Abstract
The ZENK gene, depending upon singing activity, is transcribed within all the telencephalic nuclei controlling vocal behavior in songbirds. We show here that singing by deafened or completely isolated adult zebra finches induced high levels of ZENK transcription. This mRNA however, was not translated into high levels of ZENK protein. Instead, high levels of singing-driven ZENK protein translation were found in socially interactive birds. This dissociation between ZENK mRNA and ZENK protein was regionally specific to the robust nucleus of the arcopallium (RA), a region that is well known for its control of vocal-motor behavior in birds. Our results suggest cooperation between motor and sensory processes for regulating mRNA induction and subsequent protein synthesis in socially active songbirds.
Keywords: songbird, zebra finch, motor-driven ZENK, post-transcriptional regulation, social context
INTRODUCTION
The neural basis for vocal control in songbirds involves multiple nuclei in two circuits that serve distinctly different functions: the anterior forebrain loop and the posterior descending pathway. The anterior loop is necessary for vocal plasticity, similar to the mammalian cortical-basal ganglia-loops that facilitate motor plasticity. This loop connects Area X in the striatum, to DLM [medial portion of the dorsolateral nucleus (in the thalamus)], to MAN (magnocellular nucleus of the anterior nidopallium), back to Area X. The posterior pathway confers the ability to sing, similar to the cortical-bulbar motor pathways in mammals that control production of movement behavior. This pathway connects HVC (a proper name) in the nidopallium to RA (the robust nucleus of the arcopallium), to terminate in nXIIts (the tracheo-syringeal portion of the hypoglossal nucleus) of the brainstem. Motor neurons of nXIIts innervate the syrinx, the peripheral muscles that vibrate to produce sound. The posterior pathway projects to the anterior pathway via HVC to Area X; the anterior pathway projects to the posterior pathway via lateral MAN to RA, and medial MAN to HVC (Nottebohm et al., 1976; Bottjer et al., 1984; Simpson and Vicario, 1990; Scharff and Nottebohm, 1991; Foster et al., 1997; Brainard and Doupe, 2000a; Reiner et al., 2003; Jarvis, 2004). In the two other avian species that also learn their vocalizations, parrots and hummingbirds, analogous neural circuits for vocal-control were found using ZENK (Jarvis and Mello, 2000; Jarvis et al., 2000).
ZENK protein binds to a specific nucleotide sequence motif within other genes to increase their rate of transcription (ZENK is also known as Zif268, Egr-1, Ngfi-a, Krox-24, and TIS8; Mello et al., 1992; Milbrandt, 1987; Lim et al., 1987; Christy et al., 1988; Lemaire et al., 1988; Sukhatme et al., 1988). Vocal-control nuclei in the avian telencephalon express ZENK mRNA when birds sing (Jarvis and Nottebohm, 1997; Jin and Clayton, 1997; Jarvis et al., 1998, 2000; Jarvis and Mello, 2000). This singing-induced ZENK mRNA expression in songbirds has been shown in normal-hearing as well as deafened birds, suggesting that ZENK expression is driven by motor activity (Jarvis and Nottebohm, 1997). The specific functional consequences of singing-induced ZENK mRNA expression are currently unknown, but others have suggested that this phenomenon plays a role in the short-term maintenance of cellular homeostasis, or perhaps a role in long-term maintenance of learned vocalizations (Jarvis and Nottebohm, 1997; Jin and Clayton, 1997; Jarvis et al., 1998). Both possibilities assume that singing-induced ZENK mRNA is faithfully translated into ZENK protein.
Here, we show that translating high levels of singing-driven ZENK mRNA into ZENK protein in the vocal nucleus RA can be regulated by sensory experience. And thus we propose a novel mechanism of motor sensory dependent gene regulation.
MATERIALS AND METHODS
Animals
This study used adult male zebra finches (120–232 days of age) that were obtained from our flight aviaries and individually caged with ad libitum access to fresh SunSeed Vita-Finch seed mix (www.sunseed.com) and local tap water (http://talgov.com/citytlh/utilities/water/). The FSU Animal Care and Use Committee approved all procedures.
Deafening
Birds were surgically deafened (n = 4) or sham operated (n = 4). To deafen birds, the cochlea was removed bilaterally. Adult sham controls were anesthetized only. Briefly described, under aseptic conditions and deep anesthesia with Equithesin (0.03–0.05 mL injected intramuscularly), an incision was made in the skin covering the middle ear to reveal the tympanic membrane. The columella was then removed exposing the oval window, through which a fine wire hook was inserted to extract the basilar papillae. The inner ear cavity was plugged with sterile gelfoam and the incision sutured with veterinary adhesive. Birds were sacrificed 7 to 10 days later for measurement of singing-induced ZENK.
