Abstract
Inducing cross-reactive broadly neutralizing antibody (bNAb) responses to HIV through vaccination remains an insurmountable challenge. T follicular helper cells (TFH) are fundamental for the development of antigen-specific antibody responses and therefore critical for anti-HIV vaccine design. Here we review recent studies supporting an intricate involvement of TFH in HIV pathogenesis and bNAb development in HIV infection. We also examine emerging data suggesting that TFH responses may be traceable in peripheral blood, and discuss the implications of these findings in the context of vaccine design and future research in TFH immunobiology.
Keywords: Follicular B helper T cell, TFH, HIV, HIV NAb, bNAb, Neutralizing antibodies, pTFH, Vaccine, T follicular helper cells
Antibody-mediated immunity and HIV
In the face of 2 million new infections per year, the stagnation of progress towards an efficacious HIV vaccine is sobering. In 2009 a double-blind phase III HIV vaccine RV144 “Thai” trial that used a combination of a recombinant canarypox vector (ALVAC-HIV [vCP1521]) and two booster injections of a recombinant glycoprotein 120 subunit vaccine (AIDSVAX B/E) showed marginal, yet significant protection from HIV acquisition[1] raising considerable hope for a protective vaccine. However, the results of follow-up assessments of the correlates of protection were surprising and unexpected[2]: Assessing multiple immunological parameters, the study found that not only HIV Envelope (Env)-specific, non-neutralizing IgG antibody (Ab) responses were correlated with protection, but also that protection was lost in the presence of high levels of anti-Env IgA Ab[2]. These findings raise questions regarding whether other Ab-related effector mechanisms [such as Ab-mediated cellular cytotoxicity (ADCC)] may have played a role in the marginal levels of protection observed in RV144, in contrast to most vaccines that commonly –yet not exclusively - induce neutralizing Ab (NAb) responses[3]. These data highlights critical gaps in our understanding of what constitutes a protective vaccine-induced immune response, and more importantly, raises questions as to the mechanisms that shape the Ab response to viral challenges and how these can be tailored by vaccination.
The large genetic diversity of global HIV variants stipulates broad reactivity of NAbs. Thus the ideal Ab response against HIV infection would have broadly neutralizing activity covering the wealth of different worldwide HIV variants. Passive transfer of such broadly cross-reactive neutralizing Abs (bNAbs) has been shown to protect both prophylactically[4, 5] and therapeutically[6, 7] in both non-human primates (NHP) and mice. Yet, no immunization strategy attempted against HIV has succeeded in inducing bNAbs, and only a fraction of HIV-infected individuals naturally develop bNAbs after years of HIV infection[8–15]. The difficulty of generating such a response may, in part, lie in the particular characteristics of bNAbs. Overall, monoclonal bNAbs isolated from infected individuals are characterized by unusual properties such as long antigen-contacting sites [complementarity determining region-3 (CDR-3)] and tremendous levels of somatic hypermutation (SHM), the extent of which is uncommon in other Ab responses[10, 16, 17]. These changes are characteristic of Ab affinity maturation processes, which occur in germinal centers (GC) within lymphoid tissues. T follicular helper cells (TFH) play a cardinal role in Ab affinity maturation by promoting immunoglobulin class switching [class-switch recombination (CSR)], SHM, and B cell differentiation and survival[18]. During this process, termed the GC reaction, TFH cells and antigen-specific B cells undergo a tight regulatory program that drives these mechanisms[19]. Thus, insight into the generation of protective Ab responses requires understanding of these cellular interactions and the underlying mechanisms.
There have been significant advances in our understanding of TFH immunobiology in both natural settings and during HIV infection. While perturbation of the B cell compartment in HIV infection is well established[20], a number of recent studies have shown a link to TFH immunopathology during HIV/SIV infection, including changes in TFH frequency, phenotype and function[21–25]. Recent studies suggest that the rare and infrequent generation of HIV-specific bNAbs may be associated with TFH frequency[12, 26]. This is further supported by mouse studies demonstrating a dynamic exchange of TFH cells between different GCs[27], ensuring the maximal diversification of T cell help. Using two-photon microscopy, it was demonstrated that the ability of TFH cells to enter ongoing GCs may accommodate antigenic variation during the immune response, maximizing the humoral response[27]. Moreover, emerging data suggest that TFH responses may be traceable in peripheral blood[26, 28–31], providing a potential tool to monitor vaccine efficacy. In this article, we therefore focus on the immunobiology of TFH cells in natural HIV infection and their involvement in HIV pathogenesis and bNAb development. We discuss studies that examine whether peripheral blood TFH counterparts (pTFH) may serve as surrogates for TFH cells in lymphoid tissues, and provide an outlook on how these and other recent findings might inform HIV vaccine design.
T follicular helper cells and antibody-mediated immunity
Burnet’s proposal for the clonal selection theory of Ab formation[32] launched the modern age of cellular immunology, in which lymphocytes were established as the agents of immunity and divided into distinct humoral and cellular components. Subsequently, in the 1960s, Miller and Claman described for the first time a dependence of Ab production on the presence of T cells[33, 34], termed T-dependent responses. Then in 1986, Velardi and colleagues described a CD4+ T cell subset within the GC[35]. While this subset showed properties distinct from the known T helper 1 (Th1) and Th2 CD4 T cell subsets, these cells were formally recognized as a distinct subset only in early 2000, taking center stage as a key player for T-dependent responses[18]. Since then our understanding of the development and function of TFH cells in both health and disease has grown rapidly in the past decade.
The transcriptional repressor B-cell lymphoma-6 (Bcl6) facilitates transcription of signature TFH markers that mediate TFH migration and function[36–38] while antagonizing other CD4 T cell differentiation programs[37, 38]. Expression of Bcl6 is initiated by DC-mediated priming of naïve CD4 T cells, triggering differentiation into TFH cells in T cell zones of lymphoid follicles[39]. Bcl6 induction promotes CXCR5 expression, which in turn facilitates TFH migration to the T–B cell border, promoting encounters with B cells specific for the same antigen (cognate) that reinforce TFH lineage commitment[40]. Inducible T-cell costimulator (ICOS), a surface protein expressed on activated T cells, is central to all phases of TFH development and GC formation[41, 42]. Additionally, in conjunction with IL-6, ICOS also promotes expression of IL-21, which is the cardinal cytokine for TFH cells[43, 44]. IL-21 further augments Bcl6 expression to drive TFH lineage differentiation[44–46] as shown by the inability of IL-21−/− mice to develop TFH cells with full helper activity and maintain GCs[45, 46]. These various cellular and molecular signals together contribute to B cell responses by initiating the TFH program.
