Abstract
Background. Protease inhibitor (PI)–based combination antiretroviral therapy (cART) is administered during pregnancy to prevent perinatal human immunodeficiency virus (HIV) transmission. However, PI use has been associated with adverse birth outcomes, including preterm delivery and small-for-gestational-age (SGA) births. The mechanisms underlying these outcomes are unknown. We hypothesized that PIs contribute to these adverse events by altering progesterone levels.
Methods. PI effects on trophoblast progesterone production were assessed in vitro. A mouse pregnancy model was used to assess the impact of PI-based cART on pregnancy outcomes and progesterone levels in vivo. Progesterone levels were assessed in plasma specimens from 27 HIV-infected and 17 HIV-uninfected pregnant women.
Results. PIs (ritonavir, lopinavir, and atazanavir) but not nucleoside reverse transcriptase inhibitors (NRTIs) or nonnucleoside reverse transcriptase inhibitors reduced trophoblast progesterone production in vitro. In pregnant mice, PI-based cART but not dual-NRTI therapy was associated with significantly lower progesterone levels that directly correlated with fetal weight. Progesterone supplementation resulted in a significant improvement in fetal weight. We observed lower progesterone levels and smaller infants in HIV-infected women receiving PI-based cART, compared with the control group. In HIV-infected women, progesterone levels correlated significantly with birth weight percentile.
Conclusions. Our data suggest that PI use in pregnancy may lead to lower progesterone levels that could contribute to adverse birth outcomes.
Keywords: progesterone, protease inhibitors, lopinavir, small for gestational age, low birth weight, HIV, pregnancy
(See the editorial commentary by Powis and Shapiro on pages 4–7.)
Combination antiretroviral therapy (cART) is recommended for all human immunodeficiency virus (HIV)–infected pregnant women for prevention of perinatal HIV transmission and maintenance of maternal health. Although the benefits of cART far outweigh the potential adverse effects, the rapidly increasing and earlier use of cART makes it urgently important that we improve our understanding of the safety of antiretrovirals in pregnancy. cART has been associated with several adverse pregnancy outcomes, including preeclampsia, preterm delivery (PTD), low birth weight, and small-for-gestational-age (SGA) birth, conditions that increase the risk morbidity and mortality in newborns, especially in low-resource settings [1–8]. Protease inhibitor (PI)–based cART in particular has been associated with significantly higher PTD rates [6]. Although controversy exists [9–11], most studies demonstrate a higher number of adverse birth outcomes in this population, underscoring the importance of understanding the underlying mechanisms.
Mechanistic data addressing antiretroviral toxicity during pregnancy is limited. These include nucleoside reverse transcriptase inhibitor (NRTI)–associated mitochondrial toxicity [8, 12], immune reconstitution, and a shift from T-helper type 2 (Th2) cytokines to Th1 cytokines in cART-treated HIV-infected women [4, 13]. However, direct data linking these mechanisms to clinical outcomes are limited or lacking, and additional mechanisms are likely to contribute to the adverse pregnancy outcomes experienced by HIV-infected women.
Progesterone is a sex steroid hormone that is essential for the maintenance of pregnancy. Low levels of progesterone have been associated with increased incidence of pregnancy loss, placental abnormalities, prematurity, fetal distress, and fetal growth restriction [14, 15], while progesterone supplementation has shown some efficacy in preventing PTD in high-risk patients [16–19]. Progesterone is synthesized from maternal cholesterol, and its synthesis, metabolism, and clearance depend on a variety of enzymes, many belonging to the cytochrome (CYP) family. PIs, especially ritonavir (RTV), affect the expression and activity of CYP enzymes [20], and it has been speculated that this is a mechanism through which PIs could alter sex steroid hormone levels [21, 22]. Elevated 17-hydroxyprogesterone and dehydroepiandrosterone-sulfate levels suggestive of adrenal dysfunction have been reported in neonates exposed perinatally and postnatally to RTV-boosted lopinavir [23]. Case reports of adrenal suppression requiring glucocorticoid replacement therapy with RTV-boosted PI use in conjunction with synthetic steroids have been described [24]. However, only limited data exist on the impact of antiretrovirals on progesterone, and these come primarily from studies investigating the interaction between antiretrovirals and contraceptives [21, 22].
The purpose of this study was to investigate the effect of antiretrovirals on progesterone levels in the context of pregnancy and to determine whether antiretroviral-induced alterations in progesterone levels influence pregnancy outcome. Deciphering the mechanisms by which antiretrovirals affect pregnancy outcome would facilitate the rational selection of optimal therapy during pregnancy and could inform the design of diagnostic tools and interventions to identify and treat those at high risk of adverse outcomes.
METHODS
Reagents
Zidovudine (ZDV), lamivudine (3TC), nevirapine (NVP), lopinavir (LPV), atazanavir (ATV), and RTV (National Institutes of Health AIDS Reagent Program) were dissolved in dimethyl sulfoxide in 100 mM stocks, and stored at −20°C until use. The concentration of dimethyl sulfoxide in the final medium was <0.1%. Combivir (ZDV + 3TC) and Kaletra (LPV + RTV) were purchased as prescription drugs. Progesterone and injectable corn oil were obtained from Sigma Aldrich.
In Vitro Assays
BeWo cells (ATCC CCL98) were seeded into 12-well plates (50 000 cells/mL) in Kaighn's modification of Ham's F-12 medium (ATCC catalogue no. 30-2004) and cultured at 37°C in a 5% CO2 incubator for 24 hours. Syncytialization was induced by exposure to 100 µM forskolin for 72 hours, followed by exposure to antiretrovirals at 10 times the minimum effective concentration (MEC), either singly or in combinations used in pregnancy, for 24 hours (drug concentrations are specified in the Supplementary Materials). Cells incubated under hypoxic conditions (1% O2 and 99% N2) served as a positive control. No toxicity was observed under these conditions. Cell supernatants were collected and stored at −80°C until testing. To control for differences in cell count at the end of the experiment, AlamarBlue (Life Technologies) was used according to the manufacturer's instructions to determine the number of live cells. Experiments were performed in triplicate and repeated 3–6 times.
