Abstract
Background
The objectives of this randomized comparative effectiveness study conducted by members of the Practitioners Engaged in Applied Research and Learning (PEARL) Network were to determine whether using a resin-modified glass ionomer (RMGI) liner reduces postoperative hypersensitivity (POH) in dentin-bonded Class I and Class II resin-based composite (RBC) restorations, as well as to identify other factors (putative risk factors) associated with increased POH.
Methods
PEARL Network practitioner-investigators (P-Is) (n = 28) were trained to assess sensitivity determination, enamel and dentin caries activity rankings, evaluation for sleep bruxism, and materials and techniques used. The P-Is enrolled 341 participants who had hypersensitive posterior lesions. Participants were randomly assigned to receive an RBC restoration with or without an RMGI liner before P-Is applied a one-step, self-etching bonding agent. P-Is conducted sensitivity evaluations at baseline, at one and four weeks after treatment, and at all visits according to patient-reported outcomes.
Results
P-Is collected complete data regarding 347 restorations (339 participants) at baseline, with 341 (98 percent) (333 participants) recalled at four weeks. Treatment groups were balanced across baseline characteristics and measures. RBC restorations with or without an RMGI liner had the same one-week and four-week POH outcomes, as measured clinically (by means of cold or air stimulation) and according to patient-reported outcomes.
Conclusions
Use of an RMGI liner did not reduce clinically measured or patient-reported POH in moderate-depth Class I and Class II restorations. Cold and air clinical stimulation findings were similar between groups.
Practical Implications
The time, effort and expense involved in placing an RMGI liner in these moderate-depth RBC restorations may be unnecessary, as the representative liner used did not improve hypersensitivity outcomes.
Keywords: Postoperative hypersensitivity, sensitivity, resin-modified glass ionomer liner, resin-based composite, restorative dentistry, posterior restorations
The Practitioners Engaged in Applied Research and Learning (PEARL) Network is a Good Clinical Practice–based research network whose members, identified as practitioner-investigators (P-Is), voted to conduct a two-armed, randomized comparative effectiveness study to determine whether adding a resin modified glass ionomer (RMGI) liner eliminates or reduces postoperative hypersensitivity (POH) in dentin-bonded Class I or Class II resin-based composite (RBC) restorations, as well as to identify other factors (putative risk factors) associated with increased POH.
Postoperative hypersensitivity
POH is defined as pain associated with mastication or sensitivity to heat, cold and sweet foods or beverages that is present at one week or more after treatment and related to the tooth’s having undergone restoration. (Pain that occurs during clenching only, indicating a restoration in hyperocclusion, usually is excluded from the definition of POH.) This sensitivity can be measured clinically, by the participant’s own report (best done anonymously via survey) or both, and results of these measures have been shown to correspond.1
Managing POH can be an especially taxing proposition for clinicians because pinpointing its underlying trigger or triggers and predicting its occurrence can be complicated by several technical and material factors. Moreover, persistent POH may require retreatment, which has oral health–related quality-of-life (QoL) and financial implications for the patient and, later, for the dental practice. The results of a recent PEARL study showed substantial POH in patients queried anonymously after receiving an RBC restoration.2 At four weeks after restoration placement, approximately 18 percent of study teeth had appreciable hypersensitivity (AH) (as measured by the patient’s indication of 3 or higher on a 0- to 10-point pain scale), and 10 percent of study teeth with no baseline hypersensitivity developed AH after restoration. In study teeth with AH at baseline, only about 63 percent experienced elimination of AH after restoration.2 On further analysis, the study investigators found no relationship in AH outcomes between materials, including consideration of types of liners and bonding agents, and techniques used by the 45 dental practices involved.3 On the basis of these findings, PEARL clinicians were eager to determine whether including an RMGI liner would reduce POH more than would use of a dentin bonding agent (DBA) alone in RBC restorations. If it did not, reductions in both RBC restoration chair time and cost would be possible.
In addition, PEARL Network P-Is believed the study could provide valuable information as to possible risk factors for POH, which included enamel caries stage, radiographic lesion depth, dentin caries activity (DCA) ranking, preparation depth and sleep bruxism (SB). We proposed that SB may contribute to the fatigue of the internal bond, leading to gaps between the restorative material and the dentin (particularly the pulpal floor); fluid filling these gaps results in occlusal loading sensitivity.4 By identifying patients at risk of experiencing POH, clinicians might be able to take steps to manage these factors and reduce the incidence and severity of POH.
Published studies of patients who have experienced POH after receiving posterior RBC restorations have widely varying results, although most indicated some level of transient response among some proportion of patients. The majority of studies consisted of small samples and were associated with evaluating a particular bonding agent or RBC formulation, as we describe below.
RMGI liners
A significant number of PEARL Network dentists used RMGI or glass ionomer (GI) as a liner to reduce the possibility of POH.3,5 Evidence as to the effectiveness of such a liner is mixed. Comparing the use of an RMGI liner with the direct application of a DBA, Akpata and Sadiq6 found less patient-reported hypersensitivity seven days after treatment with the RMGI (22 percent) than with the DBA alone (47 percent). POH was reduced, respectively, to 10 percent and 26 percent among patients overall at 30 days. These results contrast with those of a combined Class I and Class II study of a packable RBC in which 4.8 percent (n = 12) of restorations had been replaced within three years (10 within the first six months) as a result of POH on mastication.7 According to the results of this study, 5 percent of Class I restorations failed as a result of this form of POH, and the majority of all failed restorations were those lined with GI. In addition to using RMGI, in a study examining the use of a calcium hydroxide (Ca[OH]2) liner in deep areas of the preparation, Turkun and colleagues8 found no instances of patient-reported hypersensitivity at six months, one year or three years after treatment in 16 Class I restorations (39 Class II restorations also were included). Investigators in studies involving dental patients who received posterior RBC restorations with or without an RMGI liner (no use of Ca[OH]2) found no difference in these treatment groups.9,10
Sobral and colleagues11 suggested that POH may occur regardless of the use of a liner or a DBA. In Class II restorations (three per patient, each with a different liner approach: DBA only or either an aldehyde-based desensitizer or chlorhexidine antibacterial treatment before placement of the DBA), the investigators found that all materials and techniques tested were associated with some degree of POH. The authors concluded, “Postoperative sensitivity resulting from Class II restorations using composite resin cannot be completely eliminated with the prior use of a dentinal desensitizer or a cavity disinfectant. In day-to-day clinical treatment, postoperative sensitivity may possibly be related to the technique employed” (that is, an RBC restoration).11
Opdam and colleagues12 included POH as a secondary outcome of interest in a study of premolar restorations scheduled for extraction in which they used two bonding agents and two composite placement techniques. During the first recall appointment, which occurred five to seven weeks after restoration placement, 14 percent of restorations exhibited hypersensitivity, whereas a surprising 56 percent of restorations exhibited occlusal loading (mastication) hypersensitivity.