The coalescence of TFH and B cells initiates the GC reaction, during which TFH cells become fully polarized, highly activated GC TFH cells[19]. GC TFH cells are exposed to ongoing stimulation by cognate B cells presenting antigen and co-stimulatory signals resulting in heightened expression of TFH markers, including ICOS and PD-1, in GC TFH compared to TFH cells[27]. ICOS signaling in GC TFH cells induces expression of the transcription factor c-Maf[47], which synergizes with Bcl6 to orchestrate further initiation and maintenance of the TFH program[48]. TFH lineage differentiation is therefore tightly linked to its functional role in B cell differentiation, survival, and longevity of Ab responses.
Upon encountering antigen, B cells can differentiate directly into short-lived plasma cells (PC) or migrate into the lymphoid follicles to receive TFH-derived help. Here B cells compete for a limiting source of TFH signals based on the amount of MHC-II:antigen complexes presented on B cells[49]. The degree of TFH signal is dictated by the quality and duration of B cell interactions with molecules expressed on the surface of TFH cells[50]. These TFH signals are critical for differentiation of GC B cells into long-lived PCs and memory B cells, as well as the ensuing maturation of Ab affinity through CSR and SHM[19]. Thus B cells with the strongest interactions with TFH cells subsequently either become memory B cells or leave the GCs and differentiate into long-lived PCs[51], colonizing the bone marrow or tissues underlying epithelial surface. Several surface receptors govern this interaction in addition to MHC-II: First, the signaling lymphocytic activation molecule (SLAM) family of receptors and the SLAM-associated protein (SAP) family of intracellular adaptors are responsible for maintaining long-duration contacts with antigen-specific B cells[52]. SLAM-bound SAP triggers positive signaling through Src-family kinases while inhibiting negative signaling through the negative signaling receptor, LY108[50, 52]. Another TFH surface molecule, the inhibitory receptor programmed death-1 (PD-1) is highly expressed on TFH cells and actively involved in sustaining the GC reaction by inhibiting follicular regulatory T cells that suppress the GC response[53, 54]. Among other molecules, the interaction between CD40 on activated B cells and its ligand (CD40L) expressed on TFH cells is one of the most crucial receptors for the development of T-dependent Ab responses and the generation of affinity-matured Abs. CD40 ligation leads to expression of activation-induced cytidine deaminase (AID), which is a critical enzyme regulating CSR, SHM and likely many other additional aspects in Ab affinity maturation[55]. Whether HIV-specific TFH responses –including CD40L expression and cytokine secretion - differ between individuals that develop bNAbs or those that fail to do so remains unknown.
Another prominent factor for Ab affinity maturation is the cardinal TFH cytokine, Interleukin-21 (IL-21). In addition to its central role in TFH development, IL-21 is indispensable for Ab affinity maturation. IL-21 and Interleukin-4 have been shown to cooperatively direct Ab responses in both mice and humans[56], and further instructs the differentiation of B cells by stimulating expression of B cell master transcription factors[57]. Together, data suggest TFH cells provide critical help to GC B cells and promote Ab affinity maturation by providing IL-21 in the presence of CD40 ligation. Additionally, SAP/SLAM-dependent IL-4 production provides further survival signaling[58]. Interestingly, IL-21-secreting CD4 T cells have also been shown to help sustain CD8 T cell effector function during chronic viral infections[59, 60] and are associated with HIV control[61]. However, whether TFH cells, or a distinct IL-21-secreting CD4 subset, mediate this IL-21-dependent CD8 T cell “help” remains to be established.
TFH cells in HIV pathogenesis
A fundamental characteristic of HIV pathogenesis is widespread immune activation and perturbation of the B cell compartment[20]. That CD4 T cells are the primary targets of HIV[21] and the specific induction of TFH cells through vaccination may (temporarily) increase the pool of HIV target cells represents a duality in harnessing TFH cells for HIV vaccinology. Thus understanding the immunobiology of TFH cells in HIV infection and their involvement in the generation of bNAbs during natural HIV infection –in spite of a preferential depletion - is a fundamental question for HIV vaccine development. Currently, several studies have begun to explore TFH cells in the context of HIV/SIV.
All studies of these cells to date have consistently demonstrated an unexpected expansion of both general and virus-specific TFH frequency in both chronic HIV[21, 23, 24] and SIV[22, 25] infection, despite potential preferential depletion of antigen-specific CD4 T cells[62]. While no direct relationship with viral loads was observed[22, 23, 25], TFH frequencies substantially decreased with ART treatment, suggesting a role for antigen levels in driving TFH differentiation[21, 23]. Similarly, TFH expansion has been previously reported in the lymphocytic choriomeningitis virus (LCMV) mouse model where it has been suggested that viral persistence and prolonged T cell receptor stimulation may progressively direct T cells towards a TFH program[63]. However, another study of LCMV infection demonstrated a biphasic secretion of IL-6, the strongest driver of TFH development, during acute infection and again during the chronic phase. Yet, only the chronic phase of IL-6 secretion seems to be responsible for driving subsequent TFH expansion[64]. Interestingly, a similar biphasic IL-6 response has been demonstrated in acute HIV infection[65], but an association with human TFH cell expansion in HIV remains to be shown. Nevertheless, studies of SIV infection have shown that TFH expansion is strongly linked to a skewing in the IL-6 signaling axis[22]. Likewise, they have also reported elevated TFH levels in chronic, compared to acute, infection suggesting that immune activation, rather than antigen levels may be driving TFH expansion.
Expansion of TFH cells has been associated with perturbation of the B cell compartment. In particular, GC B cell and PC frequency was positively correlated with TFH frequency, which is not surprising given TFH cells’ involvement in the development of these cells. Moreover, memory B cells in HIV+ Lymph Nodes (LN) were negatively correlated to TFH frequency[23, 24]. In addition, aside from a general expansion of TFH frequency, TFH function seems to also be altered in HIV/SIV infection. Cubas et al. demonstrated that TFH cells from HIV+ LNs less-efficiently promoted B cell survival and differentiation, likely due to deficient IL-21 secretion. However, TFH dysfunction was primarily attributed to B cell dysregulation in that excess PD-1–PD-L1 ligation by dysregulated B cells impaired the overall TFH cell response[24].
