Abstract
Objectives
Several controversies surround lymphadenectomy for endometrial cancer; surgical approach, who to stage, and the anatomic borders of the lymphadenectomy. The purpose of this study was to identify practice patterns among gynecologic oncologists when performing a lymph node evaluation during staging for endometrial cancer.
Methods
A self-administered survey was sent via email to all SGO members on 3 occasions between 2/09 and 4/09. The survey addressed surgical approach, algorithms used to determine staging, and anatomic landmarks defining lymphadenectomy.
Results
Four hundred and six members (40%) responded. Eighty-two percent completed fellowship and 14% were fellows. Thirty-four percent finished fellowship in 2000 or later. Eighty-five percent educate fellows/residents in either academic (65%) or private practice settings (20%). For a majority of cases 40% prefer laparotomy, 31% perform robotic surgery, and 29% use laparoscopy. Minimally invasive surgery was associated with university-based practice (p=0.048). Most (53%) never/rarely use frozen section to determine whether or not to perform lymphadenectomy. A majority perform staging on all grade 2 and grade 3 cancers (66% and 90%, respectively). When performing paraaortic lymphadenectomy, 50% of respondents use the IMA as the upper border and 11% take the dissection to the renal vessels. Participants who completed fellowship in 2000 or later were less likely to go to the renal vessels (p=0.002).
Conclusion
Current controversies in surgical staging for endometrial cancer are reflected in the practice patterns among gynecologic oncologists. At this point it is unclear if standardizing surgical practice patterns will improve outcomes for patients with endometrial cancer.
Introduction
Endometrial cancer is the most common gynecologic malignancy and the fourth most common cancer among women in the United States. It is estimated that 42,160 new cases and 7,780 deaths from endometrial cancer will have occurred during 2009[1]. The majority of patients are diagnosed with early stage disease and the incidence of endometrial carcinoma has remained stable over the past decade, however, the annual number of deaths from this disease has more than doubled since 1987 [2]. While the role of adjuvant radiation therapy and/or chemotherapy is under continued investigation, surgical staging continues to be the most important part of treatment for a majority of patients.
In 1988, the International Federation of Gynecology and Obstetrics (FIGO) adopted a surgically based staging system for endometrial cancer [3]. This paradigm shift from clinical staging was based on prospective surgical staging studies conducted by the Gynecologic Oncology Group (GOG) demonstrating a relationship between prognosis and surgically determined risk factors [4]. In 2009, FIGO staging for endometrial cancer was further modified to better classify patients into prognostic risk groups based on pathologic information obtained at initial surgical staging[5]. One of these modifications included the separation of node positive patients, previously stage IIIC, into IIIC1 pelvic node positive and IIIC2 paraaortic node positive, suggesting that these groups may have differences in prognosis.
Current recommendations for the surgical management of endometrial cancer include exploratory laparotomy, pelvic washings, hysterectomy, bilateral salpingo-oophorectomy, selective biopsies of suspicious areas, and lymph node sampling in patients at risk for extra-uterine disease. Most gynecologic oncologist would agree that patients with high risk subtypes such as uterine papillary serous, clear cell, or mixed mullerian malignant tumors (MMMT) should undergo a complete surgical staging. However, there appears to be significant variability in the staging and treatment algorithms used at different institutions and even among individual physicians within the same practice in regard to endometrioid endometrial adenocarcinoma. In addition, the inclusion of a systematic pelvic and para-aortic lymphadenectomy in the surgical management of all patients, although part of the FIGO staging, remains controversial.
The purpose of this study is to evaluate the current clinical practice patterns among gynecologic oncologists at the time of surgical staging of endometrial cancer including surgical approach, algorithms used to determine surgical staging, and anatomic landmarks identified during lymphadenectomy.