Animal Pregnancy Model
Animal experiments were approved by the University Health Network Animal Use Committee and performed according to the policies and guidelines of the Canadian Council on Animal Care. C57BL/6 mice (Jackson Laboratory) were housed under a 12-hour light/dark cycle, with free access to food and water. Animals were acclimated for 1 week prior to experiment initiation. Males were isolated for 7 days prior to mating. Females were trained on gavage (using water) for 7 days prior to mating, to minimize potential stress during pregnancy. Female virgin mice aged 8–10 weeks were mated with males at a 2 to 1 ratio. Mating was monitored by the presence of vaginal plugs. Females with plugs were considered pregnant, with the day on which the plug was detected referred to as gestational day 1 (GD1), and were housed together (4 dams/cage). Pregnancy was confirmed by weight gain on GD9 (>1.5 g) and GD13 (>3 g).
Drug doses that would yield clinically relevant plasma drug levels were determined in pilot studies. Mice were administered escalating drug doses starting on GD11 for 4 days to allow drugs to reach steady-state levels. On GD15, blood was collected 1 hour and 24 hours after drug administration. Drug levels were assessed by liquid chromatography–tandem mass spectrometry as previously described [25]. Drug doses resulting in values approximating the MEC at 1 hour and in detectable levels at 24 hours were selected for all following experiments. As NRTI plasma concentrations do not appear to correlate well with efficacy or toxicity [26], we instead selected a dose of ZDV/3TC (Combivir) that accounted for the faster metabolic rate of mice, compared with humans, and was not associated with maternal toxicity.
Animals were exposed to 100/50 mg/kg/day of ZDV/3TC (Combivir) alone, ZDV/3TC plus 33/8.3 mg/kg/day LPV/RTV (Kaletra), or water as a control. Pulverized tablets were dissolved in sterile water and administered by oral gavage (100 µL/mouse).
Animals were euthanized at GD15 by CO2 inhalation. Blood (heparinized) was collected by cardiac puncture. The following parameters were recorded: number and location of fetuses and resorptions (residues from fetal demise), fetal and placenta weights, fetal viability (assessed by pedal reflex), and fetal malformations. Experiments were repeated 2–3 times.
In progesterone supplementation experiments, 0.5 mg of progesterone in 100 µL of corn oil was administered subcutaneously on GD1, GD5, GD9, and GD13. Control animals received 100 µL of corn oil.
Patient Samples
Plasma samples were obtained from women who consented to participate in a biobank program supporting research relevant to HIV infection during pregnancy. The institutional review boards of University Health Network, St. Michael's Hospital, Mount Sinai Hospital, and Women's College Hospital (Toronto, Canada) approved the study. All participants gave written informed consent.
Inclusion criteria for the biobank were: >18 years age at recruitment, ability to give informed consent, confirmed singleton pregnancy, and confirmed HIV infection (the HIV-infected group) or confirmed HIV-negative status (the control group). Exclusion criteria were multiple pregnancies, preexisting hypertension (defined as a blood pressure of >140/90 mm Hg), diabetes (type I and II), renal disease, autoimmune diseases, collagen vascular disease, documented opportunistic infection (in the HIV-infected group), and current illicit-drug use. None of the women were current tobacco smokers. Matching between HIV-infected and HIV-uninfected women was performed on the basis of race, age (±5 years), parity (0, 1, or >1), and body mass index (defined as the weight in kilograms divided by the height in meters squared; <25 or >25). Recruitment occurred in Toronto at St. Michael's Hospital, Mount Sinai Hospital, Toronto General Hospital Immunodeficiency Clinic, and Maple Leaf Medical Clinic.
Participants included in this study were recruited between September 2010 and May 2013. We included all women from whom a blood sample was collected between gestational weeks 25 and 28. This period gave us the largest set of available paired samples from an HIV-infected women and a matched control. Selection was performed without prior knowledge of the birth outcome. Twenty-seven HIV-infected women met these criteria. An identified match was not available for the entire HIV-infected cohort but was available for 17 of the 27 HIV-infected women.
All HIV-infected women received cART. Twenty-one women began therapy prior to becoming pregnant, while 6 began therapy during pregnancy (therapy initiation occurred between gestational weeks 8 and 23). Selection of cART regimen was at the discretion of their physician.
Gestational age was determined on the basis of maternal reporting of the last menstrual period and was confirmed by ultrasonography. PTD was defined as spontaneous delivery before 37 weeks of gestation. Birth weight percentiles were calculated after accounting for gestational age, infant sex, and mother's world region of birth, using the birth weight percentile curves developed by Ray et al [27]. These curves were used to avoid biasing our calculations toward a lower percentile, because a large proportion of our participants were born in Africa or the Caribbean, and women from these regions tend to deliver infants with lower birth weights than Canadian or European standards [27].
Blood samples (heparinized) were processed within 1 hour of collection and spun at 1000 ×g for 15 minutes. Plasma was collected, aliquoted, and frozen at −80°C until testing.
Measurement of Progesterone Levels
Progesterone levels were assessed by a competitive enzyme immunoassay performed according to the manufacturer's instructions. For culture supernatants (diluted 1:2) and mouse plasma samples (diluted 1:25), the Cayman Chemical kit (Ann Arbor, MI) was used. The lower limit of detection was 10 pg/mL, with a calibration range of up to 1000 pg/mL. Mean inter-assay and intra-assay variabilities (±SD) were 14.8% ± 7.7% and 6.6% ± 2.1%, respectively. For human plasma samples (diluted 1:1000), total progesterone levels were assessed using the EnzoLife Sciences kit (Farmingdale, NY). The lower limit of detection was 8.57 pg/mL with a calibration range of up to 500 pg/mL. Mean interassay and intraassay variabilities (±SD) were 10% ± 3% and 5% ± 3%, respectively. All samples were assayed in triplicate.