Despite compromised TFH-derived help, TFH expansion correlates with enhanced circulating SIV-specific IgG in NHP [22, 25] and hypergammaglobulinemia in humans[23]. Petrovas et al. described a positive association between TFH frequency, expansion of the B cell compartment and increased circulating high-avidity SIV-specific Abs in chronic SIV infection; this finding suggests that chronic SIV infection may not disrupt TFH-derived Ab affinity maturation and SIV-specific Ab production[22], but Ab affinity maturation may be impaired overall. Indeed, while higher TFH frequency drives B cell proliferation, constant antigenic stimulation may lead to the selection of low affinity B cells[25]. Thus, it is likely that the low-quality Ab production described in HIV/SIV infection may be induced by a combination of factors, including TFH /B cell dysfunction, high levels of antigenemia, and immune activation.
Expansion of virus-specific TFH cells seems to conflict with the profound depletion of activated antigen-specific CD4 T cells seen in HIV infection, raising the question of whether TFH cells are somewhat protected from infection/depletion. However, it has been demonstrated that SIV readily infects TFH cells in GCs[22] and the T cell population within the lymph node that corresponds to TFH cells (CXCR5+PD-1+Bcl6+) serves as the major T cell compartment for HIV infection, replication, and production[21]. Interestingly, a study of pigtail macaques found infection of TFH cells in the absence of CCR5 or other HIV/SIV co-receptor expression[66]; however, whether infection of TFH cells occurs through a different co-receptor or whether TFH cells are infected prior to differentiation and subsequent CCR5 downregulation remains to be seen. Overall, the current data suggest that TFH cells may represent a continually replenishing pool of target cells for SIV[22, 25, 66] and HIV[21]. Taken together, these data indicate a model in which HIV promotes B cell dysfunction while securing a viral reservoir in lymphoid tissues that are relatively devoid of CD8 CTLs[66] (Figure 1).
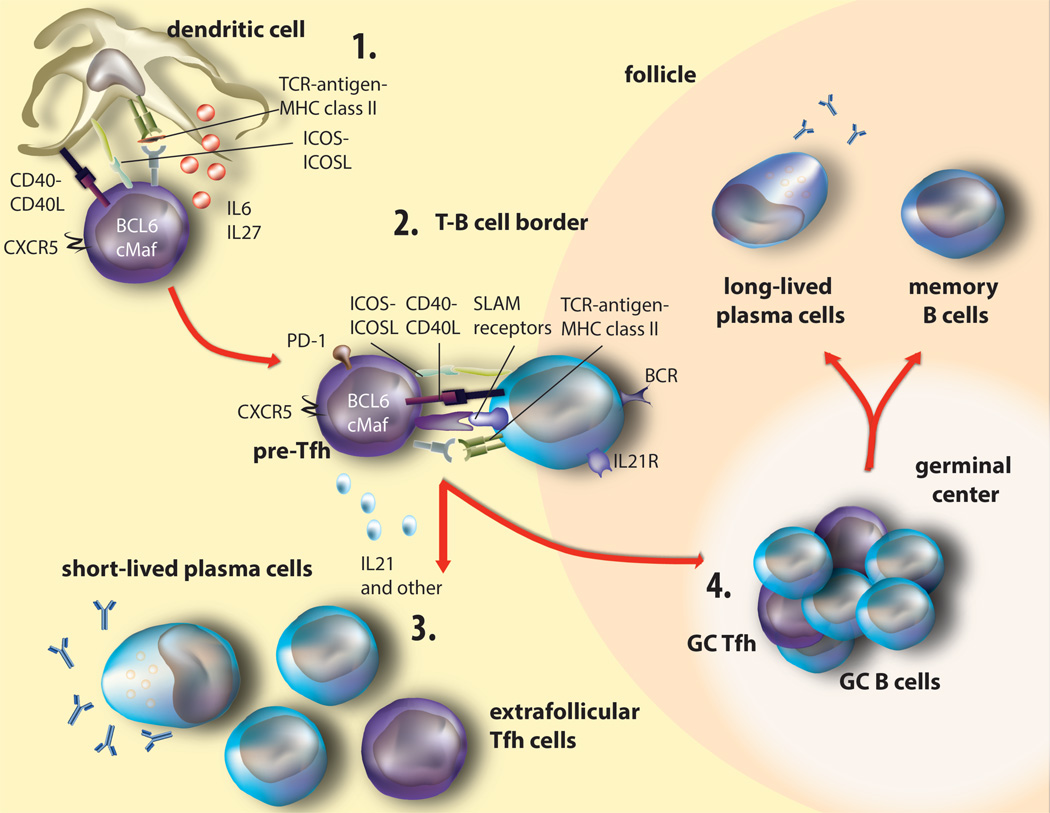
Figure 1. Development of TFH cells and the germinal center reaction.
(1) Dendritic cells present antigen and activate naïve CD4 T cells via TCR:MHC-II, ICOS-ICOS-L, and CD40:CD40L interactions to initiate the TFH program. DC- and B cell-derived IL-6 and IL-27 cytokines maintain and reinforce this lineage, leading to upregulation of the TFH transcription factors Bcl6, cMaf, and CXCR5. (2) Expression of CXCR5 facilitates the migration of pre-TFH cells toward the T—B cell interphase. Here, cognate pre-TFH and B cells exchange molecular signals including ICOS:ICOS-L, CD40:CD40L, TCR:MHC-II, and several SLAM receptor family members, as well as several cytokines including the cardinal cytokine IL-21, that reinforce expression of Bcl6 and c-Maf that further increase expression of TFH surface receptors. B cells simultaneously undergo diverse signals that instruct their maturation pathway: either extrafollicular or GC. (3) Extrafollicular activated B cells then convert to short-lived plasma cells and secrete Ab with or without extrafollicular TFH help. (4) In the GC, prolonged interactions between GC TFH and GC B cells further confirm the TFH lineage commitment, resulting in mutual exchange of survival signals that result in GC maintenance. GC TFH cells provide a limiting source of antigen for which B cells compete for survival signals based on BCR affinity. Furthermore, here GC TFH cells instruct GC B cells to phosphorylate and express Activation-induced Deaminase (AID) that mediates SHM, CSR, memory cell and long-lived plasma cell formation.