Auditory Context
The deafened birds described above sang in complete isolation, acoustic and visual. In contrast, sham-operated birds sang in the auditory presence of a counter-singing bird while remaining visually isolated. A group of solo context normal-hearing birds (n = 4) sang in a context like the deafened group, in complete acoustic and visual isolation. A male duo context group of normal-hearing birds (n = 4) sang in a context similar to the sham-operated group, in the auditory presence of a counter-singing bird. Birds housed in visual isolation only (sham-op and male duo context birds) were separated by a thin opaque partition; they could hear the vocalizations of a conspecific male neighbor. Birds were housed in these conditions for 3 to 5 days before sacrifice for measurement of singing-induced ZENK.
Singing Behavior
We observed all birds in the morning for a 45 min period that began with their first utterance of song (~8 AM). No females were present. During this time male birds sang spontaneously and their songs were recorded for later analysis. Immediately after 45 min of singing, birds were sacrificed and their brains prepared for assaying ZENK expression. The average number of song bouts produced by each experimental group was as follows: sham-operated, 79 (range = 70–89); deafened, 67 (range 62–76); solo context, 85 (range = 77–92); male duo context, 88 (range = 79–101). In the male duo context the neighboring birds sang an average of 81 bouts (range = 72–86). Birds in the male duo context began singing within 15 min of each other. They typically alternated spontaneous bursts of singing, overlapping their starting and ending song bouts. A spectro-temporal analysis of 10 song bouts from birds in the solo context and both birds in the male duo contexts revealed that the song behavior of all these birds was similar in terms of the following averages: number of introductory notes (range = 2–3), number of song notes (range = 4– 6), motifs per bout (range = 3–4), motif duration (range = 0.68–0.64), and amplitude (range = 67–70 dB). Most of these macrostructural features of song behavior are different when birds sing solo, versus directing singing toward a nearby female (Jarvis et al., 1998). Therefore, the song behavior of birds in either the solo or male duo context was considered undirected. However, it is possible that undirected singing in a solo versus male duo context differs in some respect at the microstructural level. We could not determine visually whether male duo context birds behaved differently when singing. All song bouts were observed by the experimenter as real-time generated spectrograms and were recorded as .WAV files using Avisoft Recorder sound-activated recording software (www.avisoft.de). Spectro-temporal analysis of recorded songs was done with Avisoft SASlab Pro. Song bouts were recorded from birds housed in sound-attenuating environmental chambers (built by the FSU Neuroscience program engineering faculty). These chambers were computer controlled to maintain a 26 ± 2°C ambient temperature, 78% relative humidity, and a 14:10 light/dark photoperiod.
ZENK In Situ Hybridization, Immunocyto-chemistry, and Quantification
After singing, birds were overdosed with 0.08 mL of Equithesin and transcardially perfused with 20 mL of diethyl pyrocarbonate-treated, 0.02 M phosphate buffered saline followed by 60 mL of 4% paraformaldehyde. The caudal telencephalon was sectioned coronally at 20 μm on a Leica VT1000S vibratome (www.leica.com) so that individual tissue sections contained both HVC and RA. Alternate serial sections were processed for ZENK mRNA by in situ hybridization and ZENK protein by immunocytochemistry, as previously reported (Whitney et al., 2000, 2003). Briefly described, to detect ZENK mRNA, in situ hybridization was performed using a [32P]-labeled cDNA probe encoding a 1.1 kb fragment of zebra finch ZENK. This probe was hybridized overnight to free-floating tissue sections at 48°C. Next, tissue sections were washed in SSC solution and mounted onto gelatin-coated slides for emulsion auto-radiography using Kodak NTB2. A Nissl stain (thionin) was used to visualize individual neurons. To detect ZENK protein, free-floating alternate tissue sections were quenched for endogenous peroxidase with 1% H2O2 before incubation in 5% normal goat serum solution. Sections were then incubated overnight in a solution containing an egr-1 antibody (C19) purchased from Santa Cruz Biotechnology (www.scbt.com). The following day, the tissue was incubated in biotinylated antirabbit antibody solution and then in avidin-biotin peroxidase reagent. Finally, primary antibody labeling was visualized with a 0.05% 3,3′-diaminobenzi-dine solution. Kits and reagents for ZENK protein detection were purchased from Vector Laboratories (www.vectorlabs.com) and Fisher Scientific (www.fishersci.com).
