miR-181a regulate cocaine-induced plasticity. Molecular and Cellular Neurosciences. 2009;42(4):350–362. doi: 10.1016/j.mcn.2009.08.009. http://dx.doi.org/10.1016/j.mcn.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Chandrasekar V, Dreyer JL. Regulation of MiR-124, Let-7d, and MiR-181a in the accumbens affects the expression, extinction, and reinstatement of cocaine-induced conditioned place preference. Neuropsychopharmacology. 2011;36(6):1149–1164. doi: 10.1038/npp.2010.250. http://dx.doi.org/10.1038/npp.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan VS, Kluttz JM, Bost KL, Marriott I. Prophylactic and therapeutic targeting of the neurokinin-1 receptor limits neuroinflammation in a murine model of pneumococcal meningitis. Journal of Immunology. 2011;186(12):7255–7263. doi: 10.4049/jimmunol.1100721. http://dx.doi.org/10.4049/jimmunol.1100721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XM, Splinter PL, O’Hara SP, LaRusso NF. A cellular micro-RNA, let-7i, regulates Toll-like receptor 4 expression and contributes to cholangiocyte immune responses against Cryptosporidium parvum infection. The Journal of Biological Chemistry. 2007;282(39):28929–28938. doi: 10.1074/jbc.M702633200. http://dx.doi.org/10.1074/jbc.M702633200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RB. The role of PPARs in inflammation and immunity. Journal of Leukocyte Biology. 2002;71(3):388–400. [PubMed] [Google Scholar]
- Cohen JE, Lee PR, Chen S, Li W, Fields RD. MicroRNA regulation of homeostatic synaptic plasticity. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(28):11650–11655. doi: 10.1073/pnas.1017576108. http://dx.doi.org/10.1073/pnas.1017576108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F, Nixon K, Kim D, Joseph J, Shukitt-Hale B, Qin L, et al. BHT blocks NF-kappaB activation and ethanol-induced brain damage. Alcoholism, Clinical and Experimental Research. 2006;30(11):1938–1949. doi: 10.1111/j.1530-0277.2006.00239.x. http://dx.doi.org/10.1111/j.1530-0277.2006.00239.x. [DOI] [PubMed] [Google Scholar]
- Crews FT, Qin L, Sheedy D, Vetreno RP, Zou J. High mobility group box 1/Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biological Psychiatry. 2013;73(7):602–612. doi: 10.1016/j.biopsych.2012.09.030. http://dx.doi.org/10.1016/j.biopsych.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nature Reviews. Immunology. 2002;2(10):748–759. doi: 10.1038/nri912. http://dx.doi.org/10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Xuei X, Wetherill LF, Bierut L, Bucholz K, Dick DM, et al. Association of NFKB1, which encodes a subunit of the transcription factor NF-kappaB, with alcohol dependence. Human Molecular Genetics. 2008;17(7):963–970. doi: 10.1093/hmg/ddm368. http://dx.doi.org/10.1093/hmg/ddm368. [DOI] [PubMed] [Google Scholar]
- Farris SP, Miles MF. Ethanol modulation of gene networks: Implications for alcoholism. Neurobiology of Disease. 2012;45(1):115–121. doi: 10.1016/j.nbd.2011.04.013. http://dx.doi.org/10.1016/j.nbd.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson LB, Most D, Blednov YA, Harris RA. PPAR agonists regulate brain gene expression: Relationship to their effects on ethanol consumption. Neuropharmacology. 2014 doi: 10.1016/j.neuropharm.2014.06.024. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Espejo E, Ramiro-Fuentes S, Rodriguez de Fonseca F. The absence of a functional peroxisome proliferator-activated receptor-alpha gene in mice enhances motor sensitizing effects of morphine, but not cocaine. Neuroscience. 2009;164(2):667–675. doi: 10.1016/j.neuroscience.2009.08.023. http://dx.doi.org/10.1016/j.neuroscience.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Montesinos J, Guerri C. Ethanol induces TLR4/TLR2 association, triggering an inflammatory response in microglial cells. Journal of Neurochem-istry. 