Statistical Analysis
Cell culture experiments were performed in triplicate and repeated 3–6 times. Animal experiments were repeated 2–3 times. Fetal and placenta weights were calculated for each litter, and the average weight per litter was used for statistical comparisons. Comparisons were assessed using the Mann–Whitney test or Kruskal–Wallis test with the Dunn post hoc test, unless otherwise noted. χ2 or Fisher exact tests were used for categorical variables. Correlation was assessed using the Spearman rank correlation coefficient (r). A P value of < .05 was the threshold for 2-sided statistical significance. Analyses were performed using GraphPad Prism (La Jolla, CA).
RESULTS
PIs Decrease Progesterone Levels In Vitro
We first investigated the impact of HIV antiretrovirals on progesterone levels in vitro, using BeWo cells, a third-trimester human cytotrophoblast cell line capable of sex steroid production [28]. Antiretrovirals were tested singly and in clinically relevant combinations, at 10 times the MEC for 24 hours. These conditions did not result in cytotoxicity or inhibition of proliferation. Of the 3 different classes of drugs tested—NRTIs, nonnucleoside reverse transcriptase inhibitors (NNRTIs), and PIs—only PIs resulted in reduced progesterone levels (Figure 1A). RTV, known for its significant impact on CYP enzymes [20], had the strongest inhibitory effect on progesterone levels. ATV and LPV also decreased progesterone levels, while darunavir (DRV) was the only PI tested that did not significantly affect progesterone release. Nevirapine, ZDV, and 3TC had no effect on progesterone levels.
Figure 1.
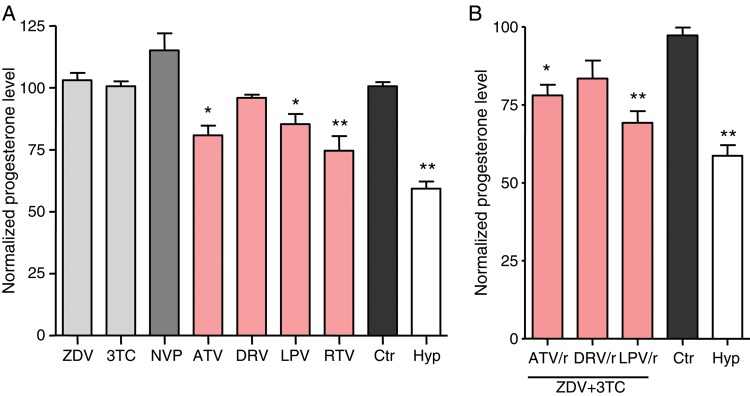
Protease inhibitors decrease trophoblast progesterone production in vitro. A, BeWo cells, a human cytotrophoblast cell line, were treated for 24 hours with 10 times the minimal effective dose (see Methods) of zidovudine (ZDV), lamivudine (3TC), nevirapine (NVP), ritonavir (RTV), atazanavir (ATV), darunavir (DRV), or lopinavir (LPV). B, BeWo cells were treated for 24 hours with combinations of ZDV plus 3TC and RTV-boosted ATV (ATV/r), DRV (DRV/r), or LPV (LPV/r). Control cells (Ctr) were treated with dimethyl sulfoxide at a final concentration of <0.1%. Cells incubated in hypoxic conditions (1% oxygen; Hyp) were used as a positive control. Progesterone levels were measured by competitive enzyme immunoassay and were corrected for the number of cells per well. Progesterone levels are expressed as percentage of the median control value. Data displayed are means±standard errors of the mean for 3–6 independent experiments. *P < .05 and **P < .01 for comparisons of each value with the control, by analysis of variance with the Dunnett post hoc test.
Drug combinations frequently used in pregnancy were also tested (Figure 1B). ZDV and 3TC were used as the NRTI backbone in combination with RTV-boosted LPV, RTV-boosted ATV, or RTV-boosted DRV. Similar to the single-drug studies, combinations containing LPV and ATV resulted in significant decreases in progesterone levels, while progesterone levels were not significantly affected by the DRV-based combination.
PI-Based cART but Not Dual NRTI Therapy Is Associated With More Adverse Pregnancy Outcomes and Lower Progesterone Levels in a Mouse Pregnancy Model
To extend our in vitro findings to an in vivo model, we treated pregnant mice with a dual-NRTI regimen, ZDV + 3TC, provided as Combivir (dual-NRTI), or a PI-based cART regimen, ZDV + 3TC with RTV-boosted LPV, provided as Combivir/Kaletra (PI-cART). This PI-cART regimen was selected because it is a recommended first-line regimen in pregnancy and the most commonly used PI-based regimen in both high and low-income countries [29]. Pilot studies were performed to optimize PI dosing. Drug doses administered to the mice yielded human pregnancy-relevant [30] plasma drug concentrations (0.4 µg/mL for LPV and 0.1 µg/mL for RTV).
Mice received antiretrovirals or, as a control, water by gavage once daily beginning on GD1, and pregnancy outcomes were assessed on GD15. None of the treatments resulted in signs of maternal toxicity or distress.