We quantified singing-induced ZENK expression for each bird without knowledge of their experimental group. In HVC and RA, density estimates were generated for neurons, ZENK mRNA-labeled cells, and ZENK protein-labeled cells using an established stereological technique (Johnson and Bottjer, 1994; Tramontin et al., 1998; Whitney et al., 2000, 2003). Neuron densities and ZENK mRNA-labeled cell densities were estimated from emulsion dipped, Nissl stained sections. First, a transmitted-light microscope fitted with a 100X objective and an ocular grid was used to define a 0.0002 mm3 volume high-power frame for stereological counting. This frame was randomly placed within the central portion of either HVC or RA. Two high-power frames were counted per HVC or RA in five different tissue sections from a bird (a total of 10 grids per HVC or RA). Neurons were identified by their nucleoli, and total number of neurons in HVC or RA was then divided by the volume of the 10 grids to estimate neuron density. ZENK mRNA- and protein-labeled cell densities were estimated similarly except that, for ZENK mRNA, digital images of the high power frames described above were captured for analysis with the Scion image analysis program (www.scioncorp.com). Cells were counted as ZENK mRNA-labeled based on a Poisson distribution of a nonspecific background signal. This required that silver grain labeling over individual cells exceeded a 99% confidence interval (Arnold, 1980; Dittrich et al., 1999). For the density estimates of ZENK protein-labeled cells all immunoreactive nuclei (light and dark) were counted. To determine the percentage of HVC and RA cells that expressed ZENK mRNA and protein, neuron density estimates were divided by the density estimates of ZENK protein and mRNA. These percentages were then normalized to the total number of bouts each bird sang in 45 min prior to sacrifice, because the number of song bouts is known to be closely related to the amount of singing-induced ZENK expression (Jarvis and Nottebohm, 1997). These values express induction rates for ZENK mRNA and protein.
Hearing status and auditory context group data were analyzed by one-way ANOVAs and Student-Neuman- Keuls posthoc tests using GraphPad Prism (www.graphpad.com).
RESULTS
Comparable induction of ZENK mRNA was observed in singing birds from all the experimental groups after mRNA expression levels were normalized to the number of song bouts each bird produced prior to sacrifice. For the deafened, sham-operated, solo context, and the male duo context groups, the mean percentage of cells expressing ZENK mRNA per song bout in HVC were 0.41% (range = 0.36– 0.45), 0.44% (range = 0.33–0.47), 0.46% (range = 0.30–0.49), and 0.45% (range = 0.32–0.51), respectively. Corresponding values for RA were also similar: 0.35% (range = 0.28–0.39), 0.31% (range = 0.26–0.37), 0.28% (range = 0.24–0.33), and 0.32% (range = 0.29–0.41) for deafened, sham-operated, solo context, and duo context groups, respectively. We next examined the functional significance of this singing-induced ZENK mRNA by measuring the efficiency of ZENK translation in HVC and RA. This was measured by generating “translation ratios,” defined as the percentage of cells labeled for ZENK protein per song bout divided by the percentage of cells labeled for ZENK mRNA per song bout.
In HVC, regardless of hearing status or auditory context, we observed robust antibody labeling of ZENK after singing (Fig. 2). Consequently, ZENK translation ratios for HVC were ≥ 1 and did not differ between groups (F < 1, see Fig. 4) indicating that singing-driven ZENK mRNA is reliably translated to ZENK protein in HVC regardless of sensory cues.
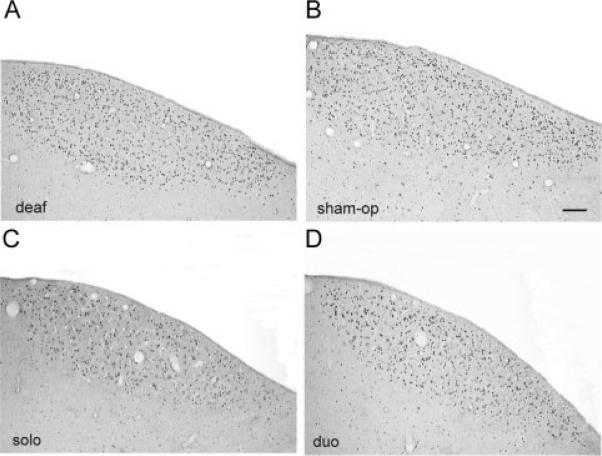
Figure 2.
In HVC singing drives ZENK protein translation, regardless of hearing status or sensory context. Whether deafened (A), sham operated (B), in social isolation (C), or in a male duo context (D), all birds showed similar levels of ZENK expression within the borders of HVC after singing; mRNA data are not shown. Tissue sections are 20 μM and coronal, medial is right. Scale bar in (B) = 100 μm.
Figure 4.
Translation of motor-driven ZENK mRNA in RA, but not HVC, depends upon sensory context. HVC showed no significant differences in the ZENK translation ratio (protein to mRNA) after deafening or manipulation of the auditory context. However, in RA the translation ratios for ZENK differed significantly among the hearing-manipulated and auditory context groups. Translation ratios were low for deafened and solo context birds compared to birds that sang and heard a singing conspecific, the sham-operated group and the male duo context group. Histogram bars are means ± SEM.