While significant progress has been made in understanding the immunbiology of TFH cells in HIV infection, several outstanding questions remain regarding how TFH cells are involved in B cell dysfunction and/or the rare generation of bNAbs that occur after years of infection. Of particular interest is the causality of these observations: that is, it is unknown whether TFH expansion directly promotes the perturbation of the B cell compartment or whether these are two simultaneously occurring infection-mediated mechanisms. Moreover, it remains to be shown whether the described alterations are overall beneficial or disadvantageous overall for the generation of bNAbs. As all HIV-infected individuals develop NAbs but only a fraction develops bNAbs, it will be critical to understand whether antigen-specific TFH function, HIV antigenemia, immune activation, other unknown factors, or a combination of these drives the extensive levels of SHM observed in bNAbs. It is likely that multiple factors synergize to dampen or delay bNAb generation; for example, low-affinity Ab responses to linear epitopes may arise from lowered selection and competition threshold for TFH help (from TFH expansion) between B cells (with low-affinity BCR) specific for different viral quasispecies[49]; this may divert the Ab response away from neotopes. Coincidentally, HIV- or Env-derived B cell deregulation may impair TFH-derived B cell help, disrupting affinity maturation, and further promoting low-affinity Ab production. However, this hypothesis is challenged by findings describing associations of TFH-like counterparts circulating in peripheral blood with bNAb development (see below). Overall, dissecting the signals involved in promoting SHM for the generation of bNAbs and understanding which TFH functions and B cell perturbations are unique to HIV (or other chronic infections) will be critical hurdles to overcome for effective vaccine design.
Broadly cross-reactive NAbs and TFH cells
In recent years, there has been substantial progress in the identification and isolation of bNAb-producing B cells from chronically infected individuals. The profound SHM displayed by most bNAbs[67] suggests extended affinity maturation signals, likely mediated by TFH cells, thus implicating a prominent role of TFH cells in bNAb development.
In most patients, bNAbs develop only after years of chronic infection[9, 11, 12] and show remarkable properties including extremely high levels of SHM[10, 16, 68, 69], long CDR3 regions[70], and, in certain cases, polyreactivity[71]. For example, Klein et al. showed that monoclonal bNAbs undergo SHM in Ab framework regions that control molecular structure. Furthermore, these mutations were essential for bNAb function, as removing point mutation occurring in these regions ablated neutralizing activity[67]. Similarly, restoring monoclonal bNAbs to their germline sequences removes neutralization potency and Env specificity[72]. These high levels of Ab rearrangements (up to 42%) are not commonly found in other Abs[10, 16, 17], suggesting a constant and prolonged activation of AID. Activation and phosphorylation processes of AID occur through CD40-CD40L signaling[55] provided by TFH cells suggesting an intimate role for TFH cells in bNAb development as they undergo multiple rounds of selection in response to a continually evolving pathogen[73]. While these observations provide a glimpse into the difficulty of bNAb development, the mechanisms behind this process, the extent of TFH involvement and the specific signals provided by TFH cells remain unknown.
In this regard, optimal antigen recognition and presentation mechanisms would likely optimize TFH B helper activity[51]. Interestingly, a retrospective study conducted on uninfected RV144 vaccine recipients demonstrated that patients carrying HLA-DQA1*05:01 and DQB1*03:01 alleles were less likely to produce NAbs[74] suggesting a direct involvement of antigen presentation to the induction of NAbs in vaccine recipients. No association between bNAb generation and HLA-II expression has yet been described in natural HIV infection, however. The extent to which HLA-II alleles influence B cell selection and whether HLA-DR or HLA-DP plays a more predominant role over HLA-DQ remains unknown. Moreover, it is also unknown whether different epitope-specificities of CD4 T cell responses are involved in NAb development and whether the abundance of Gag-specific responses over Env-specific TFH responses impacts the development of NAbs in natural HIV infection[23].
Recent landmark studies reporting the isolation of potent bNAbs from HIV-infected patients with broad neutralization breadth and advances in deep sequencing of single-cell bNAb-secreting memory B cells and PCs have provided important insights into their maturation pathways[16, 75, 76] as well as bNAb binding mechanisms[10]. While these illustrate the profound intricacies of bNAb development, the hope is that they will inform rational vaccine design to harness TFH cells to mediate targeted affinity maturation, PC differentiation and stimulation of bNAb responses. However, many questions surround this approach, particularly regarding what signals are required for optimized TFH generation, and how to induce them through vaccination (Box 1).
Box 1: Critical questions regarding TFH cells in HIV vaccine design.
Translating lessons from natural HIV infection to vaccination-induced NAb development is a hurdle that over 30 years of active research has yet to overcome. The resolution of several key issues, particularly regarding the duration of NAb development, is imperative to achieve this feat: (1) What is the relationship between TFH cells, B cell dysfunction, and NAb development in natural infection; (2) Does viral quasispecies evolution promote continuous priming of Ab responses to linear epitopes, leading to constant production of low affinity antibodies rather than affinity maturation to conserved regions (or neotopes), and is this a result of B cell competition; (3) Does the number and location of SHM that are required to convey bNAb activity solely explain the delay in NAb development or is the SHM/AID machinery impaired by HIV pathogenesis, thus affecting the efficiency of TFH:B cell help and the ensuing B cell proliferation, differentiation, and Ab affinity maturation; (4) Is the trimeric, highly glycosylated nature of HIV Env detrimental to affinity maturation and SHM by activating T-independent Ab responses or short-lived plasma cells; (5) Are certain Env variants more elusive than others at inducing beneficial TFH responses; (6) Is this an issue of epitope miscommunication between TFH cell and B cells or is it an issue of immunogenic silence; (7) Does antigenemia promote SHM in an individual with a healthier B cell compartment and how can this be achieved through vaccination; (8) Are TFH cells a major latent reservoir during HIV infection; (9) Is TFH cell dysfunction/expansion influencing the lack of bNAb development in the majority of patients and can TFH functionality/dynamics account for the rapid bNAb development in certain subjects; (10) Can the induction of specific TFH functions drive bNAb development; and (11) Can Gag-specific TFH responses provide help for Env-specific Abs.
Peripheral TFH cells in infection and vaccination
One inherent problem to studying co-dynamics of TFH and Ab development in infection and vaccines in human patients is the paucity of lymphoid tissue available for experimental analyses. Thus, the ability to define a population of memory TFH cells in peripheral blood (pTFH) would facilitate our understanding of CD4 T cell dynamics within lymphoid tissue during vaccination and infection. Initially, Mikell et al. reported higher frequencies of circulating CD4 T cells expressing PD-1 in individuals with broad neutralizing serum activity[12], thus potentially associating the emergence of a TFH-like population in peripheral blood with bNAb development, as PD-1 is highly expressed on TFH cells[53, 54]. While this study was not designed to investigate pTFH cell immunobiology, it was the first study to shine a spotlight on the potential associations between TFH (and pTFH) cells and bNAb development. Given the importance of such findings for the design of potential vaccines to specifically promote bNAb development, several groups have subsequently focused on characterizing the association between GC TFH and peripheral counterparts during HIV-specific and other types of Ab responses[26, 28–31, 77].
