(See the HIV/AIDS Major Article by Rokx et al on pages 143–53.)
Over the last decade, World Health Organization (WHO) guidelines for human immunodeficiency virus (HIV)/AIDS treatment and care have evolved toward simplifying recommendations for first-line antiretroviral therapy (ART) to promote a public health approach to treatment scale-up [1]. Whereas in 2002, WHO recommended 5 different treatment options for first-line ART, the latest guidelines released in 2013 recommend a single first-line regimen comprising tenofovir, efavirenz, and either lamivudine or emtricitabine, preferably as fixed-dose combinations [2]. These last 2 nucleoside analogues were recommended as interchangeable based on the results of a systematic review and meta-analysis of randomized trials showing no difference in rates of virological suppression, virological failure, or the development of resistance mutations [3]. Lamivudine is available from multiple generic sources and is cheaper than emtricitabine [4].
In this issue of Clinical Infectious Diseases, an analysis from Rokx et al and the AIDS Therapy Evaluation in the Netherlands nationwide HIV cohort (ATHENA) HIV treatment cohort in the Netherlands suggests better virological responses to emtricitabine compared with lamivudine as part of first-line ART. The authors of this nonrandomized cohort study conclude that using lamivudine instead of emtricitabine may result in additional morbidity and costs associated with virological failure and the development of drug resistance [5].
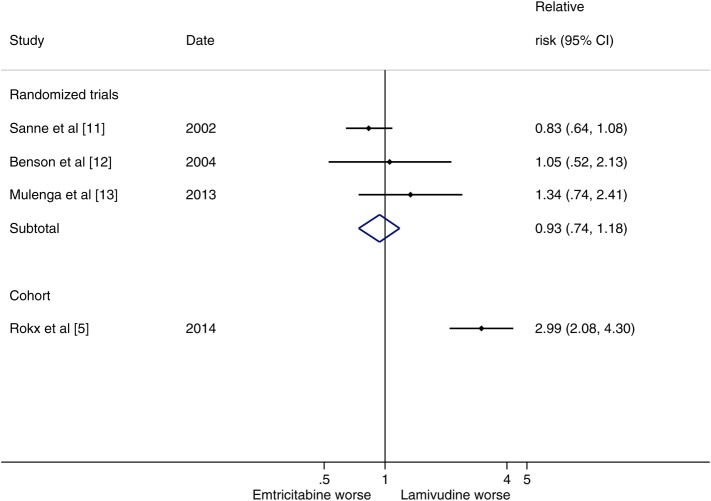
To date, 3 randomized clinical trials (n = 1242) have directly compared lamivudine and emtricitabine; the pooled results of these trials found no difference in terms of virological suppression (relative risk [RR], 1.03; 95% confidence interval [CI], .96–1.10) or virological failure (RR, 0.93; 95% CI, .74–1.18). In comparison, the risk of virological failure in the ATHENA cohort was three times higher for patients receiving lamivudine compared with efavirenz (RR, 2.99; 95% CI, 2.08–4.30).
How should clinical guidelines respond to this seemingly contradictory evidence from 3 randomized trials vs a nonrandomized cohort study? The development of clinical guidelines by WHO and an increasing number of national guidelines follows the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach, which rates the quality of evidence supporting a recommendation according to 5 criteria: risk of bias, imprecision, inconsistency, indirectness, and publication bias [6]. Randomized trials are generally rated as high-quality evidence but can be downgraded if key methodological issues arise. According to the GRADE approach, observational studies are usually rated as low-quality evidence but can be upgraded based on the quality of evidence under certain exceptional conditions [7]. Adequately powered randomized clinical trials are also required for the marketing approval of new antiretrovirals by the US Food and Drug Administration and other regulatory authorities; cohort studies in which different treatments are compared are not acceptable for such approvals [8].
Observational studies have made invaluable contributions in the field of HIV/AIDS, providing evidence of the feasibility of delivering ART at scale, giving insights into innovations in service delivery that could help efforts to support further ART scale-up, and generating datasets on adverse drug reactions that may not be apparent in trial settings. However, randomized trials remain the gold standard for assessing comparative drug efficacy for the simple reason that cohort studies can never ensure that observed differences in treatment efficacy are not in fact due to measured or unmeasured differences between patient populations.