2013;126(2):261–273. doi: 10.1111/jnc.12276. http://dx.doi.org/10.1111/jnc.12276. [DOI] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Pascual M, Gascon MS, Blanco A, Guerri C. Lipid rafts regulate ethanol-induced activation of TLR4 signaling in murine macrophages. Molecular Immunology. 2008;45(7):2007–2016. doi: 10.1016/j.molimm.2007.10.025. http://dx.doi.org/10.1016/j.molimm.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Pascual M, Guerri C. Critical role of TLR4 response in the activation of microglia induced by ethanol. Journal of Immunology. 2009;183(7):4733–4744. doi: 10.4049/jimmunol.0803590. http://dx.doi.org/10.4049/jimmunol.0803590. [DOI] [PubMed] [Google Scholar]
- George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C, et al. Neu-rokinin 1 receptor antagonism as a possible therapy for alcoholism. Science. 2008;319(5869):1536–1539. doi: 10.1126/science.1153813. http://dx.doi.org/10.1126/science.1153813. [DOI] [PubMed] [Google Scholar]
- Gorini G, Harris RA, Mayfield RD. Proteomic approaches and identification of novel therapeutic targets for alcoholism. Neuropsychopharmacology. 2014;39(1):104–130. doi: 10.1038/npp.2013.182. http://dx.doi.org/10.1038/npp.2013.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorini G, Nunez YO, Mayfield RD. Integration of miRNA and protein profiling reveals coordinated neuroadaptations in the alcohol-dependent mouse brain. PLoS One. 2013;8(12):e82565. doi: 10.1371/journal.pone.0082565. http://dx.doi.org/10.1371/journal.pone.0082565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorini G, Roberts AJ, Mayfield RD. Neurobiological signatures of alcohol dependence revealed by protein profiling. PLoS One. 2013;8(12):e82656. doi: 10.1371/journal.pone.0082656. http://dx.doi.org/10.1371/journal.pone.0082656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlicher M, Widmark E, Li Q, Gustafsson JA. Fatty acids activate a chimera of the clofibric acid-activated receptor and the glucocorticoid receptor. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(10):4653–4657. doi: 10.1073/pnas.89.10.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Cooper ZA, Tulapurkar ME, Potla R, Maity T, Hasday JD, et al. Toll-like receptor agonists and febrile range hyperthermia synergize to induce heat shock protein 70 expression and extracellular release. The Journal of Biological Chemistry. 2013;288(4):2756–2766. doi: 10.1074/jbc.M112.427336. http://dx.doi.org/10.1074/jbc.M112.427336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SC, Sundaram C, Reuter S, Aggarwal BB. Inhibiting NF-kappaB activation by small molecules as a therapeutic strategy. Biochimica et Biophysica Acta. 2010;1799(10-12):775–787. doi: 10.1016/j.bbagrm.2010.05.004. http://dx.doi.org/10.1016/j.bbagrm.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RA, Blednov YA. Neuroimmune genes and alcohol drinking behavior. In: Cui C, Grandison L, Noronha A, editors. Neural-immune interactions in brain function and alcohol related disorders. New York: Springer; 2013. pp. 425–440. [Google Scholar]
- He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Experimental Neurology. 2008;210(2):349–358. doi: 10.1016/j.expneurol.2007.11.017. http://dx.doi.org/10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Yang C, Kirkmire CM, Wang ZJ. Regulation of opioid tolerance by let-7 family microRNA targeting the mu opioid receptor. The Journal of Neuroscience. 2010;30(30):10251–10258. doi: 10.1523/JNEUROSCI.2419-10.2010. http://dx.doi.org/10.1523/JNEUROSCI.2419-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth EB, McNeal ET, Burton JL, Williams RJ, Daly JW, Creveling CR. Biochemical characterization of a filtered synaptoneurosome preparation from guinea pig cerebral cortex: Cyclic adenosine 3’:5’-monophosphate-generating systems, receptors, and enzymes. The Journal of Neuroscience. 1985;5(8):2240–2253. doi: 10.1523/JNEUROSCI.05-08-02240.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Li MD. Nicotine modulates expression of miR-140*, which targets the 3’-untranslated region of dynamin 1 gene (Dnm1) International Journal of Neuropsychopharmacology. 2009;12(4):537–546. doi: 10.1017/S1461145708009528. http://dx.doi.org/10.1017/S1461145708009528. [DOI] [PubMed] [Google Scholar]