Investigators in a 2010 study found that 3 percent (one each) of 35 Class I restorations of microhybrid, packable or nanofilled material were replaced within six months as a result of POH (evaluated at baseline and at two and six months after treatment).13 In another study of several variables, including random use of Ca(OH)2 liner on POH (123 patients with one restoration each), logistic regression showed no statistically significant influence of any of three variables—cavity depth, Ca(OH)2 liner and restorative material— on the occurrence of pain or hypersensitivity.14
In the results of the study completed by members of the PEARL Network, of which part 2 was published in 2013,3 more than 46 percent of restorations were placed with some type of liner, and 57 percent of the liners used were RMGI or GI types. The vast majority of the remaining 54 percent of restorations involved the use of DBA alone with no liner. There was no difference in the patient-reported AH outcomes between the two approaches or between three-step and self-etching bonding agents.3
There are limited scientific data regarding the relationships among a patient’s caries risk profile, DCA, treatments performed and patient-reported POH outcomes. Therefore, the PEARL Network’s randomized comparative effectiveness study was designed to provide critically important clinical and patient-centered data to substantiate treatment standards for a commonly encountered clinical problem: early Class I and Class II caries.
METHODS
The study was approved by the New York University School of Medicine institutional review board (IRB), New York City, and monitored by a data and safety monitoring board appointed by the National Institute of Dental and Craniofacial Research, National Institutes of Health. It required the recruitment and training of dentists in 34 PEARL Network practices, 28 of which enrolled patients.
The PEARL Network executive management team trained P-Is through use of videos, animated slide presentations and online quizzes in the identification of lesions, caries classification,15,16 measurement of hypersensitivity to air and cold, SB evaluation,17 DCA ranking18 and measurement of preparation dimensions and depth,2 as well as in treatment procedures. Study participants at baseline and at each recall appointment rated their hypersensitivity to air and cold stimuli by using the Numeric Pain Assessment Scale (NPAS)19 and completed questionnaires about stimuli that cause hypersensitivity and how the condition affects their QoL.18 In addition, participants were asked about their pain medication usage. P-Is in this study were limited to those who had engaged in at least one PEARL Network study, so they were experienced in administering participant surveys regarding hypersensitivity and QoL.
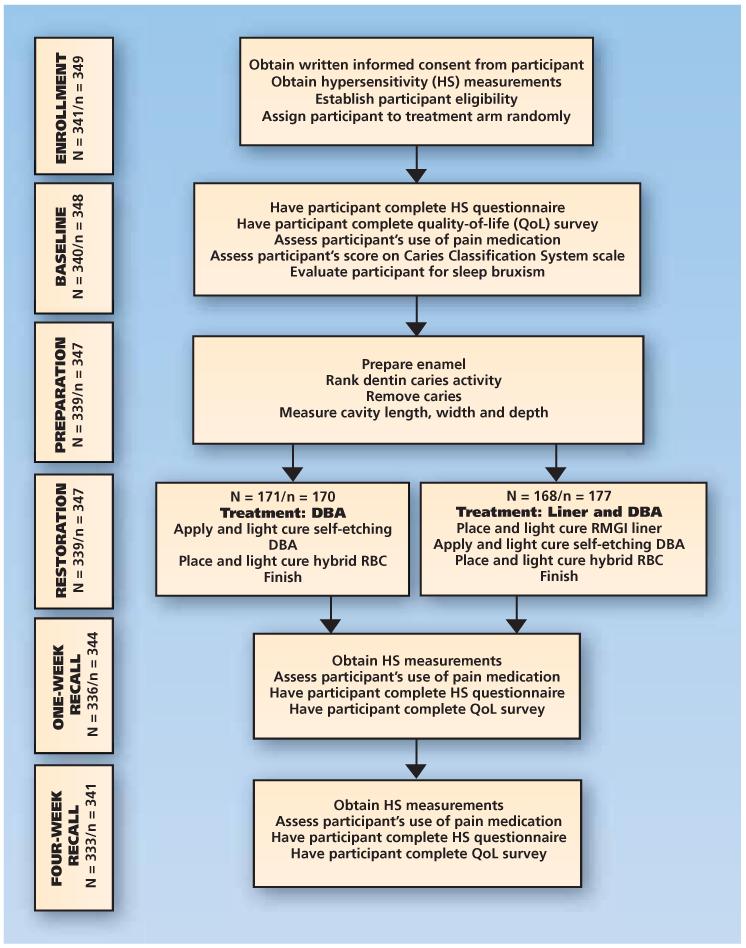
Figure 1 shows the study flow diagram, and Table 115 (page 890) shows the schedule of patient events. On enrollment, eligible participants were randomly assigned one to one (blocking within practice, using random block sizes) between the following treatment arms:
-
–
restoration with a DBA and a hybrid RBC, involving use of a one-step, self-etching DBA (Clearfil SE Bond, Kuraray America, Houston) followed by placement of an RBC (Herculite Ultra, Kerr/Sybron, Orange, Calif.), thus creating a state-of-the-art bonding and restoration system for Class I and Class II lesions. P-Is used light curing for all materials, with the light source having a minimum output of 400 milliwatts per square centimeter.
-
–
restoration involving the placement of an RMGI liner (Vitrebond Light Cure Glass Ionomer Liner/Base, 3M ESPE, St. Paul, Minn.) on the pulpal floor, the axial wall or both at a thickness of approximately 0.5 millimeter followed by application of the one-step, self-etching DBA and RBC. This RMGI is a self-adhesive formulation that can be light cured (as in this study) or self-cured over a longer period.
Figure 1.
Flow diagram of study events for Class I and Class II restoration treatment postoperative hypersensitivity outcomes, according to the randomized comparative effectiveness study protocol. N denotes the number of participants, and n denotes the number of study teeth. Asterisks indicate that participants in these categories were randomly assigned, underwent baseline assessments and were treated. Two participants (each having one tooth being used in the study) dropped out after the baseline assessment but before they received treatment. Of the 339 participants who received restorations (in one tooth in the study), one received a restoration but did not complete any baseline assessments before terminating her involvement in the study. Consequently, the effective sample size for the baseline comparisons was 338 participants, who contributed 346 teeth to the study, and the effective sample size for the primary outcome (week four) was 333 participants, who contributed 341 teeth to the study. DBA: Dentin bonding agent. RBC: Resin-based composite. RMGI: Resin-modified glass ionomer.
TABLE 1. Schedule of events and key data collected at each*.
| PROCEDURE | BASELINE | FOLLOW-UP | ||
|---|---|---|---|---|
|
| ||||
| Visit One | Visit Two | Visit Three | ||
|
| ||||
| Before intervention at baseline visit (day 0) |
Intervention at baseline visit (day 0) |
One week after restoration placement (day 7 ± 2†) |
Four weeks after restoration placement (day 28 ± 5†) |
|
|
| ||||
|
Signed Consent
Form |
X | |||
|
| ||||
| Oral Examination | ||||
| Complete | X | |||
| Air response | X | X | X | |
| Cold response | X | X | X | |
| Assessment of eligibility |
X | |||
| Sleep bruxism | X | |||
| Caries classification‡ |
X | |||
|
| ||||
|
Random
Assignment |
X | |||
|
| ||||
|
Pain Medication
Usage |
X | X | X | |
|
| ||||
| Patient Survey | ||||
| Hypersensitivity questionnaire |
X | X | X | |
| Quality of life survey |
X | X | X | |
|
| ||||
|
Intervention: Restoration of a Carious Lesion |
||||
| Caries activity ranked on opening of the lesion |
X | |||
| Preparation depth and width |
X | |||
| Treatment with DBA§ or with liner and DBA |
X | |||
|
| ||||
|
Assessment of
Adverse Events |
X | X | X | |
Empty cells indicate items that were not applicable.