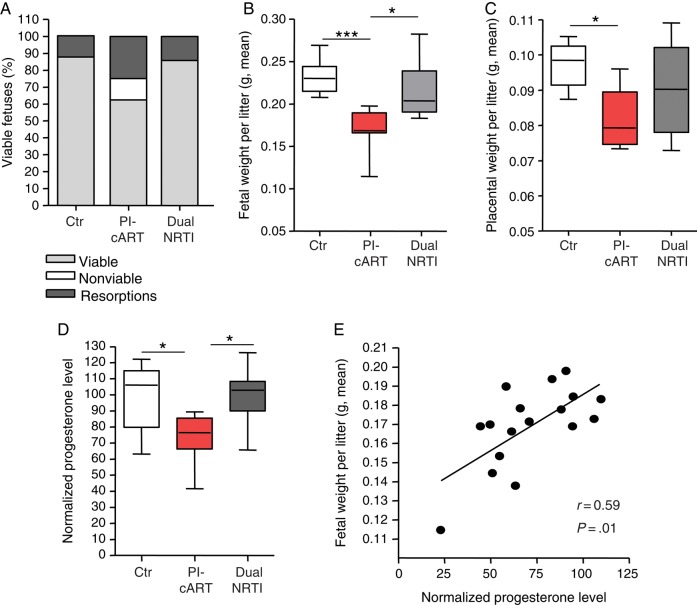
PI-cART was associated with significantly more fetal resorptions and lower fetal viability, compared with control (Figure 2A). PI-cART was also associated with significantly lower fetal and placental weights, compared with control (Figure 2B and 2C). In contrast, fetal weight, placental weight, fetal resorptions, and fetal viability were not significantly affected by dual-NRTI therapy (Figure 2A–C).
Figure 2.
Mouse plasma progesterone levels are decreased in protease inhibitor (PI)–exposed pregnant mice and correlated with fetal weight. Mated mice were exposed to either Combivir alone (zidovudine plus lamivudine; dual nucleoside reverse transcriptase inhibitor [NRTI]), Combivir plus Kaletra (ritonavir-boosted lopinavir; PI based combination antiretroviral therapy [PI-cART]), or water as a control (Ctr) by gavage once daily starting on gestational day 1. A, The percentage of fetuses that were viable (light grey), nonviable (as assessed by pedal reflex) (white), or resorbed (dark grey) for each treatment group is shown. χ2 analysis yielded the following findings: Ctr vs PI-cART, P < .001; dual NRTI vs PI-cART, P < .01; and Ctr vs dual NRTI, P = not significant. B, Average fetal weight per litter. C, Average placenta weight per litter. D, Progesterone levels in maternal plasma. Levels normalized to the control median are shown. In panels B, C, and D, data are shown as box and whisker plots, with medians, interquartile ranges, and ranges. Statistical comparisons were assessed by the Kruskal–Wallis test with the Dunn post hoc test. Data in panels A–D were acquired from the same experiment with 10 values for the Ctr group, 8 for the PI-cART group, and 8 for the dual NRTI group. Experiments were repeated 2 times. *P < .05, **P < .01, and ***P < .001. E, Progesterone levels were plotted against the average fetal weight per litter for the PI-cART group. Correlation was assessed by Spearman r. Trend line was calculated by linear regression analysis. Data represent 17 values from 3 combined experiments.
To assess whether ART had an impact on progesterone levels in vivo, we measured peripheral progesterone levels on GD15. Plasma progesterone levels were significantly lower in the PI-cART group, compared with the control group, but were unaffected in the dual-NRTI group (Figure 2D). Progesterone levels in the PI-cART group also correlated significantly with fetal weight (Figure 2E), suggesting an association between progesterone levels and fetal growth.
Progesterone Supplementation Partially Compensates for cART-Associated Fetal Weight Decrease
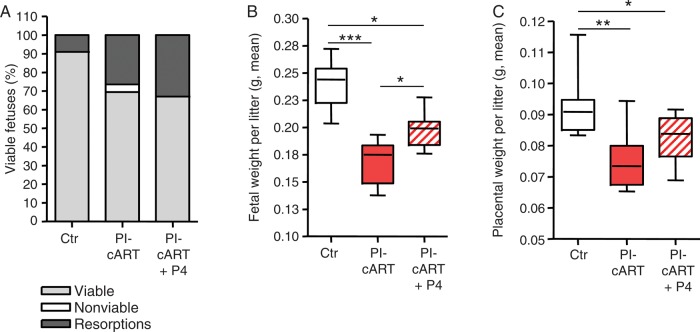
To investigate whether decreases in progesterone levels directly contributed to reduced fetal weight, we supplemented PI-cART with 0.5 mg progesterone every 4 days, beginning on GD1. We observed a significant increase in fetal weight at GD15 in mice that received PI-cART supplemented with progesterone (Figure 3B), although fetal weight did not recover to the same levels as in the control. There was also a trend toward higher placental weight in the progesterone-supplemented group (Figure 3C). However, the number of resorptions was unaffected by the supplementation (Figure 3A), suggesting that cART must exert additional progesterone-independent effects during pregnancy that require further investigation. Progesterone administration did not significantly alter the birth outcomes of control mice (Supplementary Materials).
Figure 3.
Progesterone supplementation partially reverses protease inhibitor (PI)–induced fetal weight defect. Mated mice were exposed to Combivir plus Kaletra (PI based combination antiretroviral therapy [PI-cART]) or water as a control (Ctr) by gavage once daily starting on gestational day 1 (GD1). Mice were then injected subcutaneously with either 0.5 mg progesterone (P4) suspended in corn oil or corn oil as a control on GD1, GD5, GD9, and GD13. All fetal and placenta parameters were assessed on GD15. A, Percentage of fetuses that were viable (light grey), nonviable (as assessed by pedal reflex; white), or resorbed (dark grey) for each treatment group is shown. χ2 analysis yielded the following findings: Ctr vs PI-cART, P < .001; PI-cART vs PI-cART + P4, P = not significant; and Ctr vs PI-cART + P4, P < .05. B, Average fetal weight per litter. C, Average placental weight per litter. Data were acquired from the same experiment, with 8 values for the Ctr, 10 for the PI-cART group, and 11 for the PI-cART + P4 group. Experiments were repeated once. *P < .05, **P < .01, and ***P < .001, by the Kruskal–Wallis test with the Dunn post hoc test.