While most studies agree that CXCR5+CD4 T cells in peripheral blood represent a counterpart of TFH cells, their relationship to GC TFH cells has been challenged based on differences in surface marker phenotypes and transcription factor expression levels [18]. Indeed, a large heterogeneity of circulating CXCR5+CD4 T cell subsets has been described in terms of gene expression[26, 31, 77], surface marker expression, and function[26, 28, 29, 31, 77], complicating clear conclusions on this issue. Indeed, emerging studies describe biologically relevant B helper activity in various subsets of CXCR5+CD4 pTFH cells; moreover, these subsets have been defined using different groups of cell surface markers, further complicating comparisons between these studies (see below and Table 1). Emerging evidence suggests that TFH memory is generated along with B cell memory, and combinations of phenotypic markers likely represent different layers of TFH differentiation and effector function that is reflected in peripheral blood[28, 29]. Indeed, evidence shows that pTFH cells (CXCR5+CD4+) are central memory cells comprised of Th1-, 2-, and 17-like subsets (based on CXCR3 and CCR6 expression) in healthy adults, each with diverse B helper functions in co-culture and in vivo[29, 30]. Of these, the Th1-like, CXCR3+CCR6−CXCR5+CD4 subset lacks naïve or memory B helper capacity in mice[29]. However, upon activation by trivalent influenza vaccine in humans, activated Th1-like ICOS+CXCR3+CCR6−CXCR5+CD4 subset efficiently induced memory, but not naïve, antigen-specific B cells to proliferate, differentiate, and produce Igs[30]. In contrast, the Th2- and −17-like pTFH (CXCR3−CCR6+/−) supported naïve B cell differentiation in mice[29] and humans[30]. Supporting these observations, evidence from studies of healthy and HIV-infected human specimens suggests CCR6highCXCR3+/−CXCR5+CD4 pTFH induce Ab secretion from naïve B cells, although helper capacity is impaired in HIV-infected samples[77]. Indeed, this pTFH subset (CCR7highCXCR5highCCR6highPD-1high) displayed the highest IL-21 production and naïve B cell help, inducing the greatest production of IgG1, IgG3, and IgA compared to other subsets in co-culture with naïve B cells[77]. These data suggest that perhaps CXCR3−CXCR5+CD4 pTFH cells are potent inducers of naïve B cell differentiation while CXCR3+CXCR5+CD4 pTFH primarily induce memory B cell differentiation.
Table 1.
Divergent surface marker profile of pTFH cells with B helper activity.
| Morita (2011) pTfh |
Mikell (2011)*- |
Chevalier (2011) pTfh |
Bentebibel (2013) pTfh |
Locci (2013) pTfh |
He (2013) pTfh & GC Tfh |
Boswell (2014) pTfh & GC Tfh |
|
|---|---|---|---|---|---|---|---|
| CXCR5 | + | + | + | + | + | hi**# | |
| CXCR3 | − | + | − | ±/− | |||
| PD-1 | + | + | + | hi | hi | ||
| ICOS | + | ||||||
| CCR6 | + | hi | hi | ||||
| CCR7 | low | low | hi |
study was not designed to interogate Tfh phenotype
CCR7high CXCR5low cells had limited naïve B helper activity
CXCRSlow cells in HIV viremic subjects displayed limited naïve B helper activity

Other studies have explored the relatedness of pTFH with GC TFH in the lymphoid follicle, based on other combinations of surface marker and gene expression profiles. These studies demonstrate that CXCR5+CD4 pTFH cells are either activated (PD-1+ICOS+[30] or PD1+CCR7lo[28]), or quiescent (PD-1+/−ICOS−[26, 28]) cells. In this regard, CXCR3− (PD-1+CXCR3−CXCR5+CD4+) pTFH were described as memory cells with highly functional B helper activity in co-culture that share a transcriptional profile signature with GC TFH cells, including expression of MAF and SLAMF6[26]. On the other hand, a slightly different pTFH subset (CXCR5highCCR6highPD-1high) was concluded to more closely resemble a memory, non-TFH CD4 T cell population from the tonsil compared to non-GC and GC TFH cells (based on gene expression analyses), as transcript levels of MAF, BCL6, IL-21 were lower in pTFH than tonsillar TFH cells[77]. While Boswell et al. suggested that the pTFH subset they identified was predominantly CXCR3−CCR6high, Locci et al. did not incorporate CCR6 in their phenotypic panel[26, 77]. Yet a separate study using both mouse and human specimens found high CCR6 expression in the PD-1hiCCR7lo subset of CXCR5+CD4 pTFH; this subset, but not PD-1loCCR7hi reportedly indicates active GC TFH differentiation[28]. Further studies are required to reconcile the various surface markers used to derive the conclusions described above.
Interestingly, in vivo functional relevance was shown for each of the particular pTFH subsets identified. Both subsets of activated pTFH (PD-1+ICOS+ and PD-1+CCR7lo) cells were associated with Ab responses to influenza vaccination[28, 30] and autoimmune Ab production[28]. In co-culture assays, PD-1hiCCR7lo pTFH potently induced plasmablast and PC differentiation as well as total antigen-specific IgG production to Influenza[28, 30] and doublestranded DNA[28]. Similarly, the ratio of Th1-, 2-, and 17-like pTFH subsets were reportedly skewed in patients with dermatomyositis, an autoantibody-mediated autoimmune disease, compared to healthy controls. This resulted in increased frequency of naïve B cell helper vs. non-helper pTFH cells that further correlated with disease severity and circulating plasmablasts[29].