In RA, singing-induced ZENK protein varied as a function of hearing status and auditory context, with reduced antibody labeling in the deafened group [Fig. 3(A,B)] and solo context group [Fig. 3(G,H)]. This resulted in significant group differences in ZENK translation ratios for RA [F(3, 14) = 10.09; p < 0.01]. Pairwise comparisons revealed significantly lower ZENK translation ratios in RA (p < 0.05) for the deafened and solo context groups (i.e., birds in complete isolation) when compared to either the sham-operated or the male duo context groups (i.e., birds in visual isolation only; see Fig. 4). Deafened and solo context groups were not different from each other, nor were there significant differences between the sham-operated and male duo context groups.
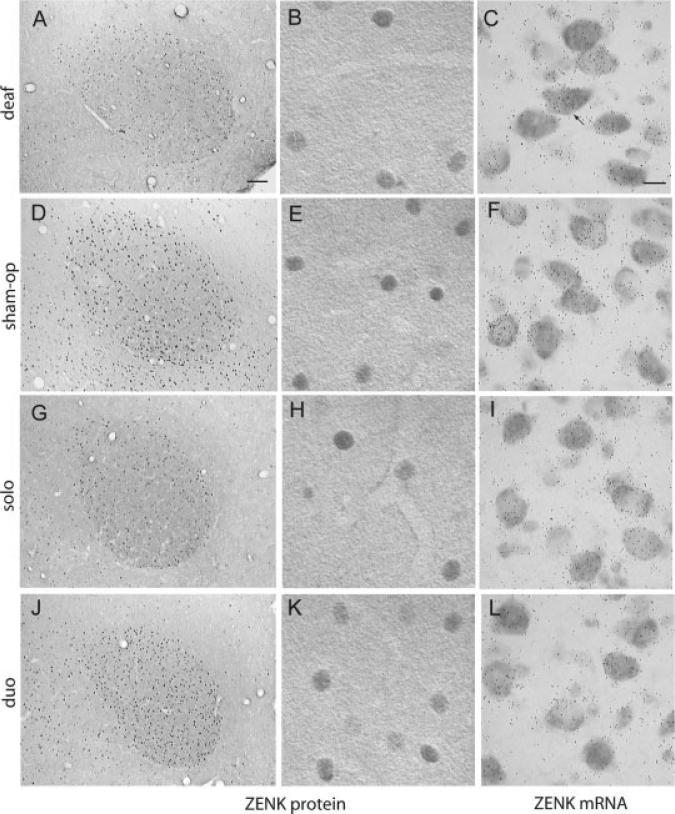
Figure 3.
In RA singing drives ZENK mRNA, while ZENK protein varies as a function of sensory context. ZENK protein-labeled cells are shown at low and high magnification in RA for birds that sang after being deafened (A,B) or sham-operated (D,E). Similar photomicrographs are shown for birds that sang in social isolation (G,H) or in the acoustic presence of a singing conspecific (J,K). For these birds a mean of 89 bouts were sung in a 45 min period. Immunoreactivity for ZENK protein in RA is low after singing in deafened and isolated birds compared to sham-operated and male duo birds [compare (A,G) to (D,J)]. The latter two groups of birds sang and heard a singing conspecific before measurement of ZENK. For comparison, ZENK mRNA-labeled cells in RA are shown in high power brightfield autoradiograph images of captured silver grains over thioninstained cells [(C,F,I,L); an example ZENK mRNA-labeled cell is denoted by the arrow in (C)]. The brightfield images show that singing induced ZENK mRNA-labeled cells regardless of hearing status or auditory context. Sections are 20 μM, and coronal, right is medial. Scale bar in (A) = 100 μm and in (C) = 10 μm.
Further analysis was conducted on the intensity of ZENK mRNA labeling in RA. That is, although the percentage of RA cells labeled per song bout did not differ between groups (see above), we asked whether cells in the deafened and solo context groups were less intensely labeled. Reduced levels of ZENK transcript might account for the observed reduction in ZENK immunoreactivity. However, after normalization to the number of song bouts produced prior to sacrifice, the fold induction of silver grains per labeled cell was similar across all groups (deafened X̄ = 0.025, SD = 0.0038; sham-operated X̄ = 0.031, SD = 0.0031; solo context X̄ = 0.027, SD = 0.0031; duo context X̄ = 0.023, SD = 0.0033).
DISCUSSION
In HVC of all birds, ZENK translation ratios were high, reflecting the expected steady translation of singing-induced ZENK mRNA. However in RA, after birds were deafened, translation ratios were low. This auditory regulation of ZENK translation in RA was investigated further by manipulating the social environment in which birds sang. Birds singing in complete isolation from other conspecifics had low translation ratios in RA. In contrast, birds that sang in visual isolation only had significantly higher translation ratios in RA.
Previous studies in songbirds by Jarvis and Nottebohm (1997) and Jarvis et al. (1998) suggest that ZENK is a motor-driven gene, as auditory feedback of song is unnecessary to drive ZENK mRNA expression in the telencephalic songbird vocal nuclei. Furthermore, these studies demonstrated that the social context in which a male zebra finch sings, that is, whether song behavior is directed toward a female versus undirected (i.e., singing alone or in the company of other males), can have a profound influence on the transcription of ZENK in key song nuclei, namely Area X, MAN, and RA. Similar to these studies, we observed that auditory input does not influence ZENK mRNA levels within telencephalic songbird vocal nuclei. However, our further analysis revealed that translation of ZENK in a vocal-motor brain region appears to be regulated by sensory experience.