In summary, adverse effects on fetal weight, placental weight, and fetal viability were associated with PI-containing cART but not with the NRTI backbone. Supplementation with progesterone throughout pregnancy resulted in a significant recovery in fetal weight, suggesting that PI-induced decreases in progesterone levels contributed to fetal growth restriction.
Progesterone Levels Are Decreased in HIV-Infected Women Receiving PI-Based cART
To extend our data to a clinically relevant population, we used plasma samples from a total of 27 HIV-infected pregnant women and 17 HIV-uninfected controls, collected between gestational weeks 25 and 28. This period was equivalent to our mouse sampling point, and it is during a time of uniformly increasing progesterone levels that is sufficiently distant from parturition, during which fluctuations in progesterone levels could occur. The mean gestational week of plasma collection (±SD) was 26.61 ± 0.99 for the HIV-infected samples and 26.67 ± 0.84 for the HIV-uninfected samples (P = .84). Demographic data, birth outcomes, CD4+ T-cell count, and HIV viral load are shown in Table 1.
Table 1.
Characteristics of Human Immunodeficiency Virus (HIV)–Infected and Matched HIV-Uninfected Pregnant Women
| Characteristic | HIV Infected (n = 27) | HIV Uninfected (n = 17) | P Valuea |
|---|---|---|---|
| Maternal age, y | 33 (29–37) | 32 (27–34) | .20 |
| Race | |||
| Black | 21 (77.8) | 14 (82.4) | 1.0 |
| White | 6 (22.2) | 3 (17.6) | |
| Parity | |||
| 0 | 10 (37) | 9 (52.9) | .56 |
| 1 | 10 (37) | 5 (29.4) | |
| ≥2 | 7 (26) | 3 (17.6) | |
| Mode of delivery | |||
| Cesarean section, no labor | 7 (25.9) | 7 (41.2) | .28 |
| Cesarean section, labor | 6 (22.2) | 1 (5.9) | |
| Vaginal | 14 (51.9) | 9 (52.9) | |
| Infant sex | |||
| Male | 18 (54.5) | 9 (45) | .58 |
| Female | 15 (45.5) | 11 (55) | |
| Preterm delivery | 4 (14.8) | 0 (0) | .15 |
| Gestational age at birth, wk | 38.5 (37.9–39.4) | 40.0 (39.0–40.9) | .0034 |
| Small for gestational age | 9 (33.3) | 0 (0) | .0076 |
| Birth weight, g | 2938 (2398–3441) | 3572 (3227–3635) | .0076 |
| Birth weight, percentile | 28.8 (8.5–65.2) | 53.4 (28.3–74.7) | .038 |
| HIV load near delivery, copies/mL | <40 (40–68) | NA | |
| CD4+ T-cell count near delivery, cells/µL | 589 (439–710) | NA | |
| Time since HIV diagnosis, y | 7 (5–10) | NA | |
Data are no. (%) of subjects or median (interquartile range).
Abbreviation: NA, not applicable.
a Race, parity, and mode of delivery comparisons were assessed by χ2 analysis; small for gestational age, preterm delivery, and infant sex comparisons were assessed by the Fisher exact test; and comparisons of median values were assessed by the Mann–Whitney test.
Maternal age, race, parity, mode of delivery, and infant sex were similar between the groups (Table 1). All HIV-infected women received cART containing a dual-NRTI backbone. Twenty-two of 27 (81.5%) received PI-based cART, and 5 of 27 (18.5%) received NNRTI-based cART. Of the 22 women receiving PI-based regimens, 12 (54.5%) received RTV-boosted LPV, 9 (40.9%) received RTV-boosted ATV, and 1 (4.5%) received RTV-boosted DRV. Of the 5 women receiving NNRTI-based regimens, 3 received NVP, 1 received efavirenz, and 1 received etravirine.
Birth weight, gestational age at delivery, and birth weight percentile were significantly lower in the HIV-infected group, compared with the control (Table 1). The rate of PTD did not differ significantly between groups, but the rate of SGA births (defined as a birth weight in the lowest 10th percentile) was significantly higher in the HIV-infected group (Table 1). Infants born to HIV-infected mothers weighed on average >600 g less than control infants (Table 1, P = .0076).
Progesterone levels were assessed in plasma samples collected during gestational weeks 25–28. In agreement with our in vitro and mouse data, progesterone levels were significantly lower in the HIV-infected group, compared with the control group. Mean progesterone levels were 132.2 ng/mL (95% confidence interval, 117.3–147.1) for HIV-infected women, compared with 179.8 ng/mL (95% CI, 141.4–218.1) for controls (Figure 4A). Although the number of women receiving non-PI regimens was very limited, progesterone levels in these women were significantly higher than those in women receiving PI-based regimens (Figure 4B). While supporting of our in vitro observations, these data must be treated with caution, given the small sample size. Progesterone levels correlated significantly with birth weight percentile in PI-treated HIV-infected women (r = 0.49; P = .018; Figure 4C) but not in the HIV-uninfected group (r = 0.23; P = .37). Progesterone levels did not correlate with gestational age at birth (Supplementary Figure 2).
Figure 4.