Establishing a significant association between HIV-specific NAb and bNAb development, however, is much more convoluted. On one hand, higher frequencies of quiescent pTFH (PD-1+CXCR3−CXCR5+CD4+)[26] and PD-1+CD4 T cells[12] were described in HIV-infected donors exhibiting broad and potent serum neutralization activity[12, 26], while no association was observed between pTFH frequency (irrespective of phenotype) and HIV Env-specific Ab titers, total IgG levels, or HIV-specific serum neutralizing activity in HIV-infected individuals exhibiting normal serum neutralization activity[77]. The difference in breadth and potency between the donors used in these studies may contribute to the discordant results. However, similarly discordant associations exist even in analyses using only samples from HIV-infected donors with broad and potent neutralizing activity, further complicating the situation. Indeed, while Mikell et al. found higher frequencies of PD-1+CD4 T cells in these patients[12], subsequent analyses failed to corroborate this association using a slightly further delineated PD-1+ pTFH subset (PD-1hiICOS+CXCR5+CD4+)[26]. The complex nature of bNAb development is confounded by classification of “good neutralizers” and subjects with broad and potent neutralizing serum activity[12, 26, 77]. Indeed, while all HIV-infected individuals develop patient-specific NAb, only a minority develops broad and potent neutralization activity[73]. Furthermore, an important caveat to consider is that broad and potent neutralizing activity appears in serum of chronically infected subjects, meaning bNAb development likely takes years to develop under normal conditions; thus, perhaps pTFH:B cell dynamics should be monitored during the acute, rather than chronic, phase of HIV infection. In this regard, Locci et al. observed a correlation between the frequency of PD-1+CXCR3−CXCR5+ pTFH cells at approximately 4 months post infection and the capacity of individuals to subsequently develop bNAbs during the chronic phase[26]. Although this association remained during the time of bNAb development months later, examining the pTFH:B cell dynamics may elucidate early events that facilitate the ensuing development of bNAbs, and explain why this occurs in only a minority of individuals. While the evidence presented thus far is quite encouraging, the novelty of using pTFH cells to track bNAb responses necessitates further experimentation to reconcile the different conclusions described. It may be possible, however, that all these subsets play varying roles in different facets of Ab responses. Indeed, recent evidence suggests that surface expression of certain TFH markers is downregulated in memory TFH cells after antigen clearance, while the TFH lineage of these cells remains intact[78].
Collectively, most recent studies reinforce the notion that a circulating pool of memory TFH cells exists in both humans and mice. Indeed, in an elegant study using both mice and human specimens, He et al. demonstrated that PD-1hi(ICOS+CCR7lo) pTFH are activated cells that identify active GC TFH differentiation[28]. Furthermore, these authors demonstrated that pTFH cells are antigen-experienced precursors of GC TFH cells, as pTFH generation occurred independent of GC-forming factors (SAP) but still required TFH differentiating factors (ICOS and Bcl6)[28]. While the relatedness of pTFH and TFH cells in lymphoid follicles was challenged by another study based on naïve B cell helper capacity, CCR6 expression, and gene expression profiles[77], it did not directly address the relationship between GC TFH in LN and pTFH from matched donors as was done by He et al. It may be possible that TFH marker expression and B helper function is affected by phase of infection or nature of immunogen. Indeed, Bentebibel et al. suggested that different vaccination modalities and/or adjuvants might stimulate divergent subsets of pTFH, skewing the Ab response to the vaccine[30]. Thus future studies investigating the pTFH profiles induced by various previously tested vaccines would be informative in this regard. Importantly, by showing that CCR7loPD-1hi pTFH are precursor TFH cells with prominent helper capacity that circulate through nondraining LN, He et al. proposed that pTFH cells can rapidly differentiate into mature TFH cells upon antigen re-exposure to support GC formation and mediate B cell help, thus implying a mechanism by which TFH cells exit the secondary LN and are poised to accelerate Ab responses in non-draining LN, likely to contain a spreading infectious agent[28]. These studies provide a better-defined phenotype with which to monitor the status and specificities of TFH cells and provide critical support to using pTFH to study the development of affinity matured Abs, such as bNAbs.
A key question that remains is the role of antigen persistence in vaccine responses and whether and how this contributes to divergent pTFH phenotypes, function, Ab potency and duration. Moreover, it may be possible that all identified potential pTFH characteristics are correct in different settings of diseases, vaccination or populations. Indeed, while antigen specificity of TFH cells in bNAb development remains to be established[26], a possible explanation for the divergent results between in vivo HIV bNAb and influenza-specific Ab development is that the CXCR3+CXCR5+CD4+ T cell population induced by influenza vaccination may provision suboptimal B cell help by only promoting memory responses[29, 30], thus potentially explaining the low efficiency and duration of seasonal influenza vaccine campaigns. Future studies exploring the interplay between pTFH cells, B cells and Ab production will likely provide insight into how to best design more effective vaccines.
Concluding remarks
Current research highlights the tight involvement of TFH cells in the development of bNAbs during natural HIV/SIV infection despite evidence of dysregulated TFH-mediated B cell help. Exploring pTFH dynamics may facilitate the dissection of TFH:B cell interactions that culminate in bNAb development during chronic infection, providing insight into how TFH responses can be manipulated to optimize AID expression, SHM, and CSR and long-lived B cell responses. Knowledge of how to tailor TFH responses through vaccination will inform vaccine development for HIV and others diseases.
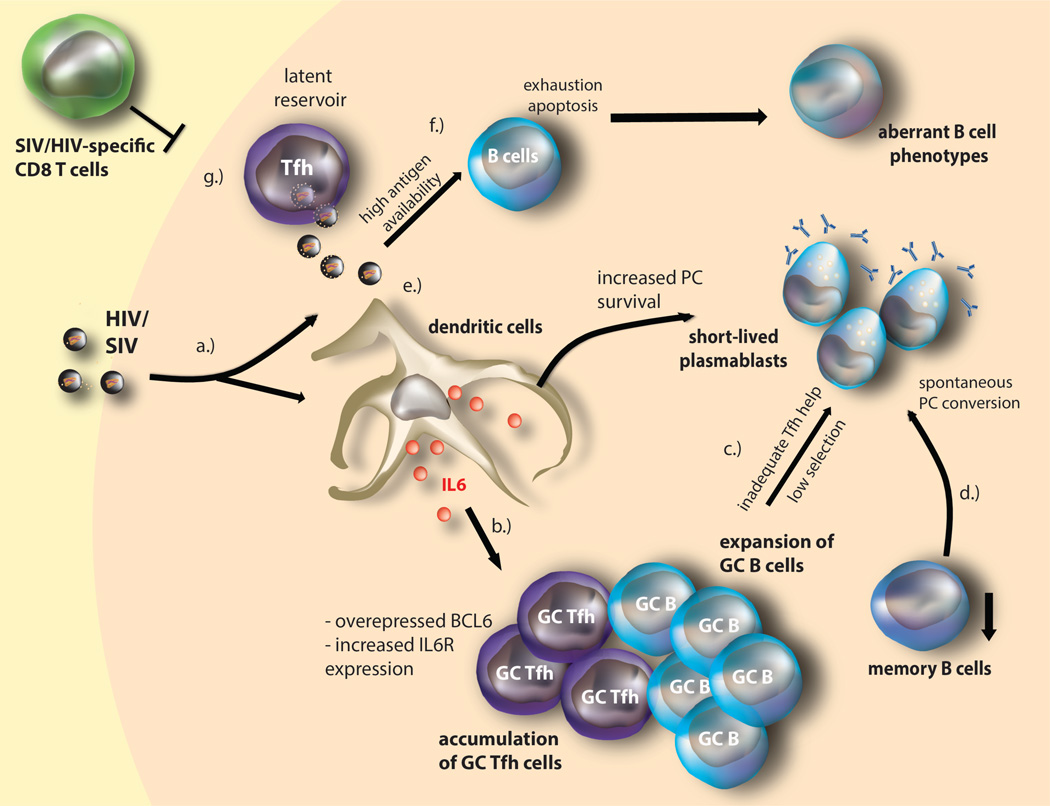
Figure 2. HIV/SIV-mediated TFH dysfunction and immunopathogenesis.
