Abstract
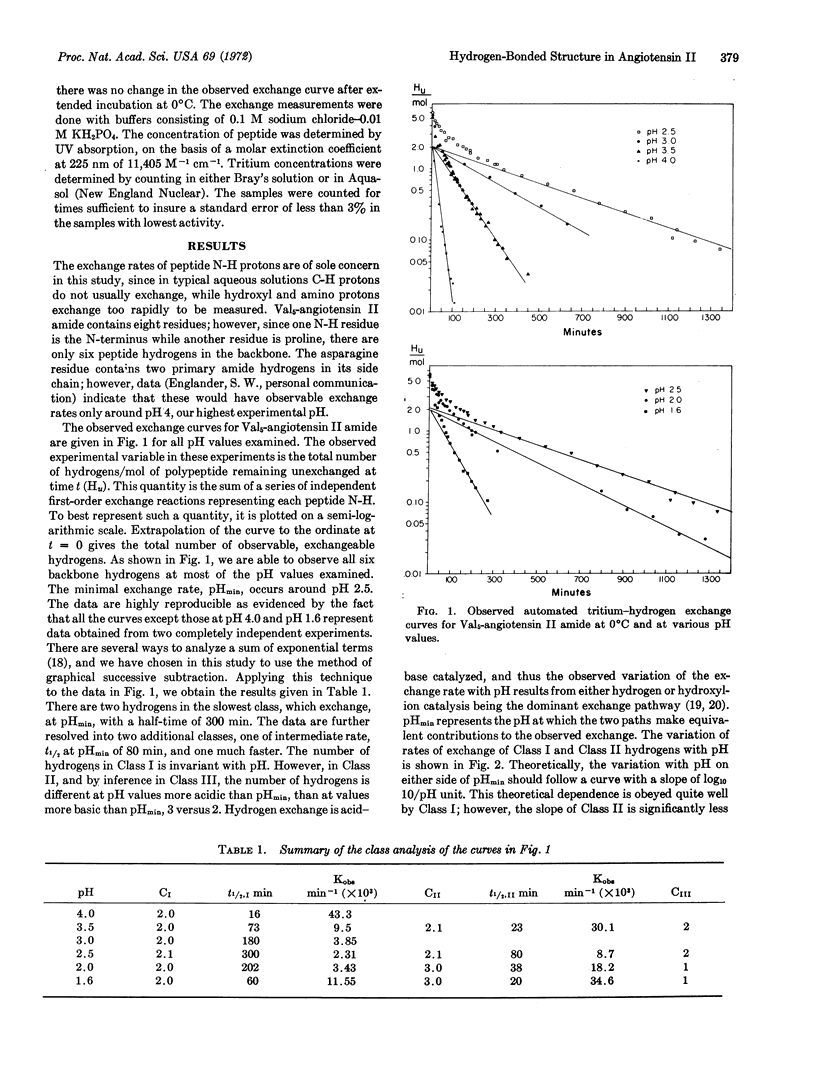
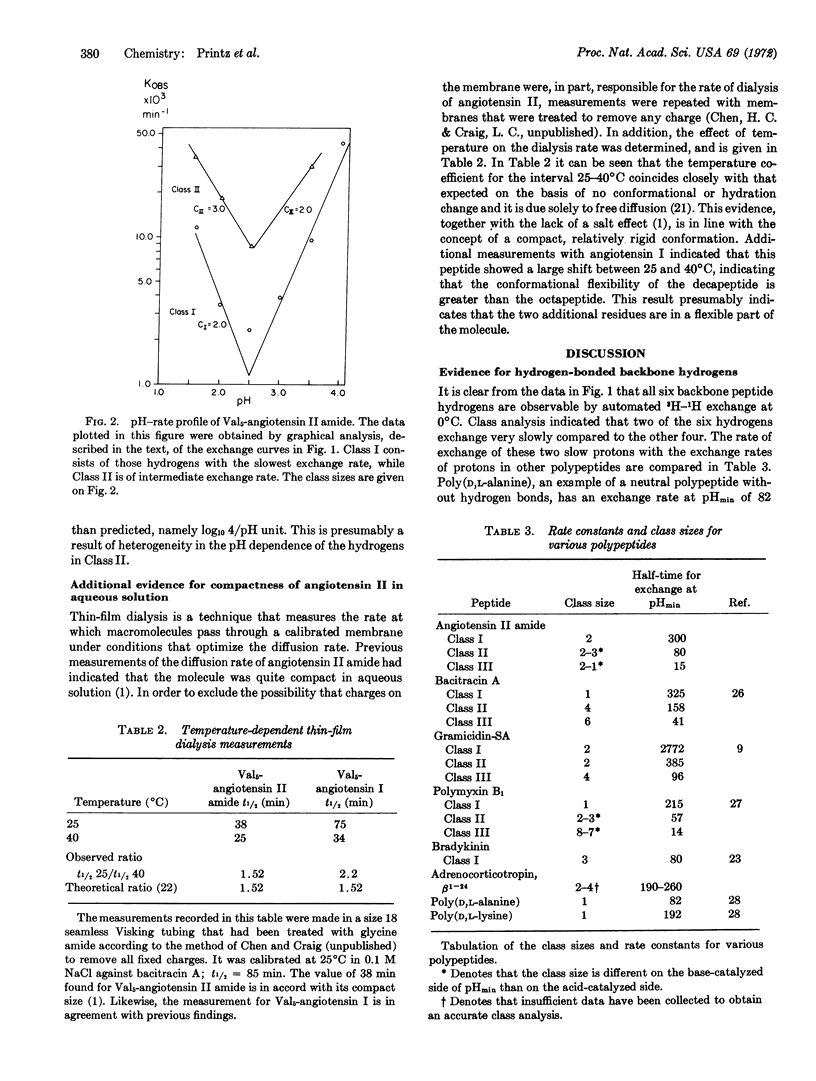
Automated tritium-hydrogen exchange measurements have been made on the linear octapeptide Val5-angiotensin II amide. All six amide hydrogens of the peptide backbone are observable, and are resolved into three classes according to their exchange rates. The rate of exchange of the slowest class, t1/2 of 300 min at 0°C (pH 2.5), is compared with that of hydrogens that exchange abnormally slowly in other peptides. It is concluded that these slow hydrogens in angiotensin II are involved in secondary structure with either one or both forming stable, intramolecular hydrogen bonds. This finding demonstrates that linear peptides may have hydrogen-bonded conformations in aqueous solutions. Analysis of the pH dependence of the rate of exchange indicates that one peptide amide hydrogen, namely that of the Asn1-Arg2 peptide bond, is not involved in hydrogen bonding and is freely accessible to the solvent. Thus, the finding of internal hydrogen bonding, together with the assignment of the environment of one peptide bond, places major constraints on the number of allowable conformations of this linear polypeptide hormone.
Keywords: tritium-hydrogen exchange, thin-film dialysis, polypeptide hormone, linear peptide, conformation
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brewster A. I., Bovey F. A. Conformation of cyclolinopeptide a observed by nuclear magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1199–1202. doi: 10.1073/pnas.68.6.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAIG L. C., HARFENIST E. J., PALADINI A. C. DIALYSIS STUDIES. 7. THE BEHAVIOR OF ANGIOTENSIN, OXYTOCIN, VASOPRESSIN, AND SOME OF THEIR ANALOGS. Biochemistry. 1964 Jun;3:764–769. doi: 10.1021/bi00894a005. [DOI] [PubMed] [Google Scholar]