- Hutt D, Balch WE. Cell biology the proteome in balance. Science. 2010;329(5993):766–767. doi: 10.1126/science.1194160. http://dx.doi.org/10.1126/science.1194160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139(4):693–706. doi: 10.1016/j.cell.2009.10.014. http://dx.doi.org/10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im HI, Hollander JA, Bali P, Kenny PJ. MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nature Neuroscience. 2010;13(9):1120–1127. doi: 10.1038/nn.2615. http://dx.doi.org/10.1038/nn.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347(6294):645–650. doi: 10.1038/347645a0. http://dx.doi.org/10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- Kane CJ, Phelan KD, Douglas JC, Wagoner G, Johnson JW, Xu J, et al. Effects of ethanol on immune response in the brain: Region-specific changes in adolescent versus adult mice. Alcoholism, Clinical and Experimental Research. 2014;38(2):384–391. doi: 10.1111/acer.12244. http://dx.doi.org/10.1111/acer.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: A treasure trove for drug development. Nature Reviews. Drug Discovery. 2004;3(1):17–26. doi: 10.1038/nrd1279. http://dx.doi.org/10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nature Reviews. Neuroscience. 2007;8(11):844–858. doi: 10.1038/nrn2234. http://dx.doi.org/10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kong EC, Allouche L, Chapot PA, Vranizan K, Moore MS, Heberlein U, et al. Ethanol-regulated genes that contribute to ethanol sensitivity and rapid tolerance in Drosophila. Alcoholism, Clinical and Experimental Research. 2010;34(2):302–316. doi: 10.1111/j.1530-0277.2009.01093.x. http://dx.doi.org/10.1111/j.1530-0277.2009.01093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krey G, Braissant O, L’Horset F, Kalkhoven E, Perroud M, Parker MG, et al. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Molecular Endocrinology. 1997;11(6):779–791. doi: 10.1210/mend.11.6.0007. http://dx.doi.org/10.1210/mend.11.6.0007. [DOI] [PubMed] [Google Scholar]
- Krichevsky AM, King KS, Donahue CP, Khrapko K, Kosik KS. A microRNA array reveals extensive regulation of microRNAs during brain development. RNA. 2003;9(10):1274–1281. doi: 10.1261/rna.5980303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq S, Cani PD, Neyrinck AM, Starkel P, Jamar F, Mikolajczak M, et al. Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain, Behavior, and Immunity. 2012;26(6):911–918. doi: 10.1016/j.bbi.2012.04.001. http://dx.doi.org/10.1016/j.bbi.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Leclercq S, De Saeger C, Delzenne N, de Timary P, Starkel P. Role of inflammatory pathways, blood mononuclear cells, and gut-derived bacterial products in alcohol dependence. Biological Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.02.003. http://dx.doi.org/10.1016/j.biopsych.2014.02.003. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- Lee RC, Ambros V. An extensive class of small RNAs in Caenorhabditis elegans. Science. 2001;294(5543):862–864. doi: 10.1126/science.1065329. http://dx.doi.org/10.1126/science.1065329. [DOI] [PubMed] [Google Scholar]