Materials and Methods
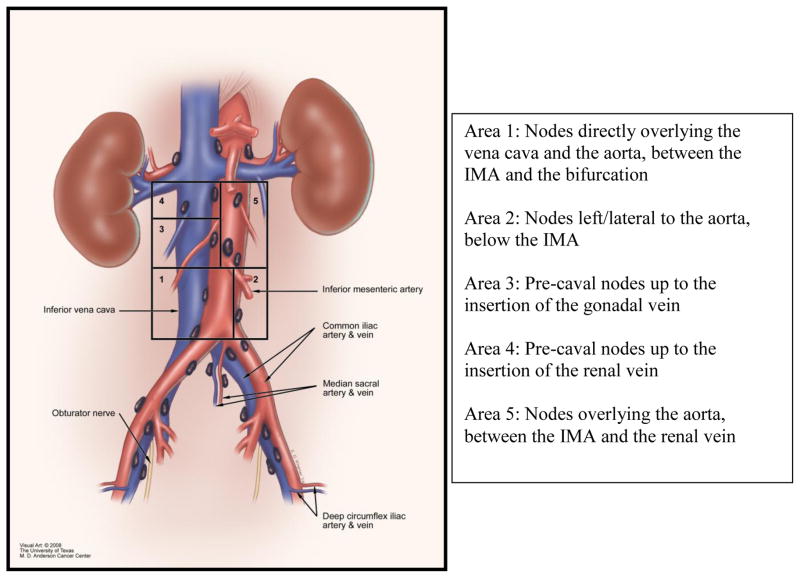
After obtaining approval from the Institutional Review Board of the University of Texas M. D. Anderson Cancer Center and the Society of Gynecologic Oncologists (SGO), an email list of all full, candidate, and fellow members of the SGO was obtained. The survey questions were designed to determine practice patterns among gynecologic oncologists when performing a lymph node evaluation as part of the surgical staging for endometrial cancer. A visual aid with attached descriptions (Figure 1) was created by the Medical Graphics department at M. D. Anderson Cancer Center to provide a clearer definition of the anatomic landmarks referred to during the para-aortic lymphadenectomy. The survey was estimated to take 10 minutes to complete and was submitted electronically.
Figure 1.
Anatomic diagram of the para-aortic lymph nodes.
The survey was sent via email on three occasions between February and April of 2009. The email included a link to an online survey which was submitted electronically. All of the data were automatically stored by the Department of Institutional Research at M. D. Anderson Cancer Center. All responses were anonymous. The respondents were asked about demographic characteristics including their current practice setting, their role in fellow and resident education, and their personal training history. The respondents were asked 17 questions regarding case management, including the use of frozen section, their preferred surgical approach, and how they define pelvic and paraaortic lymph node evaluation during endometrial cancer surgical staging.
The collected data were analyzed using frequency distribution tests. The relationships between dichotomous variables of interest were assessed using the χ2 tests or Fisher’s exact test for nominal and categorical variables. Continuous variables were assessed using the Mann-Whitney U test. Two sided p-values are reported and a p-value of ≤0.05 was considered statistically significant. Case management styles were compared among demographic groups to determine any significant associations. The SPSS for Windows version 14.0 software (SPSS Inc, Chicago, IL) was used to perform all statistical analyses.
Results
The demographic characteristics of the respondents are listed in Table 1. Four hundred and six SGO members (40%) completed the electronic survey during the study period. Ten respondents reported that they were “not a practicing gynecologic oncologist”. Eighty-two percent completed a fellowship and 14% are current fellows. Thirty-four percent of respondents finished fellowship in 2000 or later. Eighty-five percent are currently involved in the education of fellows and/or residents in either academic (65%) or private practice (20%) settings.
Table 1.
Demographic characteristics of respondents
| Number N = 406 |
Percent | |
|---|---|---|
| Training | ||
| Completed fellowship | 331 | 82% |
| Currently in fellowship | 57 | 14% |
| Other/did not answer | 18 | 4% |
| Year fellowship completed | ||
| Before 1970 | 3 | 1% |
| 1970–1979 | 28 | 7% |
| 1980–1989 | 74 | 18% |
| 1990–1999 | 108 | 27% |
| 2000 or later | 138 | 34% |
| Not applicable/did not answer | 55 | 13% |
| Practice type | ||
| University-based with fellows | 170 | 44% |
| University-based with residents only | 83 | 21% |
| Private practice with residents | 78 | 20% |
| Private practice | 59 | 15% |
For endometrial cancer staging, 40% prefer laparotomy, 31% typically perform robotic surgery, 25% use transperitoneal laparoscopy and 4% use a combined extra- and transperitoneal approach for a majority of their cases. When surgical approach was evaluated by practice type, there was a significant association between minimally invasive surgical approach and university-based practice (p=0.048). The use of intra-operative frozen section of the uterus to determine whether or not to perform a lymphadenectomy or the extent of lymphadenectomy also varied among surgeons. Thirty-one percent of participants always/usually used frozen section, 16% used it sometimes, and 53% rarely/never used frozen section to determine the extent of surgical staging. Once the decision to proceed with surgical staging was made, 60% of respondents perform the same procedure for all patients, resection of both pelvic and para-aortic lymph nodes. Twenty-seven percent respondents always remove pelvic nodes, but only remove para-aortic nodes some of the time. Utilization of frozen section for determining whether or not to stage was not associated with practice setting, preferred surgical approach or when the respondent completed fellowship.