The variable time frames indicate the recall window in each case.
According to the Caries Classification System (Fisher and colleagues15).
DBA: Dentin bonding agent.
Procedures
Baseline
PEARL Network P-Is and staff members identified patients who met the eligibility criteria by having one or more unrestored posterior teeth with Class I or Class II caries, as diagnosed clinically, with or without radiographic verification. They excluded lesions judged on radiographs to be greater than one-half the distance from the dentinoenamel junction (DEJ) to the pulp. We required that any diagnostic bitewing radiographs used were obtained no more than nine months previously. No more than one tooth per quadrant could be included in the study (see inclusion criteria below). The P-I then obtained written informed consent from patients.
P-Is evaluated each selected tooth for both air- and cold-stimulated hypersensitivity. Each P-I measured air hypersensitivity first by using a calibrated air syringe (as described by Perdigão and colleagues20) to administer a blast perpendicular to the occlusal plane in a gingival direction (height determined from calibration of the syringe, as described by Veitz-Keenan and colleagues1) for five seconds or until the participant indicated a sensation. In cases in which approximal caries was present, the P-I assessed hypersensitivity by additionally administering a buccal interproximal air blast at the same distance as that used for the calibrated occlusal method. A delay of one minute was required between tests to limit sensitization.
P-Is measured cold hypersensitivity by using a refrigerant (Hygenic Endo-Ice, Coltène/Whaledent, Altstatten, Switzerland) after a delay of at least one minute following the air test. The P-I sprayed the refrigerant onto a no. 2 cotton pellet until ice crystals formed on the pellet; he or she then placed the pellet on the occlusal surface of the tooth for 10 seconds or until the participant indicated a sensation by raising a hand. The P-I recorded the duration of application of the pellet to the tooth via a digital timer supplied to each P-I. P-Is assessed interproximal hypersensitivity by placing a similarly prepared iced cotton pellet over the interproximal area, approached from the occlusal aspect while protecting the occlusal surface and adjacent tooth by using fingers, cotton rolls or both.
Participants indicated on the NPAS19 the intensity of pain for each test. The P-Is enrolled only teeth with an NPAS score of 3 or greater for air, cold or both. If there was lingering pain (lasting longer than four seconds) after testing, the patient was not eligible. If the tooth or teeth (only one tooth per quadrant was permitted) met the eligibility criteria, the P-I enrolled the participant in the study and then assigned him or her randomly to a treatment arm.
Each tooth selected for the study underwent a five-part periodontal assessment:
-
■
scoring on a plaque index on which each tooth received a score ranging from 0 (no visible plaque) to 3 (plaque on more than 50 percent of the exposed tooth);
-
■
scoring on a gingival index;
-
■
calculus presence, reported as “yes” or “no”;
-
■
degree of inflammation, represented by bleeding on probing, reported as “yes” or “no”;
-
■
periodontal probing depths, using six measurements per tooth, covering all sides.
Participants’ preoperative hypersensitivity was determined by their filling out an NPAS questionnaire, a pain medication questionnaire related to analgesic usage and a baseline oral health QoL questionnaire, which was a modified version of the Oral Health Impact Profile (OHIP)-14.21 Aside from baseline data capture, the use of these questionnaires before the patient received treatment provided firsthand experience with the survey format and content that was administered at recall visits.
P-Is evaluated each tooth selected by using the Caries Classification System (CCS), and they ranked each tooth’s caries stage as 2, 4 or 6 (as described by Fisher and colleagues15 and Ismail and colleagues16).
P-Is conducted the clinical evaluation for evidence of SB according to American Academy of Sleep Medicine criteria.1,17 The P-I inspected the participant’s dentition for evidence of tooth wear to at least approaching dentin exposure. He or she also inspected the test tooth for wear and recorded any facets of more than 1 mm in diameter. The P-I also observed the participant for hypertrophy of masseter muscles on clenching and quizzed him or her as to awareness—his or her own or that of a partner—of tooth grinding during sleep.
Preparation
The P-I initiated cavity preparations and ranked DCA according to a visual-tactile scale18 (Table 2). He or she then completed the preparation. He or she recorded the extent of caries dentin removal (whether all of it was removed or some carious dentin was left in place). The P-I recorded the preparation’s depth (deepest and shallowest), width and length to the nearest millimeter.2,22 He or she inspected the pulpal floor and the axial wall for cracks, reporting findings by indicating “yes” or “no.”
TABLE 2. Baseline characteristics, according to treatment assignment and overall.
| CHARACTERISTIC | DBA* ONLY, n (%) |
RMGI† LINER, n (%) |
OVERALL, n (%) |
|---|---|---|---|
|
| |||
| Patient-Level Characteristics (n = 339) ‡ | |||
| Sleep bruxism | 48 (28) | 46 (27) | 94 (28) |
| Risk of experiencing future caries§ | |||
| Low | 75 (44) | 74 (44) | 149 (44) |
| Moderate | 62 (36) | 70 (42) | 132 (39) |
| High | 33 (19) | 24 (14) | 57 (17) |
|
| |||
| Tooth-Level Characteristics (n = 346) ¶ | |||
| Tooth type | |||
| Premolar | 65 (37) | 66 (39) | 131 (38) |
| Molar | 111 (63) | 104 (61) | 215 (62) |
|
Visual presence of crack in pulpal floor
dentin |
2 (1) | 2 (1) | 4 (1) |
| Gingival index | |||
| 0: Normal appearance, no bleeding | 136 (77) | 123 (72) | 259 (75) |
| 1: Mild inflammation; slight change in color; mild edema |
32 (18) | 42 (25) | 74 (21) |
| 2: Moderate inflammation; redness, edema and glazing; bleeding on probing |
8 (5) | 5 (3) | 13 (4) |
| 3: Severe inflammation; marked redness and edema; ulceration; spontaneous bleeding |
0 (0) | 0 (0) | 0 (0) |
| Class of lesion | |||
| I | 149 (85) | 141 (83) | 290 (84) |
| II | 27 (15) | 29 (17) | 56 (16) |
| Caries stage | |||
| 2: Visual change in enamel or cementum | 61 (35) | 64 (38) | 125 (36) |
| 4: Localized enamel breakdown, loss of cementum, dentin shadow |
88 (50) | 89 (52) | 177 (51) |
| 6: Distinct cavity with visible dentin, extensive lesion |
27 (15) | 17 (10) | 44 (13) |
| Radiographic visibility | |||
| Visible | 80 (45) | 81 (48) | 161 (47) |
| Not visible, but confirmed clinically | 95 (54) | 89 (52) | 184 (53) |
| Not visible | 1 (1) | 0 (0) | 1 (0) |
| Rank of dentin caries activity # | |||
| 1: Soft, serous | 31 (18) | 34 (20) | 65 (19) |
| 2: Soft, dry | 60 (34) | 55 (32) | 115 (33) |
| 3: Soft, dry, granular | 45 (26) | 49 (29) | 94 (27) |
| 4: Leathery and discolored | 19 (11) | 14 (8) | 33 (10) |
| 5: Firm, but discolored | 21 (12) | 18 (11) | 39 (11) |
| Extent of caries removal | |||
| Complete | 169 (96) | 164 (96) | 333 (96) |
| Partial | 7 (4) | 6 (4) | 13 (4) |
DBA: Dentin bonding agent.