- Lee KH, Jeong J, Yoo CG. Positive feedback regulation of heat shock protein 70 (Hsp70) is mediated through Toll-like receptor 4-PI3K/Akt-glycogen synthase kinase-3beta pathway. Experimental Cell Research. 2013;319(1):88–95. doi: 10.1016/j.yexcr.2012.09.018. http://dx.doi.org/10.1016/j.yexcr.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Lesscher HM, Wallace MJ, Zeng L, Wang V, Deitchman JK, McMahon T, et al. Amygdala protein kinase C epsilon controls alcohol consumption. Genes, Brain, and Behavior. 2009;8(5):493–499. doi: 10.1111/j.1601-183X.2009.00485.x. http://dx.doi.org/10.1111/j.1601-183X.2009.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewohl JM, Nunez YO, Dodd PR, Tiwari GR, Harris RA, Mayfield RD. Up-regulation of microRNAs in brain of human alcoholics. Alcoholism, Clinical and Experimental Research. 2011;35(11):1928–1937. doi: 10.1111/j.1530-0277.2011.01544.x. http://dx.doi.org/10.1111/j.1530-0277.2011.01544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewohl JM, Wang L, Miles MF, Zhang L, Dodd PR, Harris RA. Gene expression in human alcoholism: Microarray analysis of frontal cortex. Alcoholism, Clinical and Experimental Research. 2000;24(12):1873–1882. [PubMed] [Google Scholar]
- Lippai D, Bala S, Petrasek J, Csak T, Levin I, Kurt-Jones EA, et al. Alcohol-induced IL-1beta in the brain is mediated by NLRP3/ASC inflammasome activation that amplifies neuroinflammation. Journal of Leukocyte Biology. 2013;94(1):171–182. doi: 10.1189/jlb.1212659. http://dx.doi.org/10.1189/jlb.1212659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lewohl JM, Harris RA, Iyer VR, Dodd PR, Randall PK, et al. Patterns of gene expression in the frontal cortex discriminate alcoholic from nonalcoholic individuals. Neuropsychopharmacology. 2006;31(7):1574–1582. doi: 10.1038/sj.npp.1300947. http://dx.doi.org/10.1038/sj.npp.1300947. [DOI] [PubMed] [Google Scholar]
- Liu J, Yang AR, Kelly T, Puche A, Esoga C, June HL, Jr., et al. Binge alcohol drinking is associated with GABAA alpha2-regulated Toll-like receptor 4 (TLR4) expression in the central amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(11):4465–4470. doi: 10.1073/pnas.1019020108. http://dx.doi.org/10.1073/pnas.1019020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Kiguchi N, Fukazawa Y, Yamamoto A, Ozaki M, Kishioka S. Peroxisome proliferator-activated receptor gamma activation relieves expression of behavioral sensitization to methamphetamine in mice. Neuropsychopharmacology. 2007;32(5):1133–1140. doi: 10.1038/sj.npp.1301213. http://dx.doi.org/10.1038/sj.npp.1301213. [DOI] [PubMed] [Google Scholar]
- Mascia P, Pistis M, Justinova Z, Panlilio LV, Luchicchi A, Lecca S, et al. Blockade of nicotine reward and reinstatement by activation of alpha-type peroxisome proliferator-activated receptors. Biological Psychiatry. 2011;69(7):633–641. doi: 10.1016/j.biopsych.2010.07.009. http://dx.doi.org/10.1016/j.biopsych.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield J, Ferguson L, Harris RA. Neuroimmune signaling: A key component of alcohol abuse. Current Opinion in Neurobiology. 2013;23(4):513–520. doi: 10.1016/j.conb.2013.01.024. http://dx.doi.org/10.1016/j.conb.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield RD, Harris RA, Schuckit MA. Genetic factors influencing alcohol dependence. British Journal of Pharmacology. 2008;154(2):275–287. doi: 10.1038/bjp.2008.88. http://dx.doi.org/10.1038/bjp.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles MF, Diaz JE, DeGuzman VS. Mechanisms of neuronal adaptation to ethanol. Ethanol induces Hsc70 gene transcription in NG108-15 neuroblastoma × glioma cells. The Journal of Biological Chemistry. 1991;266(4):2409–2414. [PubMed] [Google Scholar]
- Moller DE, Berger JP. Role of PPARs in the regulation of obesity-related insulin sensitivity and inflammation. International Journal of Obesity and Related Metabolic Disorders. 2003;27(Suppl. 3):S17–S21. doi: 10.1038/sj.ijo.0802494. http://dx.doi.org/10.1038/sj.ijo.0802494. [DOI] [PubMed] [Google Scholar]