When asked about surgical management of endometrioid tumors based on the grade of the cancer, a majority of respondents removed both pelvic and para-aortic lymph nodes in all grade 2 and grade 3 cancers (66% and 90%, respectively). Only 35% evaluated both pelvic and para-aortic nodes on all grade 1 cancers. When labeling their specimens 86% of respondents called the procedure a “lymph node dissection”, while 14% described it as a “lymph node sampling”. Decision to surgically stage based on tumor grade did not differ significantly based on practice setting, preferred surgical approach or when the respondent completed fellowship.
The anatomic landmarks used to define the para-aortic lymph node evaluation were addressed in several questions. In defining the upper border of the para-aortic lymph node evaluation; 50% identified the inferior mesenteric artery (IMA) as the upper border, 34% remove nodes above the IMA but not to the level of the renal vessels, and 11% remove nodes to the level of the renal vessels (Table 2). Participants who completed fellowship in 2000 or later were less likely to remove nodes above the IMA (p = 0.002).
Table 2.
Defining the upper border of a para-arotic lymph node dissection based on year of fellowship completion (P = 0.02).
| Year of fellowship completion | IMA | Between the IMA and the renal vein | Renal vein | Other |
|---|---|---|---|---|
| Before 1979 | 7 (27%) | 11 (42%) | 4 (15%) | 4 (15%) |
| 1980–1989 | 31 (43%) | 27 (37%) | 12 (16%) | 3 (4%) |
| 1990–1999 | 46 (43%) | 46 (43%) | 9 (8%) | 6 (6%) |
| 2000 or later | 87 (63%) | 36 (25%) | 12 (9%) | 4 (3%) |
|
| ||||
| Total | 171 (50%) | 120 (35%) | 37 (11%) | 17 (5%) |
Figure 1, taken directly from the survey, was included to provide a visual aid with associated descriptions of the anatomic landmarks. When performing a para-aortic lymph node dissection 91% removed the nodes directly overlying the vena cava and the aorta, between the IMA and the bifurcation (Area 1), 83% removed nodes left/lateral to the aorta, below the IMA (Area 2), 53% removed pre-caval nodes up to the insertion of the gonadal vein (Area 3), 20% removed pre-caval nodes up to the insertion of the renal vein (Area 4), and 24% removed nodes overlying the aorta, between the IMA and the renal vein (Area 5). Extent of lymphadenectomy was not associated with either surgical approach or current practice setting.
Discussion
Endometrial cancer is the most common cancer we face as gynecologic oncologists. While the majority of women are diagnosed with early stage disease limited to the uterine fundus and do not require adjuvant therapy, debate continues among practicing gynecologic oncologists regarding the optimal surgical management of this disease. These controversies include the appropriate surgical approach, algorithms used to determine surgical staging, and the anatomic borders of the paraaortic lymphadenectomy.
As expected, we found that the role of minimally invasive surgery has continued to grow among gynecologic oncologists. Sixty percent of participants use minimally invasive surgical approaches to stage a majority of their patients with endometrial cancer. This is consistent with previous data from our institution on the increasing utilization of laparoscopy in the treatment of endometrial cancer [6, 7]. While the data on robotic surgery are currently emerging [8–11], the recent publication of GOG LAP2, a prospective, randomized study comparing laparotomy to traditional laparoscopy for the treatment of endometrial cancer has shown similar operative outcomes and significant benefits in the minimally invasive surgery group [12]. For many, minimally invasive surgery is now considered standard of care for the treatment of endometrial cancer. In addition, the robotic approach may have added benefit over laparoscopy in obese women with endometrial cancer [9]. However, access to the high para-aortic lymph nodes is limited by the robotic approach. Therefore, for surgeons who believe that a para-aortic lymph node dissection should extend up to the renal vessels, the robotic approach may have some limitations.
In addition to variability in surgical approach, the use of frozen section as part of an algorithm to determine surgical staging also differed among respondents. Only 31% of surgeons routinely used frozen section to determine the extent of surgical staging. A majority of participants staged all patients with grade 2 and grade 3 cancers. In a retrospective review of women with endometrial cancer, Mariani et al. identified a subgroup of patients that were considered “low risk” and could safely forgo lymph node evaluation. In their study, women with grade 1 or 2 endometrioid tumors, with less than 50% myometrial invasion, and tumor size less than 2 cm had a 0% risk of lymph node involvement [13]. These data have been confirmed in a follow up prospective study from the same institution [14]. While this algorithm could potentially save 27% of women with endometrial cancer and 33% of patients with endometrioid tumors a comprehensive surgical staging, the quality of frozen section can be very institution dependent [15].