RMGI: Resin-modified glass ionomer.
Missing data for one randomly assigned patient who terminated her involvement in the study before receiving treatment.
No definitions as to risk of future caries were provided to the practitioner-investigators.
Missing data for three randomly assigned participants who terminated their involvement in the study during the baseline visit.
According to scale described by Lehmann and colleagues.18
Restoration
P-Is completed restorations according to the treatment arm assigned and liner (if used). They cured DBA and RBC materials by using a calibrated light source (minimum 400 mW/cm2 radiometer [Demetron L.E.D. Radiometer, Kerr], supplied to all practices).
Recall
P-Is saw participants for evaluation at one and four weeks after treatment. This included hypersensitivity measurement involving the use of air and cold stimuli per baseline procedures. We considered lingering pain after removal of the stimulus an adverse event, necessitating the participant’s early termination of study involvement. During these recall evaluation visits, participants again completed the NPAS19 questionnaire regarding POH, the accessory questionnaire regarding analgesic usage and the OHIP survey.
Inclusion criteria
Participants had to have solely adult dentition; be 15 to 60 years of age (at ages older than 60 years, the pulp space is limited and POH less likely); be available for contact for at least four weeks after treatment; and be able to understand and willing to sign the IRB-approved informed consent form (for patients 18 years or younger, P-Is used an assent form). We required that one or more permanent posterior teeth (in different quadrants, with third molars excluded) have the clinical diagnosis of new Class I or Class II caries extending into dentin, with or without radiographic confirmation. Up to four teeth (one per quadrant) could be enrolled. The lesion depth, if visible on radiographs, had to be less than one-half the distance from the DEJ to the pulp, and we required that the radiograph have been obtained no more than nine months previously. In addition, the tooth had to be in occlusion with a natural tooth, be free of evidence of a pulpitis and require an RBC restoration as the accepted treatment for its lesion. The tooth’s baseline NPAS score for air stimulation, cold stimulation or both had to be 3 or higher, but the participant could not exhibit pain lasting more than four seconds.
Exclusion criteria
We excluded patients who had second molars that were not fully erupted; who were undergoing active orthodontic treatment (although we allowed patients who used retainers); who were currently enrolled in or had completed in the previous month a tooth-bleaching program or were participating in another ongoing dental research study; or who had prior reaction to or inability to tolerate any of the dental products being used, as demonstrated by severe topical or hypersensitivity reaction. Also excluded were people who were receiving treatment for medical disorders including dementia, Parkinson disease, severe depression, severe anxiety or any other medical condition that, in the P-I’s opinion, might affect the participant’s judgment of POH and ability to provide informed consent. If multiple teeth in a quadrant met the inclusion criteria, the patient was not eligible for inclusion: only one tooth per quadrant could be treated during the four weeks of the study. Also excluded were teeth that were mobile; had existing restoration(s); were assessed clinically as being fractured; served as an abutment for a removable partial denture; or displayed subgingival calculus, unless the calculus was removed during the treatment visit.
Statistical methods
The primary outcome measure was the greatest clinically measured sensitivity to cold and air stimuli at four weeks after treatment. For Class I lesions, we defined this as the highest reported pain score on the 0-10 scale in response to cold and air stimulation. For Class II lesions, we defined the stimulated pain score as the maximum reported sensitivity of both the occlusal and interproximal surfaces. We summarized categorical variables by using frequencies and percentages according to treatment assignment and overall. We summarized the continuous variables with a mean, a standard deviation, a minimum and a maximum. Owing to the zero inflation of all hypersensitivity measures, we considered several different distributions: the zero-inflated Poisson, the zero-inflated negative binomial and the β-binomial. For each measure of sensitivity, we selected the distribution with the lowest Akaike23 information criterion. Because the degree of zero inflation varies over time, we did not consider a joint model of both follow-up visits. Note that the primary outcome analysis was of the greatest clinically measured hypersensitivity at four weeks posttreatment. We fit one model for each visit for each of the following sensitivities: greatest sensitivity and clinically measured air and cold sensitivity, as well as self-reported sensitivities to cold, heat, sweet foods and beverages, chewing and clenching. Covariates included the corresponding baseline sensitivity and the treatment assignment. Owing to the large number of secondary analyses, we adjusted P values to control the false discovery rate at 5 percent by using the approach of Benjamini and Hochberg.24 We analyzed in this modeling only participants for whom we had data collected after baseline. In additional analyses, we evaluated the associations between putative risk factors (for example, deepest lesion depth, SB, caries stage, DCA ranking and radiographic visibility) and the 16 measures of POH, adjusting for baseline sensitivity. The false discovery rate was controlled at 5 percent for the 16 comparisons for each risk factor.
Sample size and power
The sample size was determined on the basis of a simulation study in which β-binomial and zero-inflated binomial distribution models were used. A sample size of 150 participants per arm would provide approximately 80 percent power to detect a shift in the distribution of sensitivity scores between treatments that would result in a 15 percentage point difference in the proportion of participants with complete elimination of sensitivity, while controlling for the type I error rate of less than 5 percent.
RESULTS
PEARL Network P-Is (n = 28) enrolled 341 patients who were randomly assigned to two groups, but three participants terminated their involvement in the study, two before receiving treatment and one at the baseline visit but after receiving treatment. Of the 339 participants who received treatment, 171 were randomly assigned to the DBA group and 168 to the RMGI group. The recall rates for these participants were 99 percent at one week and 98 percent at four weeks after treatment. This resulted in complete primary outcome data for 341 restorations. Only seven participants had multiple restorations—six with two and one with three— and all completed the study.
Table 2 presents baseline characteristics according to treatment assignment and overall. The groups were balanced as to baseline clinically measured air and cold hypersensitivity and caries classification. SB, DCA and caries removal were balanced between the treatment arms. The sex distribution also was balanced, with a 1.5 female-to-male ratio (not shown).
At baseline, a majority of patients (51 percent) had received a diagnosis of stage 4 caries, according to the classification used in our previous study.18 The P-Is ranked DCA as active (or rapidly progressing) (score of 1 or 2) in 52 percent of these lesions; this finding is in line with the majority’s being ranked as having active caries. The caries risk classification of these patients generally was low (44 percent) to moderate (39 percent).