- Moschos SA, Williams AE, Perry MM, Birrell MA, Belvisi MG, Lindsay MA. Expression profiling in vivo demonstrates rapid changes in lung microRNA levels following lipopolysaccharide-induced inflammation but not in the anti-inflammatory action of glucocorticoids. BMC Genomics. 2007;8:240. doi: 10.1186/1471-2164-8-240. http://dx.doi.org/10.1186/1471-2164-8-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Most D, Ferguson L, Blednov Y, Mayfield RD, Harris RA. The synaptoneurosome transcriptome: A model for profiling the synaptic molecular effects of alcohol. Pharmacogenomics Journal. 2014 doi: 10.1038/tpj.2014.43. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neasta J, Ben Hamida S, Yowell Q, Carnicella S, Ron D. Role for mammalian target of rapamycin complex 1 signaling in neuroadaptations underlying alcohol-related disorders. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(46):20093–20098. doi: 10.1073/pnas.1005554107. http://dx.doi.org/10.1073/pnas.1005554107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nature Reviews. Neuroscience. 2001;2(2):119–128. doi: 10.1038/35053570. http://dx.doi.org/10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Nicolas FE, Pais H, Schwach F, Lindow M, Kauppinen S, Moulton V, et al. Experimental identification of microRNA-140 targets by silencing and overexpressing miR-140. RNA. 2008;14(12):2513–2520. doi: 10.1261/rna.1221108. http://dx.doi.org/10.1261/rna.1221108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez YO, Mayfield RD. Understanding alcoholism through microRNA signatures in brains of human alcoholics. Frontiers in Genetics. 2012;3:43. doi: 10.3389/fgene.2012.00043. http://dx.doi.org/10.3389/fgene.2012.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez YO, Truitt JM, Gorini G, Ponomareva ON, Blednov YA, Harris RA, et al. Positively correlated miRNA-mRNA regulatory networks in mouse frontal cortex during early stages of alcohol dependence. BMC Genomics. 2013;14:725. doi: 10.1186/1471-2164-14-725. http://dx.doi.org/10.1186/1471-2164-14-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okvist A, Johansson S, Kuzmin A, Bazov I, Merino-Martinez R, Ponomarev I, et al. Neuroadaptations in human chronic alcoholics: Dysregulation of the NF-kappaB system. PLoS One. 2007;2(9):e930. doi: 10.1371/journal.pone.0000930. http://dx.doi.org/10.1371/journal.pone.0000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill LA, Sheedy FJ, McCoy CE. MicroRNAs: The fine-tuners of Tolllike receptor signalling. Nature Reviews. Immunology. 2011;11(3):163–175. doi: 10.1038/nri2957. http://dx.doi.org/10.1038/nri2957. [DOI] [PubMed] [Google Scholar]
- Osterndorff-Kahanek E, Ponomarev I, Blednov YA, Harris RA. Gene expression in brain and liver produced by three different regimens of alcohol consumption in mice: Comparison with immune activation. PLoS One. 2013;8(3):e59870. doi: 10.1371/journal.pone.0059870. http://dx.doi.org/10.1371/journal.pone.0059870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Justinova Z, Mascia P, Pistis M, Luchicchi A, Lecca S, et al. Novel use of a lipid-lowering fibrate medication to prevent nicotine reward and relapse: Preclinical findings. Neuropsychopharmacology. 2012;37(8):1838–1847. doi: 10.1038/npp.2012.31. http://dx.doi.org/10.1038/npp.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Balino P, Alfonso-Loeches S, Aragon CM, Guerri C. Impact of TLR4 on behavioral and cognitive dysfunctions associated with alcohol-induced neuroinflammatory damage. Brain, Behavior, and Immunity. 2011;25(Suppl. 1):S80–S91. doi: 10.1016/j.bbi.2011.02.012. http://dx.doi.org/10.1016/j.bbi.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Pascual M, Pla A, Minarro J, Guerri C. Neuroimmune activation and myelin changes in adolescent rats exposed to high-dose alcohol and associated cognitive dysfunction: A review with reference to human adolescent drinking. Alcohol and Alcoholism. 2014;49(2):187–192. doi: 10.1093/alcalc/agt164. http://dx.doi.org/10.1093/alcalc/agt164. [DOI] [PubMed] [Google Scholar]