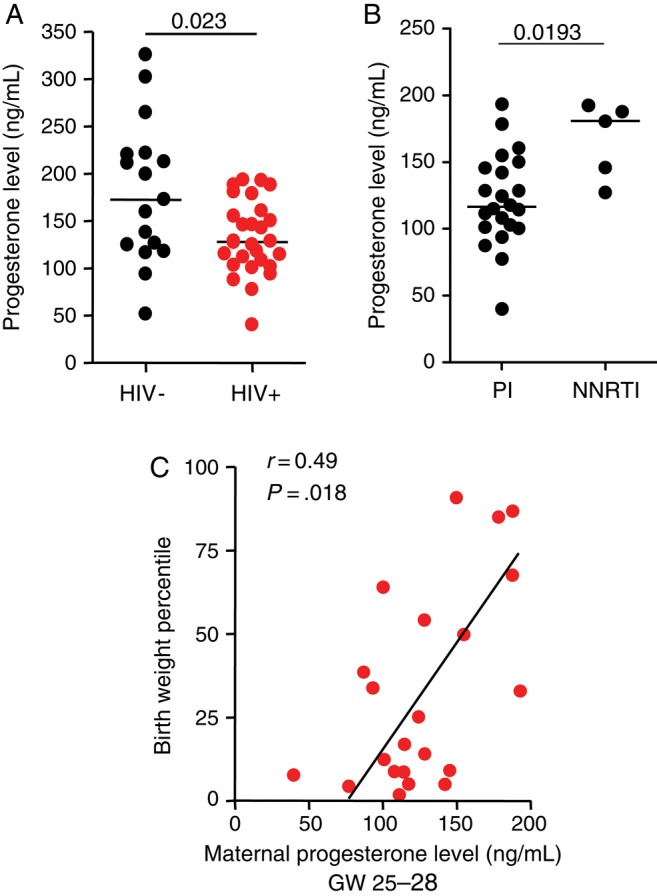
Progesterone levels are lower in protease inhibitor (PI)–exposed human immunodeficiency virus (HIV)–infected pregnant women and correlated with birth weight percentile. A, Plasma progesterone assessed by enzyme immunoassay in plasma specimens obtained during gestational weeks (GW) 25–28 from 17 HIV-negative and 27 HIV-infected pregnant women. B, Progesterone levels in 22 HIV-infected women receiving PI-based combination antiretroviral therapy (cART) and 5 receiving nonnucleoside reverse transcriptase inhibitor (NNRTI)–based cART. C, Correlation between birth weight percentile and progesterone levels in 22 HIV-infected women receiving PI-based cART. All data are shown as scatterplots with median values. Statistical comparisons were made using the Mann–Whitney test. Correlation was assessed by Spearman r.
In summary, our data are evidence that PI-based cART is associated with lower progesterone levels in HIV-infected pregnant women and that maternal progesterone levels in these women correlate with infant birth weight percentile.
DISCUSSION
In this study, we report an association between HIV PI use and lower progesterone levels in the context of pregnancy. We show that certain PIs but not NRTIs induced decreases in progesterone levels in vitro and in a mouse model and that progesterone levels were lower in HIV-infected pregnant women receiving PI-based cART, compared with uninfected controls. Lower progesterone levels, in turn, correlated with fetal growth restriction in the mouse model and with lower birth weight percentiles in HIV-infected patients. Although we focused most of our investigations on RTV-boosted LPV, as this is currently the most commonly used PI in pregnancy in both high- and low-resource settings, our in vitro data suggest that effects on progesterone may vary with different PIs. In vitro trophoblast progesterone production was significantly reduced by LPV, ATV (which is increasingly used in pregnancy), and RTV exposure but was not significantly affected by DRV treatment. Whether these differences will also hold true in HIV-infected pregnant women requires further investigation in a larger patient cohort.
Our study identifies progesterone as a probable biomarker for HIV-infected women at risk for delivering low-birth-weight infants and as a potential intervention to prevent this adverse outcome. Decreases in progesterone levels late in the third trimester (ie, after gestational week 34) have been reported for fetal growth restricted pregnancies [31]. We observed reduced progesterone levels earlier during pregnancy, between gestational weeks 25 and 28, in HIV-infected women exposed to PI-based cART, raising the possibility that progesterone could serve as an early biomarker for pregnancies at risk for growth restriction in this population. Further, progesterone supplementation strategies targeted at the second trimester could be considered as a means of increasing fetal weight in these women. Progesterone supplementation during pregnancy has been found to be safe and well tolerated [16–19].
In our mouse model, we observed lower progesterone levels with PI-exposure that were associated with fetal growth restriction. This PI-induced defect in fetal growth was partially but significantly improved when mice were supplemented with progesterone throughout pregnancy. These findings are in agreement with previous observations in both rodent and sheep models in which progesterone reduction during pregnancy was associated with fetal growth restriction [32–34], and fetal weight was significantly improved following progesterone supplementation [32, 33]. A recent meta-analysis of 36 randomized controlled trials of progesterone supplementation to prevent PTD in high-risk women also reported a significant effect for progesterone in reducing the rate of low birth weight [35].
Although our mouse data demonstrate that PI-induced low progesterone levels contribute at least in part to fetal growth restriction, our findings in humans are correlative, and a causal relationship will need to be confirmed. Factors such as placenta dysfunction may also result in both low progesterone levels and low birth weight. In addition, SGA has been reported for all cART regimens, independent of PIs [1, 2], suggesting that additional mechanisms, such as immune reconstitution and a Th2-to-Th1 cytokine shift, may also contribute to this adverse event [4, 13].
PTD is the most common adverse outcome associated with PI use [6]. Given the established relationship between progesterone and parturition, low progesterone levels may be a mechanism by which PIs increase the risk of PTD. The rate of PTD was too low in our cohort to investigate this relationship. We did not observe a significant correlation between progesterone levels and gestational age at birth, but this may be a factor of our sampling time point (ie, gestational weeks 25–28). Third trimester progesterone levels may be more reflective of PTD risk.
Our study has several strengths. We were able to demonstrate a consistent PI effect on progesterone levels in vitro in an animal model, using clinically relevant drug levels, and we were able to extend our findings to a patient population. The limitations of our study include the small number of women included in our study, particularly those receiving a PI-sparing regimen. Because of the limited number of women receiving PI-sparing regimens, we were unable to exclude the possibility that non–PI-based cART may also affect progesterone levels in HIV-infected pregnant women. Although our hypothesis could be strengthened by inclusion of an HIV-infected treatment-naive group, this would not be ethically possible given current treatment recommendations. Despite these limitations, we were able to demonstrate that our in vitro and animal model findings are relevant to HIV-infected pregnant women.