Additionally, our data distinguish a context-dependent difference in the brain of male birds singing alone versus singing in an acoustically interactive environment. This context-dependent regulation of ZENK is distinct from that observed during female-directed singing where ZENK mRNA levels are reduced in several vocal-control nuclei (e.g., RA, lateral MAN, lateral Area X; Jarvis et al., 1998). In contrast, the male only context-dependent regulation of ZENK that we observed involved protein level changes but without change at the mRNA level, and this occurred only in RA (i.e., robust ZENK protein was found in the anterior song nuclei Area X and lateral MAN; data not shown). As such, ZENK is one of the few genes to date shown to undergo such post-transcriptional regulation during the generation of behavior (Whitney et al., 2000; Quattrone et al., 2001; Pascale et al., 2004).
Low ZENK translation ratios in the RA of deafened birds initially suggested that translation of singing-driven ZENK mRNA depends on sensory feedback of a birds’ own song. But we also found low ZENK translation ratios in the RA of normal hearing solo birds. The low ZENK translation ratios in the RA of these two “sensory-deprived” adult groups resembled those we found previously in fledgling early vocal learners that were normal hearing, and that sang in a socially interactive environment (Whitney et al., 2000). A similar mechanism could inhibit translation of ZENK in the RA of these birds, but we cannot make this determination from the present data. Further developmental and comparative study is needed, which could also be useful for determining a precise role for ZENK induction in songbird vocal learning. Nonetheless, the present results demonstrate that ZENK translation ratios in the RA of adults were always high in birds that were normal hearing and sang in socially interactive settings. These data suggest that in adult birds transcription and translation of ZENK in RA are regulated cooperatively by self-generated vocal-motor behavior and sensory experience.
We have used the term translation ratio to describe our findings, suggesting that the low ZENK protein antibody labeling in RA of solo and deafened birds is the result of negative translational control. Negative translational control of ZENK would require repressor proteins (Dever, 2002; Klann et al., 2004; Weiler et al., 2004) that, once activated, bind to a sequence-specific site within the 5′ end of ZENK mRNAs. Other recent evidence suggests that endogenous microRNAs can function similarly for post-transcriptional repression of gene expression (Rogelj and Giese, 2004; Gebauer and Hentze, 2004; Bartel and Chen, 2004). However, we cannot rule out the possibility that our observed discrepancies between mRNA and protein levels of ZENK in RA were due to increased protein degradation. Rapid protein degradation of ZENK in RA could occur via the polypep-tide ubiquitin, as activity has been shown regulate protein degradation via the ubiquitin-proteosome pathway (Ehlers, 2003). Moreover, these two mechanisms for post-transcriptional gene control are not mutually exclusive.
The cellular mechanisms aforementioned could all rapidly and reversibly adjust ZENK protein levels in RA, but to what synaptic mechanism might they be linked? Catecholamine release within RA is one possibility, however the levels of catecholamines as well as the rate-limiting enzyme for their synthesis are expressed in all of the telencephalic song nuclei and this expression is not particularly robust in RA (Soha et al., 1996). Another mechanism for synaptic regulation of ZENK protein in RA could involve the differential glutamate receptor-mediated synaptic activity from HVC and MAN. Individual neurons in RA receive synapses from both HVC and lateral MAN (Mooney and Konishi, 1991; Stark and Perkel, 1999). But different ionotropic glutamate receptors mediate HVC and lateral MAN input to RA: HVC activates both NMDA and non-NMDA receptors, while lateral MAN activates NMDA receptors almost exclusively (Mooney and Konishi, 1991; Stark and Perkel, 1999; Wada et al., 2004). Thus, singing-driven depolarization at HVC synapses within RA could mediate ZENK transcription, whereas activity at NMDA synapses from lateral MAN could activate Ca++-dependent second-messenger systems involved in ZENK translation. In this framework, sensory experience would influence lateral MAN activity, but there are presently no data that address this possibility. There is evidence to suggest that lateral MAN neurons generate an error signal when the birds’ auditory input does not match vocal output (Brainard and Doupe, 2000a,b; but cf. Leonardo, 2004), which would certainly be the case when singing in a noisy environment.