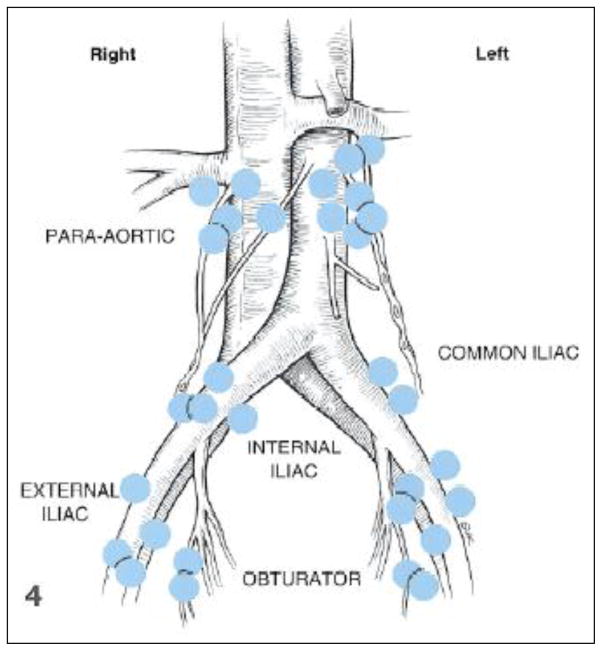
Finally, the anatomic borders of the lymphadenectomy continue to be controversial despite information available, on the lymphatic drainage of the endometrium. The goal of a lymphadenectomy at the time of surgical staging for any cancer is to identify and remove the lymph nodes that are at highest risk for local spread. There have been several lymphatic mapping studies performed in women with endometrial cancer using different techniques. Burke et al. used blue dye injected into the uterine fundus in 18 women at the time of surgical staging [16]. Figure 2 represents the sentinel lymph nodes identified in this study. As noted in the figure, sentinel nodes were identified in the pelvic lymph node basins as well as the para-aortic lymph nodes. Among those identified in the para-aortic region, all of the sentinel nodes were above the IMA, following the drainage of the gonadal vessels. Maccauro et al. described lymphatic mapping via hysteroscopic, peritumoral injection of both blue and radioactive colloid. They also identified sentinel lymph nodes in the para-aortic region above the IMA [17]. Interestingly, in both studies, there was no direct drainage to nodal basins between the bifurcation of the aorta and the IMA. While this data clearly supports that para-aortic lymph nodes above the IMA directly drain the uterine body, a large number of practicing gynecologic oncologists continue to use the IMA as the upper border of their dissection.
Figure 2.
Lymphatic drainage of the uterus and identification of the sentinel nodes.
Clinical data from the Mayo Clinic has also helped better define the risk of lymph node metastasis, as well as the patterns of spread in endometrial cancer [14]. In a prospective study of 482 patients with endometrial cancer, 281 underwent full staging to the renal vessels. Twenty-two percent had positive lymph nodes; of these 51% had both positive pelvic and para-aortic nodes, 33% had positive pelvic nodes only and 16% had isolated involvement of the para-aortic nodes. Among those with positive para-aortic nodes, 46% were only positive above the IMA and 77% had at least one metastatic node located above the level of the IMA. As seen in the results from our current survey, 50% of the gynecologic oncologists who participated used the IMA as their upper border of dissection, potentially limiting the effectiveness of detecting positive para-aortic nodes. Interestingly, completing fellowship in 2000 or later, during which time this data has been published was associated with limiting the para-aortic lymph node dissection to below the IMA. This may be based on the current guidelines for para-aortic lymphadenectomy in GOG 210 and GOG LAP2 where the upper border of the para-aortic lymph node dissection is defined as the IMA [12].
While this survey only captures the reported practice patterns of 40% of SGO members, there is clear variability among practicing gynecologic oncologist. This study is limited by both reporting bias and a limited response rate. While the true practice patterns among gynecologic oncologists may not be clearly defined in this study, it does confirm that controversy in the surgical management of endometrial cancer still exists. Ultimately, our primary goal is to improve the survival and outcome for our patients. Despite variability in practice patterns among gynecologic oncologists, the 5 year survival for patients with early stage endometrial cancer remains high. At this point it is unclear if standardizing surgical practice patterns will improve outcomes for patients with endometrial cancer.