In conclusion, we have provided evidence supporting an association between PI-based therapy (in particular, RTV-boosted LPV) and lower progesterone levels during pregnancy. We also demonstrated that lower progesterone levels are associated with fetal growth restriction and lower birth weight percentiles. We propose that PI-induced reduction in progesterone levels during pregnancy is a potential mechanism contributing to the higher incidence of SGA among infants delivered by PI-exposed HIV-infected women. While the molecular mechanisms that lead to progesterone reduction remain to be elucidated, our study provides needed information on the topic of antiretroviral safety in pregnancy that could translate into more-informed treatment choices for HIV-infected pregnant women and into potential interventions to improve pregnancy outcomes in these women.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Logan Kennedy, Kate Besel, Sheryl Lynn Hewko, Roberta Halpenny, Leanne DeSouza, and M. J. Martin, for their commitment and contributions to the study; Chloe MacDonald, Dr Mark Kibschull, Dr Oksana Shynlova, and Dr Kayla Hayford, for expert assistance; the labor and delivery staff at St. Michael's Hospital and Mount Sinai Hospital; and the women who participated in our study, for their interest and commitment to the project, which made this work possible.
E. P., M. S., and L. S. conceived and designed the experiments. E. P., H. M., and L. S. performed the experiments and analyzed the data. M. R. L., M. H. Y., K. E. M., S. L. W., R. S., and J. M. recruited patients. E. P. and L. S. wrote the manuscript, with contributions from all authors.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the Ontario HIV Treatment Network (grant G655 and JIDA award G287 to L. S. and career support grant to S. L. W.), the Canadian Institutes of Health Research (grants IHD123784 and MOP130398 to L. S.), the Canadian Foundation for AIDS Research (grant 16 to L. S.), the Interdisciplinary HIV Pregnancy Group (trainee award to E. P.), and the Toronto General Research Institute (postdoctoral fellowship to H. M.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Chen JY, Ribaudo HJ, Souda S, et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. J Infect Dis. 2012;206:1695–705. doi: 10.1093/infdis/jis553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Machado ES, Hofer CB, Costa TT, et al. Pregnancy outcome in women infected with HIV-1 receiving combination antiretroviral therapy before versus after conception. Sex Transm Infect. 2009;85:82–7. doi: 10.1136/sti.2008.032300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ekouevi DK, Coffie PA, Becquet R, et al. Antiretroviral therapy in pregnant women with advanced HIV disease and pregnancy outcomes in Abidjan, Côte d'Ivoire. AIDS. 2008;22:1815–20. doi: 10.1097/QAD.0b013e32830b8ab9. [DOI] [PubMed] [Google Scholar]
- 4.Wimalasundera RC, Larbalestier N, Smith JH, et al. Pre-eclampsia, antiretroviral therapy, and immune reconstitution. Lancet. 2002;360:1152–4. doi: 10.1016/s0140-6736(02)11195-0. [DOI] [PubMed] [Google Scholar]
- 5.Townsend CL, Cortina-Borja M, Peckham CS, Tookey PA. Antiretroviral therapy and premature delivery in diagnosed HIV-infected women in the United Kingdom and Ireland. AIDS. 2007;21:1019–26. doi: 10.1097/QAD.0b013e328133884b. [DOI] [PubMed] [Google Scholar]
- 6.Powis KM, Kitch D, Ogwu A, et al. Increased risk of preterm delivery among HIV-infected women randomized to protease versus nucleoside reverse transcriptase inhibitor-based HAART during pregnancy. J Infect Dis. 2011;204:506–14. doi: 10.1093/infdis/jir307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newell M-L, Bunders MJ. Safety of antiretroviral drugs in pregnancy and breastfeeding for mother and child. Curr Opin HIV AIDS. 2013;8:503–9. doi: 10.1097/COH.0b013e3283632b88. [DOI] [PubMed] [Google Scholar]
- 8.López M, Hernàndez S, Morén C, et al. Perinatal outcomes, mitochondrial toxicity and apoptosis in HIV-treated pregnant women and in-utero-exposed newborn. AIDS. 2012;26:419–28. doi: 10.1097/QAD.0b013e32834f3232. [DOI] [PubMed] [Google Scholar]
- 9.Kourtis AP, Schmid CH, Jamieson DJ, Lau J. Use of antiretroviral therapy in pregnant HIV-infected women and the risk of premature delivery: a meta-analysis. AIDS. 2007;21:607–15. doi: 10.1097/QAD.0b013e32802ef2f6. [DOI] [PubMed] [Google Scholar]
- 10.Townsend C, Schulte J, Thorne C, et al. Antiretroviral therapy and preterm delivery-a pooled analysis of data from the United States and Europe. BJOG. 2010;117:1399–410. doi: 10.1111/j.1471-0528.2010.02689.x. [DOI] [PubMed] [Google Scholar]
- 11.de Vincenzi I Kesho Bora Study Group. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis. 2011;11:171–80. doi: 10.1016/S1473-3099(10)70288-7. [DOI] [PubMed] [Google Scholar]
- 12.Ross AC, Leong T, Avery A, et al. Effects of in utero antiretroviral exposure on mitochondrial DNA levels, mitochondrial function and oxidative stress. HIV Med. 2012;13:98–106. doi: 10.1111/j.1468-1293.2011.00945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiore S, Newell M-L, Trabattoni D, et al. Antiretroviral therapy-associated modulation of Th1 and Th2 immune responses in HIV-infected pregnant women. J Reprod Immunol. 2006;70:143–50. doi: 10.1016/j.jri.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379–85. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- 15.Morris RK, Oliver EA, Malin G, Khan KS, Meads C. Effectiveness of interventions for the prevention of small-for-gestational age fetuses and perinatal mortality: a review of systematic reviews. Acta Obstet Gynecol Scand. 2013;92:143–51. doi: 10.1111/aogs.12029. [DOI] [PubMed] [Google Scholar]