The critical social/sensory variable for regulating ZENK protein translation in RA could be increased general arousal, hearing other conspecifics’ general vocalizations (calls and peeps), hearing other conspecifics’ songs, or some other aspect of social experience (perhaps olfaction) that was not precluded by the opaque partition that separated the duo context birds. Previous research on male-male vocal interactions in zebra finches suggests that general arousal is not a factor, because male zebra finches do not engage in counter-singing for territorial defense, nor do they alter song amplitude when in acoustic contact with each other (Cynx and Gell, 2004; Brumm and Todt, 2004). Also, playbacks of song were sufficient to induce a robust ZENK protein response in RA of singing birds (Mello and Ribiero, 1998), suggesting that specifically hearing song is a noteworthy cue for regulating ZENK translation. Still, the importance of other social variables remains to be determined.
The functional consequence of cooperative motor-sensory regulation of ZENK expression in RA was not tested in this study, but studies in rodents on a homologue of ZENK (zif-268) report that its induction is necessary for memory consolidation and memory reconsolidation. ZENK is required for long-term synaptic plasticity (LTP) and spatial memory formation (Jones et al., 2001); disruption of ZENK during recall of fear memory resulted in amnesia during later recall of that memory (Lee et al., 2004). If ZENK expression serves a similar function—to consolidate the song memory or reconsolidate the song memory after it has been recalled for the purpose of singing— then our data would suggest that more ZENK in RA is required in social environments, perhaps due to the auditory interference associated with hearing the songs of other competing vocalizing males.
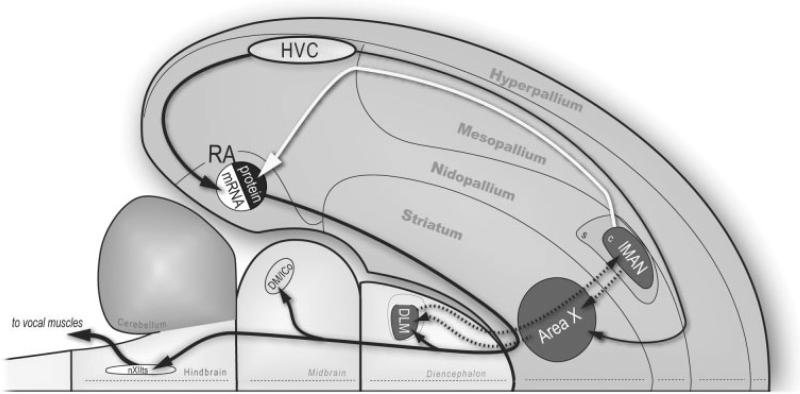
Figure 1.
Schematic view of zebra finch CNS showing the relative locations of songbird vocal nuclei and their axonal connections. Two nuclei in the caudal telencephalon (HVC and RA) are important during song learning and adult vocal behavior, whereas song regions in the anterior fore-brain (lMAN, DLM, Area X, the pallial-basal ganglia-thalamic loop) play an important role in vocal plasticity but are not necessary for maintenance of stereotyped adult vocal behavior. Here we propose that HVC input to RA drives ZENK mRNA and lMAN input modulates translation of ZENK protein (see text for additional details). Avian brain regions are referred to by their modern nomenclature (Reiner et al., 2004). Abbreviations: Area X, Area X of subpallium; DLM, medial portion of the dorsolateral nucleus of the thalamus; DM/ICo, dorsomedial nucleus of the intercollicular complex; HVC, high vocal center; lMAN, lateral magnocellular nucleus of the anterior nidopallium; RA, robust nucleus of the arcopallium; nXIIts, hypoglossal nucleus, tracheosyringeal portion.
Acknowledgments
We are grateful to Dr. ED. Jarvis who provided very insightful comments during preparation of this manuscript. Ross Henderson and Pam Maras provided experimental and technical assistance. O.W. is supported by an Individual NRSA through the American Psychological Association Diversity in Neuroscience Program.
Contract grant sponsor: American Psychological Association Diversity in Neuroscience Program (O.W.).
Contract grant sponsor: NIH; contract grant number: DC02035 (F.J.).