(a.) HIV/SIV mediated immune activation induces high IL-6 production found within infected lymph nodes. (b.) IL-6 induces expansion of (potentially dysfunctional) TFH cells expressing high levels of Bcl6 and IL-6Rα. TFH expansion is further associated with increased numbers of GC B cells. (c.) GC TFH expansion increases contact with PD-L1-expressing GC B cells, resulting in deregulated GC TFH cells and inadequate help provisioned to GC B cells, likely by lowering the selection threshold and decreasing IL-21 signaling (and other TFH cytokines such as IFN γ and IL-10) that lead B cell differentiation into short-lived PC formation and increased polyclonal and HIV/SIV-specific (primarily targeting Gag) IgG production. (d.) Similarly, direct IL-6 signaling may also mediate spontaneous terminal differentiation of memory B cell into plasma cells, resulting in observed decrease of memory B cells in HIV/SIV infection. (e.) High antigenic availability within the lymph node likely also enhances plasma cell survival. (f.) High antigenemia likely also contributes to B cell exhaustion, apoptosis, and the subsequent aberrant B cell phenotypes. (g.) HIV/SIV infection of TFH cells (enhanced by IL-6) likely maintains the viral reservoir, as infected TFH cells, may be resistant to apoptosis; this likely constitutes a latent reservoir within a privileged tissue, as HIV/SIV-specific CD8 CTL relatively seldom enters the lymphoid tissue.
Highlights.
-
-
T follicular helper cells present a latent HIV viral reservoir
-
-
Tfh cells may be intricately involved in the generation of HIV-specific broadly neutralizing Abs
-
-
Insights into Tfh immunobiology may provide inroads into effective vaccine design
-
-
Peripheral Tfh cell counterparts may facilitate studies of Tfh in lymphoid tissues
Acknowledgements
We apologize to those whose work is not mentioned in this review due to space limitations. This study was funded by the US National Institutes of Health (NIH) (R01 AI091450-01 and R01 AI094602-01) and a cooperative agreement (W81XWH-07-2-0067) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense (DOD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Note: The views expressed herein are those of the authors and should not be construed to represent the positions of the US Army or the Department of Defense.
References
- 1.Rerks-Ngarm S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 2.Haynes BF, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47:401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 4.Moldt B, et al. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci U S A. 2012;109:18921–18925. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balazs AB, et al. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2012;481:81–84. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shingai M, et al. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature. 2013;503:277–280. doi: 10.1038/nature12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barouch DH, et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013;503:224–228. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang J, et al. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature. 2012;491:406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker LM, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheid JF, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sather DN, et al. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J Virol. 2009;83:757–769. doi: 10.1128/JVI.02036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikell I, et al. Characteristics of the earliest cross-neutralizing antibody response to HIV-1. PLoS Pathog. 2011;7:e1001251–100. doi: 10.1371/journal.ppat.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray ES, et al. The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. J Virol. 2011;85:4828–4840. doi: 10.1128/JVI.00198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simek MD, et al. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J Virol. 2009;83:7337–7348. doi: 10.1128/JVI.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doria-Rose NA, et al. Breadth of human immunodeficiency virus-specific neutralizing activity in sera: clustering analysis and association with clinical variables. J Virol. 2010;84:1631–1636. doi: 10.1128/JVI.01482-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu X, et al. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science. 2011;333:1593–1602. doi: 10.1126/science.1207532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu X, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tangye SG, et al. The good, the bad and the ugly - TFH cells in human health and disease. Nat Rev Immunol. 2013;13:412–426. doi: 10.1038/nri3447. [DOI] [PubMed] [Google Scholar]
- 19.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 20.Moir S, Fauci AS. Insights into B cells and HIV-specific B-cell responses in HIV-infected individuals. Immunol Rev. 2013;254:207–224. doi: 10.1111/imr.12067. [DOI] [PubMed] [Google Scholar]
- 21.Perreau M, et al. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med. 2013;210:143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrovas C, et al. CD4 T follicular helper cell dynamics during SIV infection. J Clin Invest. 2012;122:3281–3294. doi: 10.1172/JCI63039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindqvist M, et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest. 2012;122:3271–3280. doi: 10.1172/JCI64314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cubas RA, et al. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med. 2013;19:494–499. doi: 10.1038/nm.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong JJ, et al. Spatial alterations between CD4(+) T follicular helper, B, and CD8(+) T cells during simian immunodeficiency virus infection: T/B cell homeostasis, activation, and potential mechanism for viral escape. J Immunol. 2012;188:3247–3256. doi: 10.4049/jimmunol.1103138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Locci M, et al. Human Circulating PD-1CXCR3CXCR5 Memory Tfh Cells Are Highly Functional and Correlate with Broadly Neutralizing HIV Antibody Responses. Immunity. 2013 doi: 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shulman Z, et al. T follicular helper cell dynamics in germinal centers. Science. 2013;341:673–677. doi: 10.1126/science.1241680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He J, et al. Circulating Precursor CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T Cells Indicate Tfh Cell Activity and Promote Antibody Responses upon Antigen Reexposure. Immunity. 2013;39:770–781. doi: 10.1016/j.immuni.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Morita R, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bentebibel SE, et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci Transl Med. 2013;5:176ra132. doi: 10.1126/scitranslmed.3005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chevalier N, et al. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. J Immunol. 2011;186:5556–5568. doi: 10.4049/jimmunol.1002828. [DOI] [PubMed] [Google Scholar]
- 32.Burnet FM. A modification of Jerne’s theory of antibody production using the concept of clonal selection. Australian Journal of Science. 1957:67–69. doi: 10.3322/canjclin.26.2.119. [DOI] [PubMed] [Google Scholar]
- 33.Miller JF, et al. Thymus and the production of antibody-plaque-forming cells. Nature. 1965;208:1332–1334. doi: 10.1038/2081332a0. [DOI] [PubMed] [Google Scholar]