- Pascual-Lucas M, Fernandez-Lizarbe S, Montesinos J, Guerri C. LPS or ethanol triggers clathrin- and rafts/caveolae-dependent endocytosis of TLR4 in cortical astrocytes. Journal of Neurochemistry. 2014;129(3):448–462. doi: 10.1111/jnc.12639. http://dx.doi.org/10.1111/jnc.12639. [DOI] [PubMed] [Google Scholar]
- Pietrzykowski AZ, Friesen RM, Martin GE, Puig SI, Nowak CL, Wynne PM, et al. Posttranscriptional regulation of BK channel splice variant stability by miR-9 underlies neuroadaptation to alcohol. Neuron. 2008;59(2):274–287. doi: 10.1016/j.neuron.2008.05.032. http://dx.doi.org/10.1016/j.neuron.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignataro L, Varodayan FP, Tannenholz LE, Harrison NL. The regulation of neuronal gene expression by alcohol. Pharmacology & Therapeutics. 2009;124(3):324–335. doi: 10.1016/j.pharmthera.2009.09.002. http://dx.doi.org/10.1016/j.pharmthera.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev I, Wang S, Zhang L, Harris RA, Mayfield RD. Gene coexpression networks in human brain identify epigenetic modifications in alcohol dependence. The Journal of Neuroscience. 2012;32(5):1884–1897. doi: 10.1523/JNEUROSCI.3136-11.2012. http://dx.doi.org/10.1523/JNEUROSCI.3136-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. Journal of Neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. http://dx.doi.org/10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55(5):453–462. doi: 10.1002/glia.20467. http://dx.doi.org/10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan EM, Philpot BD, Huganir RL, Bear MF. Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nature Neuroscience. 1999;2(4):352–357. doi: 10.1038/7263. http://dx.doi.org/10.1038/7263. [DOI] [PubMed] [Google Scholar]
- Raab-Graham KF, Haddick PC, Jan YN, Jan LY. Activity- and mTOR-dependent suppression of Kv1.1 channel mRNA translation in dendrites. Science. 2006;314(5796):144–148. doi: 10.1126/science.1131693. http://dx.doi.org/10.1126/science.1131693. [DOI] [PubMed] [Google Scholar]
- Redell JB, Moore AN, Ward NH, 3rd., Hergenroeder GW, Dash PK. Human traumatic brain injury alters plasma microRNA levels. Journal of Neurotrauma. 2010;27(12):2147–2156. doi: 10.1089/neu.2010.1481. http://dx.doi.org/10.1089/neu.2010.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RA, James NH, Woodyatt NJ, Macdonald N, Tugwood JD. Evidence for the suppression of apoptosis by the peroxisome proliferator activated receptor alpha (PPAR alpha) Carcinogenesis. 1998;19(1):43–48. doi: 10.1093/carcin/19.1.43. [DOI] [PubMed] [Google Scholar]
- Ron D, Messing RO. Signaling pathways mediating alcohol effects. Current Topics in Behavioral Neurosciences. 2013;13:87–126. doi: 10.1007/7854_2011_161. http://dx.doi.org/10.1007/7854_2011_161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso M, Munoz M, Berger M. The role of neurokinin-1 receptor in the microenvironment of inflammation and cancer. Scientific World Journal. 2012;2012:381434. doi: 10.1100/2012/381434. http://dx.doi.org/10.1100/2012/381434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna PP, Simpson C, Lutjens R, Koob G. ERK regulation in chronic ethanol exposure and withdrawal. Brain Research. 2002;948(1-2):186–191. doi: 10.1016/s0006-8993(02)03191-8. [DOI] [PubMed] [Google Scholar]
- Schlicht I. Enhancement of voluntary alcohol consumption in rats by clofibrate feeding. Alcohol. 1987;4(3):199–206. doi: 10.1016/0741-8329(87)90043-7. [DOI] [PubMed] [Google Scholar]
- Schoonjans K, Staels B, Auwerx J. The peroxisome proliferator activated receptors (PPARS) and their effects on lipid metabolism and adipocyte differentiation. Biochimica et Biophysica Acta. 1996;1302(2):93–109. doi: 10.1016/0005-2760(96)00066-5. [DOI] [PubMed] [Google Scholar]
- Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, et al. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439(7074):283–289. doi: 10.1038/nature04367. http://dx.doi.org/10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- Seneviratne C, Ait-Daoud N, Ma JZ, Chen G, Johnson BA, Li MD. Susceptibility locus in neurokinin-1 receptor gene associated with alcohol dependence. Neuropsychopharmacology. 2009;34(11):2442–2449. doi: 10.1038/npp.2009.65. http://dx.doi.org/10.1038/npp.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalheiser NR, Lugli G. microRNA regulation of synaptic plasticity. Neuro-molecular Medicine. 2009;11(3):133–140. doi: 10.1007/s12017-009-8065-2. http://dx.doi.org/10.1007/s12017-009-8065-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonkoly E, Stahle M, Pivarcsi A. MicroRNAs and immunity: Novel players in the regulation of normal immune function and inflammation. Seminars in Cancer Biology. 2008;18(2):131–140. doi: 10.1016/j.semcancer.2008.01.005. http://dx.doi.org/10.1016/j.semcancer.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Soreq H, Wolf Y. NeurimmiRs:microRNAs in the neuroimmune interface. Trends in Molecular Medicine. 2011;17(10):548–555. doi: 10.1016/j.molmed.2011.06.009. http://dx.doi.org/10.1016/j.molmed.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Sosanya NM, Huang PP, Cacheaux LP, Chen CJ, Nguyen K, Perrone-Bizzozero NI, et al. Degradation of high affinity HuD targets releases Kv1.1 mRNA from miR-129 repression by mTORC1. The Journal of Cell Biology. 2013;202(1):53–69. doi: 10.1083/jcb.201212089. http://dx.doi.org/10.1083/jcb.201212089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopponi S, de Guglielmo G, Somaini L, Cippitelli A, Cannella N, Kallupi M, et al. Activation of PPARgamma by pioglitazone potentiates the effects of naltrexone on alcohol drinking and relapse in msP rats. Alcoholism, Clinical and Experimental Research. 2013;37(8):1351–1360. doi: 10.1111/acer.12091. http://dx.doi.org/10.1111/acer.12091. [DOI] [PubMed] [Google Scholar]
- Stopponi S, Somaini L, Cippitelli A, Cannella N, Braconi S, Kallupi M, et al. Activation of nuclear PPARgamma receptors by the antidiabetic agent pioglitazone suppresses alcohol drinking and relapse to alcohol seeking. Biological Psychiatry. 2011;69(7):642–649. doi: 10.1016/j.biopsych.2010.12.010. http://dx.doi.org/10.1016/j.biopsych.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Sun Y, Bennett A. Cannabinoids: A new group of agonists of PPARs. PPAR Research. 2007;2007:23513. doi: 10.1155/2007/23513. http://dx.doi.org/10.1155/2007/23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Mandrekar P, Petrasek J, Catalano D. The unfolding web of innate immune dysregulation in alcoholic liver injury. Alcoholism, Clinical and Experimental Research. 2011;35(5):782–786. doi: 10.1111/j.1530-0277.2010.01398.x. http://dx.doi.org/10.1111/j.1530-0277.2010.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(33):12481–12486. doi: 10.1073/pnas.0605298103. http://dx.doi.org/10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapocik JD, Solomon M, Flanigan M, Meinhardt M, Barbier E, Schank JR, et al. Coordinated dysregulation of mRNAs and microRNAs in the rat medial prefrontal cortex following a history of alcohol dependence. The Pharmacogenomics Journal. 2013;13(3):286–296. doi: 10.1038/tpj.2012.17. http://dx.doi.org/10.1038/tpj.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsell A, Schank JR, Singley E, Hunt SP, Heilig M. Neurokinin-1 receptors (NK1R:s), alcohol consumption, and alcohol reward in mice. Psychopharmacology. 2010;209(1):103–111. doi: 10.1007/s00213-010-1775-1. http://dx.doi.org/10.1007/s00213-010-1775-1. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79(7):1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Triantafilou M, Triantafilou K. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends in Immunology. 2002;23(6):301–304. doi: 10.1016/s1471-4906(02)02233-0. [DOI] [PubMed] [Google Scholar]