REFERENCES
- Arnold AP. Quantitative analysis of sex differences in hormone accumulation in the zebra finch brain: methodological and theoretical issues. J Comp Neurol. 1980;189:421–426. doi: 10.1002/cne.901890302. [DOI] [PubMed] [Google Scholar]
- [2005 Apr 2];Avisoft Bioacoustics [Internet] Germany: Avisoft Bio-acoustics. 2005 Available from: http://www.avisoft.de./
- Bartel DP, Chen CZ. Micromanagers of gene expression: the potentially widespread influence of metazoan microRNAs. Nat Rev Genet. 2004;5:396–400. doi: 10.1038/nrg1328. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984;224:901–903. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations. Nature. 2000a;404:762–766. doi: 10.1038/35008083. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Auditory feedback in learning and maintenance of vocal behaviour. Nat Rev Neurosci. 2000b;1:31–40. doi: 10.1038/35036205. [DOI] [PubMed] [Google Scholar]
- Brumm H, Todt D. Male-male vocal interactions and the adjustment of song amplitude in a territorial bird. Animal Behav. 2004;67:281–286. [Google Scholar]
- Christy BA, Lau LF, Nathans D. A gene activated in mouse 3T3 cells by serum growth factors encodes a protein with ‘zinc finger’ sequences. Proc Natl Acad Sci USA. 1988;85:7857–7861. doi: 10.1073/pnas.85.21.7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynx J, Gell C. Social mediation of vocal amplitude in a songbird, Taeniopygia guttata. Animal Behav. 2004;67:451–455. [Google Scholar]
- Dever TE. Gene-Specific Regulation by General Translation Factors. Cell. 2002;108:545–556. doi: 10.1016/s0092-8674(02)00642-6. [DOI] [PubMed] [Google Scholar]
- Dittrich F, Feng Y, Metzdorf R, Gahr M. Estrogeninducible, sex-specific expression of brain-derived neurotrophic factor mRNA in a forebrain song control nucleus of the juvenile zebra finch. Proc Natl Acad Sci USA. 1999;96:8241–8246. doi: 10.1073/pnas.96.14.8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers M. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci. 2003;6:231–242. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- Fischer Scientific [Internet] New Hampshire. Fischer Scientific International; 2005. [2005 Apr 2]. Available from: http://www.fischersci.com/ [Google Scholar]
- Foster EF, Mehta RP, Bottjer SW. Axonal connections of the medial magnocellular nucleus of the anterior neostriatum in zebra finches. J Comp Neurol. 1997;382:364–381. doi: 10.1002/cne.903820305. [DOI] [PubMed] [Google Scholar]
- Gebauer F, Hentze MW. Molecular mechanisms of translational control. Nat Rev Mol Cell Biol. 2004;10:827–835. doi: 10.1038/nrm1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GraphPad Software [Internet] Callifornia. GraphPad Software; 2005. [2005 Apr 2]. Available from http://www.graphpad.com/ [Google Scholar]
- Jarvis ED. Learned birdsong and the neurobiology of human language. Ann NY Acad Sci. 2004;1016:746–777. doi: 10.1196/annals.1298.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED, Mello CV. Molecular mapping of brain areas involved in parrot vocal communication. J Comp Neurol. 2000;419:1–31. doi: 10.1002/(sici)1096-9861(20000327)419:1<1::aid-cne1>3.0.co;2-m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED, Nottebohm F. Motor-driven gene expression. Proc Natl Acad Sci USA. 1997;94:4097–4102. doi: 10.1073/pnas.94.8.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED, Ribeiro S, da Silva ML, Ventura D, Vielliard J, Mello CV. Behaviourally driven gene expression reveals song nuclei in hummingbird brain. Nature. 2000;6796:628–632. doi: 10.1038/35020570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED, Scharff C, Grossman MR, Ramos JA, Nottebohm F. For whom the bird sings: context-dependent gene expression. Neuron. 1998;21:775–788. doi: 10.1016/s0896-6273(00)80594-2. [DOI] [PubMed] [Google Scholar]
- Jin H, Clayton DF. Localized changes in immediate early gene regulation during sensory and motor learning in zebra finches. Neuron. 1997;19:1049–1059. doi: 10.1016/s0896-6273(00)80396-7. [DOI] [PubMed] [Google Scholar]
- Johnson F, Bottjer SW. Afferent influences on cell death and birth during development of a cortical nucleus necessary for learned vocal behavior in zebra finches. Development. 1994;120:13–24. doi: 10.1242/dev.120.1.13. [DOI] [PubMed] [Google Scholar]
- Jones MW, Errington ML, French PJ, Fine A, Bliss TV, Garel S, Charnay P, Bozon B, Laroche S, Davis S. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat Neurosci. 2001;4:289–296. doi: 10.1038/85138. [DOI] [PubMed] [Google Scholar]
- Klann E, Antion MD, Banko JL, Hou L. Synaptic plasticity and translation initiation. Learn Mem. 2004;4:365–372. doi: 10.1101/lm.79004. [DOI] [PubMed] [Google Scholar]
- Lee J, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304:839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- Leica [Internet] Leica Microsystems; Germany: 2005. [2005 Apr 2]. Available from: http://www.leica.com/ [Google Scholar]