There was evidence of SB in 28 percent of patients. This percentage is lower than that found in our noncarious cervical lesion study,1 in which 42 percent of participants had evidence of SB—something Ommerborn and colleagues25 considered to be a cofactor for the development of noncarious cervical lesions. The level of SB in this study was unexpectedly high, as only 12.1 percent of control participants in Ommerborn and colleagues’ study exhibited evidence of SB.
Complete caries removal occurred in 96 percent of teeth in the study. P-Is applied or placed, respectively, DBA and RBC, cured them and completed restorations in the 339 participants who began study treatment (recall that one participant dropped out after treatment, leaving 338 participants with baseline data).
The primary outcome of this study was the greatest week-4 pain level in response to air and cold stimulation (that is, maximum air-stimulated and cold-stimulated pain levels). In the case of Class II lesions, the outcome was the maximum of four clinically measured quantities: the occlusal and interproximal cold-stimulated pain scores and the occlusal and interproximal air-stimulated pain scores. Table 3 shows the outcomes for all participants with postrandomization data.
TABLE 3. Summary of clinically measured sensitivities according to visit and treatment assignment and overall.
| HYPERSENSITIVITY | DBA* ONLY | RMGI† LINER | OVERALL | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| n‡ | Mean (SD§) | Range¶ | n | Mean (SD) | Range¶ | n | Mean (SD) | Range¶ | |
|
| |||||||||
| Baseline | |||||||||
| Cold | |||||||||
| Occlusal | 176 | 4.8 (2.08) | 0-9 | 170 | 4.6 (2.05) | 0-9 | 346 | 4.7 (2.06) | 0-9 |
| Interproximal | 27 | 4.8 (2.50) | 0-9 | 29 | 5.4 (2.34) | 0-9 | 56 | 5.1 (2.42) | 0-9 |
| Overall | 176 | 4.9 (2.03) | 0-9 | 170 | 4.8 (2.05) | 0-9 | 346 | 4.9 (2.03) | 0-9 |
| Air | |||||||||
| Occlusal | 176 | 3.1 (1.90) | 0-8 | 170 | 3.3 (1.84) | 0-9 | 346 | 3.2 (1.87) | 0-9 |
| Interproximal | 27 | 3.7 (2.44) | 0-8 | 29 | 3.1 (1.73) | 0-8 | 56 | 3.4 (2.11) | 0-8 |
| Overall | 176 | 3.3 (1.90) | 0-8 | 170 | 3.4 (1.78) | 0-9 | 346 | 3.4 (1.84) | 0-9 |
| Greatest sensitivity | 176 | 5.3 (1.71) | 3-9 | 170 | 5.2 (1.70) | 3-9 | 346 | 5.2 (1.71) | 3-9 |
|
| |||||||||
| One Week After Restoration | |||||||||
| Cold | |||||||||
| Occlusal | 176 | 2.4 (2.45) | 0-10 | 168 | 2.2 (2.10) | 0-9 | 344 | 2.3 (2.29) | 0-10 |
| Interproximal | 27 | 2.3 (1.66) | 0-6 | 29 | 2.4 (1.95) | 0-7 | 56 | 2.3 (1.80) | 0-7 |
| Overall | 176 | 2.5 (2.43) | 0-10 | 168 | 2.3 (2.14) | 0-9 | 344 | 2.4 (2.30) | 0-10 |
| Air | |||||||||
| Occlusal | 176 | 0.8 (1.38) | 0-10 | 168 | 0.7 (1.03) | 0-4 | 344 | 0.8 (1.22) | 0-10 |
| Interproximal | 27 | 0.7 (1.10) | 0-4 | 29 | 1.1 (1.25) | 0-4 | 56 | 0.9 (1.19) | 0-4 |
| Overall | 176 | 0.8 (1.40) | 0-10 | 168 | 0.8 (1.09) | 0-4 | 344 | 0.8 (1.26) | 0-10 |
| Greatest sensitivity | 176 | 2.6 (2.37) | 0-10 | 168 | 2.4 (2.08) | 0-9 | 344 | 2.5 (2.23) | 0-10 |
|
| |||||||||
| Four Weeks After Restoration | |||||||||
| Cold | |||||||||
| Occlusal | 174 | 1.9 (2.17) | 0-9 | 167 | 1.6 (1.80) | 0-9 | 341 | 1.8 (2.00) | 0-9 |
| Interproximal | 27 | 3.0 (2.42) | 0-7 | 29 | 2.2 (2.01) | 0-6 | 56 | 2.6 (2.23) | 0-9 |
| Overall | 174 | 2.0 (2.20) | 0-9 | 167 | 1.7 (1.92) | 0-9 | 341 | 1.9 (2.07) | 0-9 |
| Air | |||||||||
| Occlusal | 174 | 0.5 (1.02) | 0-6 | 167 | 0.5 (1.18) | 0-8 | 341 | 0.5 (1.10) | 0-8 |
| Interproximal | 27 | 0.4 (0.80) | 0-3 | 29 | 0.8 (1.21) | 0-4 | 56 | 0.6 (1.04) | 0-4 |
| Overall | 174 | 0.5 (1.02) | 0-6 | 167 | 0.5 (1.22) | 0-8 | 341 | 0.5 (1.12) | 0-8 |
| Greatest sensitivity | 174 | 2.1 (2.19) | 0-9 | 167 | 1.8 (1.96) | 0-9 | 341 | 1.9 (2.08) | 0-9 |
DBA: Dentin bonding agent.
RMGI: Resin-modified glass ionomer.
n: Number of study teeth.
SD: Standard deviation.
Range: Minimum and maximum values.
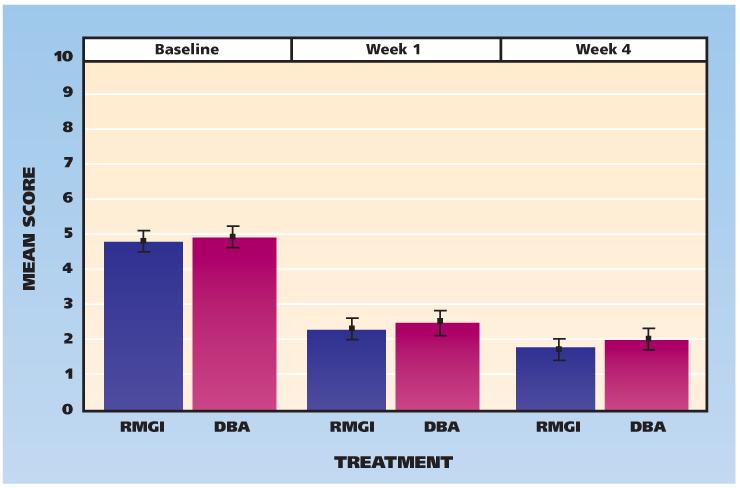
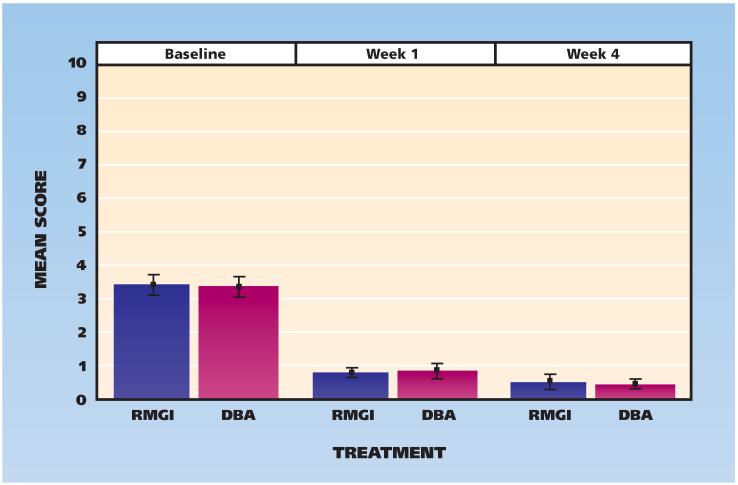
Figure 2 shows the baseline and one- and four-week results for clinically measured cold hypersensitivity; Figure 3 shows the results for air hypersensitivity. The clinically measured sensitivities to cold and air stimulation did not differ between groups.