- Varodayan FP, Pignataro L, Harrison NL. Alcohol induces synaptotagmin 1 expression in neurons via activation of heat shock factor 1. Neuroscience. 2011;193:63–71. doi: 10.1016/j.neuroscience.2011.07.035. http://dx.doi.org/10.1016/j.neuroscience.2011.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan S, Steitz JA. AU-rich-element-mediated upregulation of translation by FXR1 and Argonaute 2. Cell. 2007;128(6):1105–1118. doi: 10.1016/j.cell.2007.01.038. http://dx.doi.org/10.1016/j.cell.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318(5858):1931–1934. doi: 10.1126/science.1149460. http://dx.doi.org/10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- Vetreno RP, Qin L, Crews FT. Increased receptor for advanced glycation end product expression in the human alcoholic prefrontal cortex is linked to adolescent drinking. Neurobiology of Disease. 2013;59:52–62. doi: 10.1016/j.nbd.2013.07.002. http://dx.doi.org/10.1016/j.nbd.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RJ, Lallemand F, de Witte P. Influence of adolescent heavy session drinking on the systemic and brain innate immune system. Alcohol and Alcoholism. 2014;49(2):193–197. doi: 10.1093/alcalc/agu002. http://dx.doi.org/10.1093/alcalc/agu002. [DOI] [PubMed] [Google Scholar]
- Whitman BA, Knapp DJ, Werner DF, Crews FT, Breese GR. The cytokine mRNA increase induced by withdrawal from chronic ethanol in the sterile environment of brain is mediated by CRF and HMGB1 release. Alcoholism, Clinical and Experimental Research. 2013;37(12):2086–2097. doi: 10.1111/acer.12189. http://dx.doi.org/10.1111/acer.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson LL, Bilbo SD. Chemokines and the hippocampus: A new perspective on hippocampal plasticity and vulnerability. Brain, Behavior, and Immunity. 2013;30:186–194. doi: 10.1016/j.bbi.2013.01.077. http://dx.doi.org/10.1016/j.bbi.2013.01.077. [DOI] [PubMed] [Google Scholar]
- Wu Y, Lousberg EL, Moldenhauer LM, Hayball JD, Coller JK, Rice KC, et al. Inhibiting the TLR4-MyD88 signalling cascade by genetic or pharmacological strategies reduces acute alcohol-induced sedation and motor impairment in mice. British Journal of Pharmacology. 2012;165(5):1319–1329. doi: 10.1111/j.1476-5381.2011.01572.x. http://dx.doi.org/10.1111/j.1476-5381.2011.01572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S, Pandey A, Shukla A, Talwelkar SS, Kumar A, Pant AB, et al. miR-497 and miR-302b regulate ethanol-induced neuronal cell death through BCL2 protein and cyclin D2. The Journal of Biological Chemistry. 2011;286(43):37347–37357. doi: 10.1074/jbc.M111.235531. http://dx.doi.org/10.1074/jbc.M111.235531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovleva T, Bazov I, Watanabe H, Hauser KF, Bakalkin G. Transcrip-tional control of maladaptive and protective responses in alcoholics: A role of the NF-kappaB system. Brain, Behavior, and Immunity. 2011;25(Suppl. 1):S29–S38. doi: 10.1016/j.bbi.2010.12.019. http://dx.doi.org/10.1016/j.bbi.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain, Behavior, and Immunity. 2011;25(2):181–213. doi: 10.1016/j.bbi.2010.10.015. http://dx.doi.org/10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Zou J, Crews F. Induction of innate immune gene expression cascades in brain slice cultures by ethanol: Key role of NF-kappaB and proinflammatory cytokines. Alcoholism, Clinical and Experimental Research. 2010;34(5):777–789. doi: 10.1111/j.1530-0277.2010.01150.x. http://dx.doi.org/10.1111/j.1530-0277.2010.01150.x. [DOI] [PubMed] [Google Scholar]