- Lemaire P, Revelant O, Bravo R, Charnay P. Two mouse genes encoding potential transcription factors with identical DNA-binding domains are activated by growth factors in cultured cells. Proc Natl Acad Sci USA. 1988;85:4691–4695. doi: 10.1073/pnas.85.13.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardo A. Experimental test of the birdsong error-correction model. Proc Natl Acad Sci USA. 2004;101:16935–16940. doi: 10.1073/pnas.0407870101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim RW, Varnum BC, Herschman HR. Cloning of tetradecanoyl phorbol ester-induced ‘primary response’ sequences and their expression in density-arrested Swiss 3T3 cells and a TPA non-proliferative variant. Oncogene. 1987;1:263–270. [PubMed] [Google Scholar]
- Mello CV, Ribeiro S. ZENK protein regulation by song in the brain of songbirds. J Comp Neurol. 1998;393:426–438. doi: 10.1002/(sici)1096-9861(19980420)393:4<426::aid-cne3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proc Natl Acad Sci USA. 1992;89:6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrandt J. A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science. 1987;238:797–799. doi: 10.1126/science.3672127. [DOI] [PubMed] [Google Scholar]
- Mooney R, Konishi M. Two distinct inputs to an avian song nucleus activate different glutamate receptor subtypes on individual neurons. Proc Natl Acad Sci USA. 1991;88:4075–4079. doi: 10.1073/pnas.88.10.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Pascale A, Gusev PA, Amadio M, Dottorini T, Govoni S, Alkon DL, Quattrone A. Increase of the RNA-binding protein HuD and posttranscriptional up-regulation of the GAP-43 gene during spatial memory. Proc Natl Acad Sci USA. 2004;101:1217–1222. doi: 10.1073/pnas.0307674100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrone A, Pascale A, Nogues X, Zhao W, Gusev P, Pacini A, Alkon DL. Posttranscriptional regulation of gene expression in learning by the neuronal ELAV-like mRNA-stabilizing proteins. Proc Natl Acad Sci USA. 2001;98:11668–11673. doi: 10.1073/pnas.191388398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, et al. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogelj B, Giese KP. Expression and function of brain specific small RNAs. Rev Neurosci. 2004;3:185–198. doi: 10.1515/revneuro.2004.15.3.185. [DOI] [PubMed] [Google Scholar]
- Santa Cruz Biotechnology [Internet] Santa Cruz Biotechnology; California: 2005. [2005 Apr 2]. Available from: http://www.scbl.com/ [Google Scholar]
- Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finchsong system: implication for vocal learning. J Neurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scion Corporation [Internet] Scion Corporation; Maryland: 2005. [2005 Apr 2]. Available from: http://www.scioncorp.com/ [Google Scholar]
- Simpson HB, Vicario DS. Brain pathways for learned and unlearned vocalizations differ in zebra finches. J Neurosci. 1990;10:1541–1556. doi: 10.1523/JNEUROSCI.10-05-01541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soha JA, Shimizu T, Doupe AJ. Development of the catecholaminergic innervation of the song system of the male zebra finch. J Neurobiol. 1996;29:473–489. doi: 10.1002/(SICI)1097-4695(199604)29:4<473::AID-NEU5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Stark LL, Perkel DJ. Two-stage, input-specific synaptic maturation in a nucleus essential for vocal production in the zebra finch. J Neurosci. 1999;19:9107–9116. doi: 10.1523/JNEUROSCI.19-20-09107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhatme VP, Cao XM, Chang LC, Tsai-Morris CH, Ferreira PC, Cohen DR, Edwards SA, Curran T, LeBeau MM, Adamson ED. A zinc finger-gene coregulated with c-fos during growth and differentiation, and after cellular depolarization. Cell. 1988;53:37–43. doi: 10.1016/0092-8674(88)90485-0. [DOI] [PubMed] [Google Scholar]
- Sun Seed [Internet] Sun Seed Company; Ohio: 2005. [2005 Apr 2]. Available from: http://www.sunseed.com/ [Google Scholar]
- Tramontin AD, Smith GT, Breuner CW, Brenowitz EA. Seasonal plasticity and sexual dimorphism in the avian song control system: stereological measurement of neuron density and number. J Comp Neurol. 1998;396:186–192. doi: 10.1002/(sici)1096-9861(19980629)396:2<186::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Vector Laboratories [Internet] Vector Laboratories; California: 2005. [2005 Apr 2]. Available from: http:// www.vectorlabs.com/ [Google Scholar]
- Wada K, Sakaguchi H, Jarvis ED, Hagiwara M. Differential expression of glutamate receptors in avian neural pathways for learned vocalization. J Comp Neurol. 2004;476:44–64. doi: 10.1002/cne.20201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler IJ, Spangler CC, Klintsova AY, Grossman AW, Kim SH, Bertaina-Anglade V, Khaliq H, de Vries FE, Lambers FAE, Hatia F, et al. WT Fragile X mental retardation protein is necessary for neurotransmitter-activated protein translation at synapses. Proc Natl Acad Sci USA. 2004;101:17504–17509. doi: 10.1073/pnas.0407533101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney O, Soderstrom K, Johnson F. Post-transcriptional regulation of ZENK expression associated with zebra finch vocal development. Mol Brain Res. 2000;80:279–290. doi: 10.1016/s0169-328x(00)00178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney O, Soderstrom K, Johnson F. CB1 cannabinoid receptor activation inhibits a neural correlate of song recognition in an auditory/perceptual region of the zebra finch telencephalon. J Neurobiol. 2003;56:266–274. doi: 10.1002/neu.10233. [DOI] [PMC free article] [PubMed] [Google Scholar]