Figure 2.
Scores for clinically measured hypersensitivity to cold, according to visit and treatment assignment (0 = no pain, 10 = worst pain). Whiskers indicate standard deviations from the mean. DBA: Dentin bonding agent. RMGI: Resin-modified glass ionomer.
Figure 3.
Scores for clinically measured hypersensitivity to air, according to visit and treatment assignment (0 = no pain, 10 = worst pain). Whiskers indicate standard deviations from the mean. DBA: Dentin bonding agent. RMGI: Resin-modified glass ionomer.
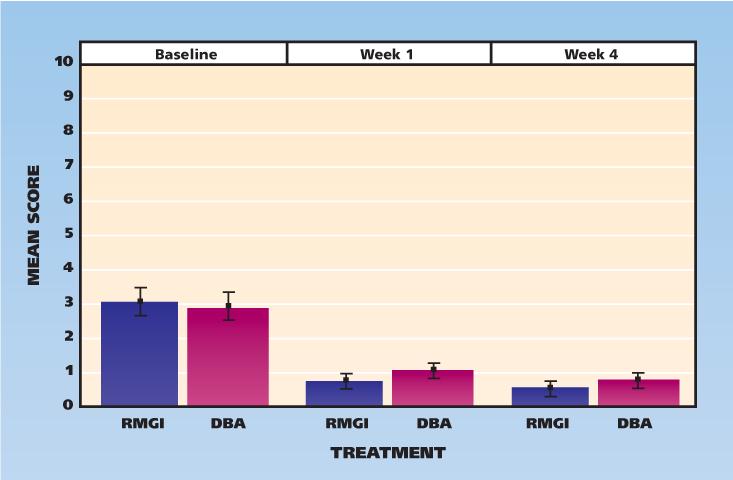
The secondary outcomes were patient-reported hypersensitivities, and these paralleled the clinically measured hypersensitivity outcomes. Patients reported the highest sensitivity to cold drinks or food (Figure 4). There was no statistically significant difference between the treatment groups for patient-reported outcomes.
Figure 4.
Participants’ scores for self-reported hypersensitivity to cold, according to visit and treatment assignment (0 = no pain, 10 = worst pain). Whiskers indicate standard deviations from the mean. DBA: Dentin bonding agent. RMGI: Resin-modified glass ionomer.
Table 4 (page 896) presents the modeling results for each hypersensitivity measure at each postoperative visit; the reported P values were adjusted so comparison could be made to a .05 significance level. There was no statistically significant difference (P < .05) between treatment groups for any POH measure at any point. POH was not associated significantly with lesion depth, SB, caries stage, DCA ranking or radiographic visibility (P < .18).
TABLE 4. Analytic results of modeling each type of hypersensitivity at each visit.
| WEEK | SENSITIVITY MEASURE |
MODELING DISTRIBUTION |
PREDICTOR VARIABLES IN MODEL |
|
|---|---|---|---|---|
| Baseline Sensitivity (P Value)* |
Treatment Arm (Adjusted P Value) |
|||
|
| ||||
| One | Greatest sensitivity | β-binomial | <.001 | .996 |
| Cold, measured | β-binomial | <.001 | .996 | |
| Air, measured | ZINB† | .001 | .996 | |
| Cold, self-reported | β-binomial | <.001 | .189 | |
| Hot, self-reported | ZIP‡ | <.001 | .996 | |
| Sweet, self-reported | β-binomial | <.001 | .958 | |
| Chewing, self-reported |
β-binomial | <.001 | .996 | |
| Clenching, self-reported |
β-binomial | <.001 | .981 | |
|
| ||||
| Four | Greatest sensitivity | β-binomial | <.001 | .442§ |
| Cold, measured | β-binomial | <.001 | .958 | |
| Air, measured | β-binomial | <.001 | .996 | |
| Cold, self-reported | β-binomial | <.001 | .671 | |
| Hot, self-reported | ZIP | <.001 | .996 | |
| Sweet, self-reported | β-binomial | <.001 | .252 | |
| Chewing, self-reported |
β-binomial | .003 | .958 | |
| Clenching, self-reported |
β-binomial | .018 | .996 | |
These P values were not adjusted for multiple comparisons because explicit testing of the relationship between baseline sensitivity and postoperative hypersensitivity (POH) was not intended. Baseline sensitivity was a highly significant predictor of POH.
ZINB: Zero-inflated negative binomial distribution.
ZIP: Zero-inflated Poisson distribution.
Because this is the primary outcome, the P value reported was not adjusted.
DISCUSSION
This study’s primary outcome of hypersensitivity at four weeks, clinically measured via air blast and cold sensitivity, exhibited no statistically significant difference between the treatment groups; both exhibited significantly reduced sensitivity, but the degree of reduction was not different. To our knowledge, this study is the first to be powered to detect a difference of 15 percentage points between treatment arms in the proportion of participants whose AH had been eliminated at four weeks after treatment. Our results led to the conclusion that among the 28 P-I practices and the 341 restorations (333 participants at four weeks), there was no evidence to support the use of an RMGI liner in moderate-depth lesions (mean greatest depth, 3.9 mm). Our results are supported by those of other studies of more limited sample size.9,10,26 Whether our results can apply to preparations approaching the pulp remains to be determined.
Critical to this study is the balance between the two randomized treatment groups before treatment. As detailed in Tables 2 and 3, there is a concordance between the treatment arms in all of the listed measures, but particularly in the cold and air response at baseline. Visual evidence of a crack in the pulpal floor, pulpal wall or both was found in 1 percent of the teeth in each group.
We compared the outcomes across the eight P-Is who had treated 15 or more teeth. There was no difference across these sites, with the medians of the clinically measured greatest sensitivity at four weeks being 2 or less in all instances; in some cases, the median was zero. This finding held for clinically measured cold and air sensitivity (a median of zero at all sites for the latter). This consistency across practices adds strength to our findings.