The ATHENA cohort study provided an adjusted analysis using propensity scoring. Although such analyses are preferable to unadjusted analyses, selection bias will remain if imbalances in unobserved confounding variables exist. The comparison groups in the ATHENA cohort study by Rokx et al are greatly unbalanced both geographically, between Western and sub-Saharan settings, and temporally, with the median ART initiation year at 2004 for lamivudine and 2009 for emtricitabine. Patients receiving lamivudine also had a higher baseline viral load and lower baseline CD4 cell counts, were more likely to be injection drug users (which could influence adherence), coinfected with hepatitis B virus, and managed within a large treatment programme (>2000 patients). These differences could be associated with important differences such as culture and clinical practice that can never be fully corrected for statistically through methods like propensity scores [9].
Thus, in light of these methodological limitations and the large discrepancy between the results reported by Rokx et al and those provided by prospective randomized controlled trials (Figure 1), it is reasonable to believe that the observed treatment differences are the result of study design rather than actual differences in efficacy between lamivudine and emtricitabine. Although many cohort studies and randomized trials result in similar effect estimates, it is not possible to know whether a cohort study is robustly valid until a randomized trial is conducted [10].
Figure 1.
Relative risk of virological failure at 48 weeks comparing lamivudine and emtricitabine. Abbreviation: CI, confidence interval.
The authors of the ATHENA cohort suggest that additional randomized trials are needed to evaluate the comparative efficacy of lamivudine and emtricitabine. We agree that such evidence would be valuable; if future trials find important differences between these 2 drugs, then this evidence should be interpreted in light of the 3 completed randomized trials, and may have implications for future guidelines. In the meantime, on the basis of the currently available randomized evidence, we conclude that lamivudine and emtricitabine can be considered to be interchangeable.
Note
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Vitoria M, Vella S, Ford N. Scaling up antiretroviral therapy in resource-limited settings: adapting guidance to meet the challenges. Curr Opin HIV AIDS. 2013;8:12–8. doi: 10.1097/COH.0b013e32835b8123. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Geneva, Switzerland: WHO; 2013. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. [PubMed] [Google Scholar]
- 3.Ford N, Shubber Z, Hill A, et al. Comparative efficacy of lamivudine and emtricitabine: a systematic review and meta-analysis of randomized trials. PLoS One. 2013;8:e79981. doi: 10.1371/journal.pone.0079981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Médecins Sans Frontières. 16th ed. Geneva, Switzerland: MSF; 2013. Untangling the web of antiretroviral price reductions. [Google Scholar]
- 5.Rokx C, Fibriani A, van de Vijver DAMC, et al. ATHENA National Observational Cohort. Increased virological failure in naive HIV-1–infected patients taking lamivudine compared with emtricitabine in combination with tenofovir and efavirenz or nevirapine in the Dutch nationwide ATHENA cohort. Clin Infect Dis. 2015;60:143–53. doi: 10.1093/cid/ciu763. [DOI] [PubMed] [Google Scholar]
- 6.Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–6. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Guyatt GH, Oxman AD, Sultan S, et al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011;64:1311–6. doi: 10.1016/j.jclinepi.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Hill A, Sabin C. Designing and interpreting HIV noninferiority trials in naive and experienced patients. AIDS. 2008;228:913–21. doi: 10.1097/QAD.0b013e3282f5556d. [DOI] [PubMed] [Google Scholar]
- 9.Austin PC, Mamdani MM. A comparison of propensity score methods: a case-study estimating the effectiveness of post-AMI statin use. Stat Med. 2006;25:2084–106. doi: 10.1002/sim.2328. [DOI] [PubMed] [Google Scholar]
- 10.Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342:1887–92. doi: 10.1056/NEJM200006223422507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanne I, van der Horst C, Shaw A, et al. Two randomized, controlled, equivalence trials of emtricitabine (FTC) to lamivudine (3TC) [abstract 4432] XIV International AIDS Conference, Barcelona, 7–12 July, 2002. [Google Scholar]
- 12.Benson CA, van der Horst C, Lamarca A, et al. A randomized study of emtricitabine and lamivudine in stably suppressed patients with HIV/AIDS. 2004;18:2269–76. doi: 10.1097/00002030-200411190-00007. [DOI] [PubMed] [Google Scholar]
- 13.Mulenga L, Muwango A, Moyo C, et al. Efficacy of tenofovir disoproxil fumarate/emtricitabine and tenofovir disoproxil both in combination with efavirenz in antiretroviral-naïve, HIV-1-infected Zambians [abstract TULBPE18]. In: 7th IAS Conference on HIV Pathogenesis, Treatment and Prevention. Kuala Lumpur, 30 June–3 July 2013, Malaysia. [Google Scholar]