Footnotes
Conflict of Interest Statement. There are no conflicts of interest among the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could a3ect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics, 2009. CA Cancer J Clin. 2009 doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Silverberg E, Lubera J. Cancer statistics, 1987. CA Cancer J Clin. 1987;37:2–19. doi: 10.3322/canjclin.37.1.2. [DOI] [PubMed] [Google Scholar]
- 3.Shepherd JH. Revised FIGO staging for gynaecological cancer. Br J Obstet Gynaecol. 1989;96:889–92. doi: 10.1111/j.1471-0528.1989.tb03341.x. [DOI] [PubMed] [Google Scholar]
- 4.Creasman WT, DeGeest K, DiSaia PJ, Zaino RJ. Significance of true surgical pathologic staging: a Gynecologic Oncology Group Study. Am J Obstet Gynecol. 1999;181:31–4. doi: 10.1016/s0002-9378(99)70431-x. [DOI] [PubMed] [Google Scholar]
- 5.Creasman W. Revised FIGO staging for carcinoma of the endometrium. Int J Gynaecol Obstet. 2009;105:109. doi: 10.1016/j.ijgo.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Frumovitz M, Ramirez PT, Greer M, Gregurich MA, Wolf J, Bodurka DC, Levenback C. Laparoscopic training and practice in gynecologic oncology among Society of Gynecologic Oncologists members and fellows-in-training. Gynecol Oncol. 2004;94:746–53. doi: 10.1016/j.ygyno.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Mabrouk M, Frumovitz M, Greer M, Sharma S, Schmeler KM, Soliman PT, Ramirez PT. Trends in laparoscopic and robotic surgery among gynecologic oncologists: A survey update. Gynecol Oncol. 2009;112:501–5. doi: 10.1016/j.ygyno.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boggess JF, Gehrig PA, Cantrell L, Shafer A, Ridgway M, Skinner EN, Fowler WC. A comparative study of 3 surgical methods for hysterectomy with staging for endometrial cancer: robotic assistance, laparoscopy, laparotomy. Am J Obstet Gynecol. 2008;199:360e1–9. doi: 10.1016/j.ajog.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Gehrig PA, Cantrell LA, Shafer A, Abaid LN, Mendivil A, Boggess JF. What is the optimal minimally invasive surgical procedure for endometrial cancer staging in the obese and morbidly obese woman? Gynecol Oncol. 2008;111:41–5. doi: 10.1016/j.ygyno.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 10.Seamon LG, Cohn DE, Richardson DL, Valmadre S, Carlson MJ, Phillips GS, Fowler JM. Robotic hysterectomy and pelvic-aortic lymphadenectomy for endometrial cancer. Obstet Gynecol. 2008;112:1207–13. doi: 10.1097/AOG.0b013e31818e4416. [DOI] [PubMed] [Google Scholar]
- 11.DeNardis SA, Holloway RW, Bigsby GEt, Pikaart DP, Ahmad S, Finkler NJ. Robotically assisted laparoscopic hysterectomy versus total abdominal hysterectomy and lymphadenectomy for endometrial cancer. Gynecol Oncol. 2008;111:412–7. doi: 10.1016/j.ygyno.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 12.Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, Spiegel G, Barakat R, Pearl ML, Sharma SK. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331–6. doi: 10.1200/JCO.2009.22.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mariani A, Webb MJ, Keeney GL, Haddock MG, Calori G, Podratz KC. Low-risk corpus cancer: is lymphadenectomy or radiotherapy necessary? Am J Obstet Gynecol. 2000;182:1506–19. doi: 10.1067/mob.2000.107335. [DOI] [PubMed] [Google Scholar]
- 14.Mariani A, Dowdy SC, Cliby WA, Gostout BS, Jones MB, Wilson TO, Podratz KC. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11–8. doi: 10.1016/j.ygyno.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frumovitz M, Slomovitz BM, Singh DK, Broaddus RR, Abrams J, Sun CC, Bevers M, Bodurka DC. Frozen section analyses as predictors of lymphatic spread in patients with early-stage uterine cancer. J Am Coll Surg. 2004;199:388–93. doi: 10.1016/j.jamcollsurg.2004.05.258. [DOI] [PubMed] [Google Scholar]
- 16.Burke TW, Levenback C, Tornos C, Morris M, Wharton JT, Gershenson DM. Intraabdominal lymphatic mapping to direct selective pelvic and paraaortic lymphadenectomy in women with high-risk endometrial cancer: results of a pilot study. Gynecol Oncol. 1996;62:169–73. doi: 10.1006/gyno.1996.0211. [DOI] [PubMed] [Google Scholar]
- 17.Maccauro M, Lucignani G, Aliberti G, Villano C, Castellani MR, Solima E, Bombardieri E. Sentinel lymph node detection following the hysteroscopic peritumoural injection of 99mTc-labelled albumin nanocolloid in endometrial cancer. Eur J Nucl Med Mol Imaging. 2005;32:569–74. doi: 10.1007/s00259-004-1709-4. [DOI] [PubMed] [Google Scholar]