- 16.Wahabi HA, Abed Althagafi NF, Elawad M, Al Zeidan RA. Progestogen for treating threatened miscarriage. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD005943.pub4. CD005943. [DOI] [PubMed] [Google Scholar]
- 17.Walch KT, Huber JC. Progesterone for recurrent miscarriage: truth and deceptions. Best Pract Res Clin Obstet Gynaecol. 2008;22:375–89. doi: 10.1016/j.bpobgyn.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Schmouder VM, Prescott GM, Franco A, Fan-Havard P. The rebirth of progesterone in the prevention of preterm labor. Ann Pharmacother. 2013;47:527–36. doi: 10.1345/aph.1R281. [DOI] [PubMed] [Google Scholar]
- 19.Norwitz ER, Caughey AB. Progesterone supplementation and the prevention of preterm birth. Rev Obstet Gynecol. 2011;4:60–72. [PMC free article] [PubMed] [Google Scholar]
- 20.Andany N, Loutfy MR. HIV protease inhibitors in pregnancy: pharmacology and clinical use. Drugs. 2013;73:229–47. doi: 10.1007/s40265-013-0017-3. [DOI] [PubMed] [Google Scholar]
- 21.Tseng A, Hills-Nieminen C. Drug interactions between antiretrovirals and hormonal contraceptives. Expert Opin Drug Metab Toxicol. 2013;9:559–72. doi: 10.1517/17425255.2013.772579. [DOI] [PubMed] [Google Scholar]
- 22.Vogler MA, Patterson K, Kamemoto L, et al. Contraceptive efficacy of oral and transdermal hormones when co-administered with protease inhibitors in HIV-1-infected women: pharmacokinetic results of ACTG trial A5188. J Acquir Immune Defic Syndr. 2010;55:473–82. doi: 10.1097/QAI.0b013e3181eb5ff5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simon A, Warszawski J, Kariyawasam D, et al. Association of prenatal and postnatal exposure to lopinavir-ritonavir and adrenal dysfunction among uninfected infants of HIV-infected mothers. JAMA. 2011;306:70–8. doi: 10.1001/jama.2011.915. [DOI] [PubMed] [Google Scholar]
- 24.Foisy MM, Yakiwchuk EMK, Chiu I, Singh AE. Adrenal suppression and Cushing's syndrome secondary to an interaction between ritonavir and fluticasone: a review of the literature. HIV Med. 2008;9:389–96. doi: 10.1111/j.1468-1293.2008.00579.x. [DOI] [PubMed] [Google Scholar]
- 25.Lorello G, la Porte C, Pilon R, Zhang G, Karnauchow T, MacPherson P. Discordance in HIV-1 viral loads and antiretroviral drug concentrations comparing semen and blood plasma. HIV Med. 2009;10:548–54. doi: 10.1111/j.1468-1293.2009.00725.x. [DOI] [PubMed] [Google Scholar]
- 26.Anderson PL, Kakuda TN, Kawle S, Fletcher CV. Antiviral dynamics and sex differences of zidovudine and lamivudine triphosphate concentrations in HIV-infected individuals. AIDS. 2003;17:2159–68. doi: 10.1097/00002030-200310170-00003. [DOI] [PubMed] [Google Scholar]
- 27.Ray JG, Sgro M, Mamdani MM, et al. Birth weight curves tailored to maternal world region. J Obstet Gynaecol Can. 2012;34:159–71. doi: 10.1016/S1701-2163(16)35159-3. [DOI] [PubMed] [Google Scholar]
- 28.Bahn RS, Worsham A, Speeg KV, Ascoli M, Rabin D. Characterization of steroid production in cultured human choriocarcinoma cells. J Clin Endocrinol Metab. 1981;52:447–50. doi: 10.1210/jcem-52-3-447. [DOI] [PubMed] [Google Scholar]
- 29.Chougrani I, Luton D, Matheron S, Mandelbrot L, Azria E. Safety of protease inhibitors in HIV-infected pregnant women. HIV AIDS (Auckl) 2013;5:253–62. doi: 10.2147/HIV.S33058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambert JS, Else LJ, Jackson V, et al. Therapeutic drug monitoring of lopinavir/ritonavir in pregnancy. HIV Med. 2011;12:166–73. doi: 10.1111/j.1468-1293.2010.00865.x. [DOI] [PubMed] [Google Scholar]
- 31.Salas SP, Marshall G, Gutiérrez BL, Rosso P. Time course of maternal plasma volume and hormonal changes in women with preeclampsia or fetal growth restriction. Hypertension. 2006;47:203–8. doi: 10.1161/01.HYP.0000200042.64517.19. [DOI] [PubMed] [Google Scholar]
- 32.van Marthens E, Zamenhof S, Firestone C. The effect of progesterone on fetal and placental development in normal and protein-energy-restricted rats. Nutr Metab. 1979;23:438–48. doi: 10.1159/000176290. [DOI] [PubMed] [Google Scholar]
- 33.Wallace JM, Bourke DA, Da Silva P, Aitken RP. Influence of progesterone supplementation during the first third of pregnancy on fetal and placental growth in overnourished adolescent ewes. Reproduction. 2003;126:481–7. doi: 10.1530/rep.0.1260481. [DOI] [PubMed] [Google Scholar]
- 34.Mark PJ, Smith JT, Waddell BJ. Placental and fetal growth retardation following partial progesterone withdrawal in rat pregnancy. Placenta. 2006;27:208–14. doi: 10.1016/j.placenta.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Dodd J, Jones L, Flenady V, Cincotta R, Crowther C. Prenatal administration of progesterone for preventing preterm birth in women considered to be at risk of preterm birth. Cochrane Database Syst Rev. 2013;7 doi: 10.1002/14651858.CD004947.pub3. CD004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.