The economic impact of the findings cannot be dismissed. It is estimated that each dentist in North America placed an average of 575 RBC restorations in 2011 (80 percent of these in the posterior region), yielding about 460 relevant restorations (George Tysowski, DDS, PhD, Ivoclar Vivadent, Amherst, N.Y., unpublished data, March 10, 2012). Assuming 100,000 dentists are active in the United States and 40 percent of them use liners,3 our findings would apply to approximately 18.4 million posterior RBC restorations. If we propose that operatory overhead is $300 per hour and the time to place a liner and cure it is at best 30 seconds, then $2.50 would be saved when the clinician does not use a liner. Given a material cost of approximately $2.00 (assuming 100 applications per paste package) per restoration for the liner, the resulting total savings per restoration is $4.50. Thus, by not using liners in posterior restorations, U.S. dentists would save approximately $82.8 million per year. This equates conservatively to more than $2,000 per year for each dentist who no longer uses an RMGI liner for these restorations.
With regard to putative risk factors for POH, we did not find that the measured lesion depth was related to any hypersensitivity outcome differences between treatments. Although the number of Class II restorations in our study was limited (16 percent), the interproximal and occlusal POH outcomes were not different. Similarly, evidence of SB was not related to outcomes. The majority of lesions were stage 4 caries, defined as enamel breakdown with evidence of dentin involvement or enamel with an underlying shadow. The treatment arms were balanced in terms of participants’ caries stage, and caries stage was not related to hypersensitivity outcomes. We informally quizzed the P-Is as to whether they found the CCS easy to comprehend and use on the basis of the online learning modules we had provided. The majority indicated it was straightforward and easy to understand and apply. They found the laminated chairside reference sheet we had provided to be helpful. Their only suggestion was that the online quiz be expanded to include more lesions for practice in determining caries stage.
The treatment groups were balanced as to the P-Is’ ranking of risk of future caries development, with the majority of participants considered to be at low or moderate risk. We did not provide P-Is with a definition of risk of developing future caries. The presence of a carious lesion puts the patient at high risk of developing future caries, according to the accepted definition.16 Our findings indicate a troublingly wide variance in practitioners’ understanding of caries risk. Members of the PEARL Network recently reported on a randomly assigned case presentation study in which they found an analogous wide variance in periodontal diagnosis and treatment recommendations among practitioners.27
The treatment groups also were balanced with regard to participants’ DCA ranking, and we determined that rankings were not related to hypersensitivity outcomes. The DCA rankings we used can be grouped as rapidly progressing or slowly progressing.18 Of interest was that the distribution of lesion rankings at baseline for these 346 posterior teeth mirrored the distribution in our previous study, in which PEARL Network P-Is ranked 671 carious posterior teeth.18 The result is data regarding more than 1,000 teeth, the majority with occlusal lesions, of which 57 percent had what was deemed as slowly progressing caries. PEARL Network P-Is diagnosed these teeth as needing restoration on the basis of visual and radiographic appearance; given the DCA findings, however, they might have treated these teeth more conservatively.
Study limitations
The study as completed has several limitations. The caries was of limited depth, and the resulting preparation generally did not approach the pulp closely; thus, the results might not apply to deep lesions. Also, the study investigators used only a self-etching bonding agent, and the results might have differed with use of a total-etch bonding agent. However, findings from our previous study3 indicated no clinically meaningful difference in postoperative AH between use of total and selfetching DBAs in moderate-depth Class I restorations. We used a single brand of RMGI, a brand we believe to be popular with U.S. dentists, and results might differ with another product. Finally, although the study was limited to posterior teeth because we believed these were most likely to experience POH, we believe our results would apply to anterior restorations as well.
CONCLUSIONS
Our results indicate that in moderate-depth Class I and Class II restorations, use of an RMGI liner did not reduce clinically measured or patient-reported POH. Cold and air clinical stimulation findings were similar and were parallelled by the anonymous patient-reported sensitivities at each time point. These findings suggest that the time, effort and expense involved in placing an RMGI liner in RBC restorations may be unnecessary, as the representative liner used did not improve hypersensitivity outcomes.
Acknowledgments
This study was supported by grant U01-DE016755, which was awarded to the College of Dentistry, New York University, New York City, by the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, Md.
ABBREVIATION KEY
- AH
Appreciable hypersensitivity
- Ca(OH)2
Calcium hydroxide
- CCS
Caries Classification System
- DBA
Dentin bonding agent
- DCA
Dentin caries activity
- DEJ
Dentinoenamel junction
- GI
Glass ionomer
- HS
Hypersensitivity
- IRB
Institutional review board
- NPAS
Numeric Pain Assessment Scale
- OHIP
Oral Health Impact Profile
- PEARL
Practitioners Engaged in Applied Research and Learning
- P-I
Practitioner-investigator
- POH
Postoperative hypersensitivity
- QoL
Quality of life
- RBC
Resin-based composite
- RMGI
Resin-modified glass ionomer
- SB
Sleep bruxism
- ZINB
Zero-inflated negative binomial distribution
- ZIP
Zero-inflated Poisson distribution
Footnotes
Disclosure. None of the authors reported any disclosures.
Contributor Information
Dr. Brad Strober, Dentist in private practice in Edison, N.J.; practitioner-investigator in the Practitioners Engaged in Applied Research and Learning Network, New York City.
Dr. Analia Veitz-Keenan, Dentist in private practice in New York City; a clinical associate professor and the academic director, Department of Oral and Maxillofacial Pathology, Radiology and Medicine, College of Dentistry, New York University, New York City; practitioner-investigator in the Practitioners Engaged in Applied Research and Learning Network, New York City.
Dr. Julie Ann Barna, Dentist in private practice in Lewisburg, Pa.; practitioner-investigator in the Practitioners Engaged in Applied Research and Learning Network, New York City.
Dr. Abigail G. Matthews, Biostatistician, The EMMES Corporation, Rockville, Md.
Mr. Donald Vena, Statistician, The EMMES Corporation, Rockville, Md..
Dr. Ronald G. Craig, Associate professor, Department of Basic Sciences and Craniofacial Biology and Department of Periodontology and Implant Dentistry, College of Dentistry, New York University, New York City; director of information dissemination, Practitioners Engaged in Applied Research and Learning Network, New York City.
Dr. Frederick A. Curro, Clinical professor, Department of Oral and Maxillofacial Pathology, Radiology and Medicine, College of Dentistry, New York University, New York City; director of recruitment, retention and operations, Practitioners Engaged in Applied Research and Learning Network, New York City.
Dr. Van P. Thompson, Director of protocol development and training, Practitioners Engaged in Applied Research and Learning Network, and a professor and the chairperson, Department of Biomaterials and Biomimetics, College of Dentistry, New York University, New York City; professor, Biomaterials, Biomimetics and Biophotonics Research Group, The Dental Institute, King’s College London.