- 34.Claman HN, et al. Thymus-marrow cell combinations. Synergism in antibody production. Proc Soc Exp Biol Med. 1966;122:1167–1171. doi: 10.3181/00379727-122-31353. [DOI] [PubMed] [Google Scholar]
- 35.Velardi A, et al. Functional analysis of cloned germinal center CD4+ cells with natural killer cell-related features. Divergence from typical T helper cells. J Immunol. 1986;137:2808–2813. [PubMed] [Google Scholar]
- 36.Johnston RJ, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nurieva RI, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu D, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Kerfoot SM, et al. Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone. Immunity. 2011;34:947–960. doi: 10.1016/j.immuni.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 41.Choi YS, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34:932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu H, et al. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature. 2013;496:523–527. doi: 10.1038/nature12058. [DOI] [PubMed] [Google Scholar]
- 43.Bauquet AT, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nurieva RI, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eto D, et al. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS One. 2011;6:e17739. doi: 10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rasheed MA, et al. Interleukin-21 is a critical cytokine for the generation of virus-specific long-lived plasma cells. J Virol. 2013;87:7737–7746. doi: 10.1128/JVI.00063-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hiramatsu Y, et al. c-Maf activates the promoter and enhancer of the IL-21 gene, and TGF-beta inhibits c-Maf-induced IL-21 production in CD4+ T cells. J Leukoc Biol. 2010;87:703–712. doi: 10.1189/jlb.0909639. [DOI] [PubMed] [Google Scholar]
- 48.Kroenke MA, et al. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. J Immunol. 2012;188:3734–3744. doi: 10.4049/jimmunol.1103246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwickert TA, et al. Germinal center reutilization by newly activated B cells. J Exp Med. 2009;206:2907–2914. doi: 10.1084/jem.20091225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kageyama R, et al. The receptor Ly108 functions as a SAP adaptor-dependent on-off switch for T cell help to B cells and NKT cell development. Immunity. 2012;36:986–1002. doi: 10.1016/j.immuni.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwickert TA, et al. A dynamic T cell-limited checkpoint regulates affinity-dependent B cell entry into the germinal center. J Exp Med. 2011;208:1243–1252. doi: 10.1084/jem.20102477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cannons JL, et al. SLAM family receptors and SAP adaptors in immunity. Annu Rev Immunol. 2011;29:665–705. doi: 10.1146/annurev-immunol-030409-101302. [DOI] [PubMed] [Google Scholar]
- 53.Sage PT, et al. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat Immunol. 2013;14:152–161. doi: 10.1038/ni.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Good-Jacobson KL, et al. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol. 2010;11:535–542. doi: 10.1038/ni.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pavri R, Nussenzweig MC. AID targeting in antibody diversity. Adv Immunol. 2011;110:1–26. doi: 10.1016/B978-0-12-387663-8.00005-3. [DOI] [PubMed] [Google Scholar]
- 56.Zotos D, et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Linterman MA, et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cannons JL, et al. Biochemical and genetic evidence for a SAP-PKC-theta interaction contributing to IL-4 regulation. J Immunol. 2010;185:2819–2827. doi: 10.4049/jimmunol.0902182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frohlich A, et al. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324:1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- 60.Elsaesser H, et al. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chevalier MF, et al. HIV-1-specific interleukin-21+ CD4+ T cell responses contribute to durable viral control through the modulation of HIV-specific CD8+ T cell function. J Virol. 2011;85:733–741. doi: 10.1128/JVI.02030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Douek DC, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 63.Fahey LM, et al. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J Exp Med. 2011;208:987–999. doi: 10.1084/jem.20101773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harker JA, et al. Late interleukin-6 escalates T follicular helper cell responses and controls a chronic viral infection. Science. 2011;334:825–829. doi: 10.1126/science.1208421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Slike B, et al. Early immune events during acute HIV infection. Retrovirology. 2012;9:181. [Google Scholar]
- 66.Xu Y, et al. Simian immunodeficiency virus infects follicular helper CD4 T cells in lymphoid tissues during pathogenic infection of pigtail macaques. J Virol. 2013;87:3760–3773. doi: 10.1128/JVI.02497-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klein F, et al. Somatic mutations of the immunoglobulin framework are generally required for broad and potent HIV-1 neutralization. Cell. 2013;153:126–138. doi: 10.1016/j.cell.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scheid JF, et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458:636–640. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- 69.Corti D, et al. Analysis of memory B cell responses and isolation of novel monoclonal antibodies with neutralizing breadth from HIV-1-infected individuals. PLoS One. 2010;5:e8805. doi: 10.1371/journal.pone.0008805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McLellan JS, et al. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature. 2011;480:336–343. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mouquet H, et al. Memory B cell antibodies to HIV-1 gp140 cloned from individuals infected with clade A and B viruses. PLoS One. 2011;6:e24078. doi: 10.1371/journal.pone.0024078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McGuire AT, et al. Engineering HIV envelope protein to activate germline B cell receptors of broadly neutralizing anti-CD4 binding site antibodies. J Exp Med. 2013;210:655–663. doi: 10.1084/jem.20122824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Klein F, et al. Antibodies in HIV-1 vaccine development and therapy. Science. 2013;341:1199–1204. doi: 10.1126/science.1241144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Paris R, et al. HLA class II restriction of HIV-1 clade-specific neutralizing antibody responses in ethnic Thai recipients of the RV144 prime-boost vaccine combination of ALVAC-HIV and AIDSVAX((R)) B/E. Vaccine. 2012;30:832–836. doi: 10.1016/j.vaccine.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 75.Liao HX, et al. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013;496:469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou T, et al. Multidonor analysis reveals structural elements, genetic determinants, and maturation pathway for HIV-1 neutralization by VRC01-class antibodies. Immunity. 2013;39:245–258. doi: 10.1016/j.immuni.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boswell KL, et al. Loss of circulating CD4 T cells with B cell helper function during chronic HIV infection. PLoS Pathog. doi: 10.1371/journal.ppat.1003853. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hale JS, et al. Distinct memory CD4+ T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity. 2013;38:805–817. doi: 10.1016/j.immuni.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]