References
- 1.Veitz-Keenan A, Barna JA, Strober B, et al. Treatments for hypersensitive noncarious cervical lesions: A Practitioners Engaged in Applied Research and Learning (PEARL) Network randomized clinical effectiveness study. JADA. 2013;144(5):495–506. doi: 10.14219/jada.archive.2013.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berkowitz GS, Speilman H, Matthews A, et al. Postoperative hypersensitivity and its relationship to preparation variables in Class I resin-based composite restorations: findings from the Practitioners Engaged in Applied Research and Learning (PEARL) Network, part 1 Compend Contin Educ Dent. 2013;34(3):E44–E52. [PMC free article] [PubMed] [Google Scholar]
- 3.Blanchard P, Wong Y, Matthews A, et al. Restoration variables and postoperative hypersensitivity in Class I restorations: PEARL Network findings, part 2. Compend Contin Educ Dent. 2013;34(4):E62–E68. [PMC free article] [PubMed] [Google Scholar]
- 4.Miguez PA, Pereira PN, Foxton RM, Walter R, Nunes MF, Swift EJ., Jr. Effects of flowable resin on bond strength and gap formation in Class I restorations. Dent Mater. 2004;20(9):839–845. doi: 10.1016/j.dental.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Christensen GJ. Preventing postoperative tooth sensitivity in Class I, II and V restorations. JADA. 2002;133(2):229–231. doi: 10.14219/jada.archive.2002.0149. [DOI] [PubMed] [Google Scholar]
- 6.Akpata ES, Sadiq W. Post-operative sensitivity in glass-ionomer versus adhesive resin-lined posterior composites. Am J Dent. 2001;14(1):34–38. [PubMed] [Google Scholar]
- 7.Ernst CP, Martin M, Stuff S, Willershausen B. Clinical performance of a packable resin composite for posterior teeth after 3 years. Clin Oral Investig. 2001;5(3):148–155. doi: 10.1007/s007840100117. [DOI] [PubMed] [Google Scholar]
- 8.Turkun LS, Turkun M, Ozata F. Clinical performance of a packable resin composite for a period of 3 years. Quintessence Int. 2005;36(5):365–372. [PubMed] [Google Scholar]
- 9.Haller B, Trojanski A. Effect of multi-step dentin bonding systems and resin-modified glass ionomer cement liner on marginal quality of dentin-bonded resin composite Class II restorations. Clin Oral Investig. 1998;2(3):130–136. doi: 10.1007/s007840050058. [DOI] [PubMed] [Google Scholar]
- 10.Burrow MF, Banomyong D, Harnirattisai C, Messer HH. Effect of glass-ionomer cement lining on postoperative sensitivity in occlusal cavities restored with resin composite: a randomized clinical trial. Oper Dent. 2009;34(6):648–655. doi: 10.2341/08-098-C. [DOI] [PubMed] [Google Scholar]
- 11.Sobral MA, Garone-Netto N, Luz MA, Santos AP. Prevention of postoperative tooth sensitivity: a preliminary clinical trial. J Oral Rehabil. 2005;32(9):661–668. doi: 10.1111/j.1365-2842.2005.01479.x. [DOI] [PubMed] [Google Scholar]
- 12.Opdam NJ, Feilzer AJ, Roeters JJ, Smale I. Class I occlusal composite resin restorations: in vivo post-operative sensitivity, wall adaptation, and microleakage. Am J Dent. 1998;11(5):229–234. [PubMed] [Google Scholar]
- 13.Sadeghi M, Lynch CD, Shahamat N. Eighteen-month clinical evaluation of microhybrid, packable and nanofilled resin composites in Class I restorations. J Oral Rehabil. 2010;37(7):532–537. doi: 10.1111/j.1365-2842.2010.02073.x. [DOI] [PubMed] [Google Scholar]
- 14.Wegehaupt F, Betke H, Solloch N, Musch U, Wiegand A, Attin T. Influence of cavity lining and remaining dentin thickness on the occurrence of postoperative hypersensitivity of composite restorations. J Adhes Dent. 2009;11(2):137–141. [PubMed] [Google Scholar]
- 15.Fisher J, Glick M. FDI World Dental Federation Science Committee. A new model for caries classification and management: the FDI World Dental Federation caries matrix. JADA. 2012;143(6):546–551. doi: 10.14219/jada.archive.2012.0216. [DOI] [PubMed] [Google Scholar]
- 16.Ismail AI, Tellez M, Pitts NB, et al. Caries management pathways preserve dental tissues and promote oral health. Community Dent Oral Epidemiol. 2013;41(1):e12–e40. doi: 10.1111/cdoe.12024. [DOI] [PubMed] [Google Scholar]
- 17.American Academy of Sleep Medicine . The International Classification of Sleep Disorders, Revised: Diagnostic and Coding Manual. 2nd ed American Academy of Sleep Medicine; Westchester, Ill: 2005. [Google Scholar]
- 18.Lehmann M, Veitz-Keenan A, Matthews AG, et al. Dentin caries activity in early occlusal lesions selected to receive operative treatment: findings from the Practitioners Engaged in Applied Research and Learning (PEARL) Network. JADA. 2012;143(4):377–385. doi: 10.14219/jada.archive.2012.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Proceedings of the International Conference on Novel Anti-caries and Remineralizing Agents; Vina del Mar, Chile. January 10-12, 2008; [PubMed] [Google Scholar]; Adv Dent Res. 2009;21(1):3–89. [PubMed] [Google Scholar]
- 20.Perdigão J, Dutra-Correa M, Castilhos N, et al. One-year clinical performance of self-etch adhesives in posterior restorations. Am J Dent. 2007;20(2):125–133. [PubMed] [Google Scholar]
- 21.Slade GD, Spencer AJ. Development and evaluation of the Oral Health Impact Profile. Community Dent Health. 1994;11(1):3–11. [PubMed] [Google Scholar]
- 22.Yip KH, Poon BK, Chu FC, Poon EC, Kong FY, Smales RJ. Clinical evaluation of packable and conventional hybrid resin-based composites for posterior restorations in permanent teeth: results at 12 months. JADA. 2003;134(12):1581–1589. doi: 10.14219/jada.archive.2003.0103. [DOI] [PubMed] [Google Scholar]
- 23.Akaike H. In: Petrov BN, Csaki F, editors. Information theory and an extension of the maximum likelihood principle; Proceedings of the Second International Symposium on Information Theory; Budapest, Hungary: Akademiai Kiado. 1973.pp. 267–281. [Google Scholar]
- 24.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Statist Soc Series B. 1995;57(1):289–300. [Google Scholar]
- 25.Ommerborn MA, Schneider C, Giraki M, et al. In vivo evaluation of noncarious cervical lesions in sleep bruxism subjects. J Prosthet Dent. 2007;98(2):150–158. doi: 10.1016/S0022-3913(07)60048-1. [DOI] [PubMed] [Google Scholar]
- 26.Turkun LS, Aktener BO. Twenty-four-month clinical evaluation of different posterior composite resin materials. JADA. 2001;132(2):196–203. doi: 10.14219/jada.archive.2001.0155. [DOI] [PubMed] [Google Scholar]
- 27.Martin JA, Grill AC, Matthews AG, et al. Periodontal diagnosis affected by variation in terminology. J Periodontol. 2013;84(5):606–613. doi: 10.1902/jop.2012.110743. [DOI] [PMC free article] [PubMed] [Google Scholar]