- Craig L. C. Conformation studies with polypeptides by rotatory dispersion and thin-film dialysis. Proc Natl Acad Sci U S A. 1968 Sep;61(1):152–159. doi: 10.1073/pnas.61.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGLANDER S. W. A HYDROGEN EXCHANGE METHOD USING TRITIUM AND SEPHADEX: ITS APPLICATION TO RIBONUCLEASE. Biochemistry. 1963 Jul-Aug;2:798–807. doi: 10.1021/bi00904a030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englander S. W., Staley R. Measurement of the free and the H-bonded amides of myoglobin. J Mol Biol. 1969 Oct 28;45(2):277–295. doi: 10.1016/0022-2836(69)90105-3. [DOI] [PubMed] [Google Scholar]
- Ferreira A. T., Hampe O. G., Paiva A. C. The conformation of angiotensin II in aqueous solution. II. Dialysis and gel filtration behavior of [Asn 1-Val 5]-angiotensin II. Biochemistry. 1969 Aug;8(8):3483–3487. doi: 10.1021/bi00836a052. [DOI] [PubMed] [Google Scholar]
- Franze de Fernandez M. T., Delius A. E., Paladini A. C. Influence of pH on the conformation of angiotensin II and analog peptides. Biochim Biophys Acta. 1968 Jan 22;154(1):223–225. doi: 10.1016/0005-2795(68)90275-4. [DOI] [PubMed] [Google Scholar]
- Galardy R. E., Printz M. P., Craig L. C. Tritium--hydrogen exchange of bacitracin A. Evidence for an intramolecular hydrogen bond. Biochemistry. 1971 Jun 22;10(13):2429–2436. doi: 10.1021/bi00789a001. [DOI] [PubMed] [Google Scholar]
- HVIDT A. A DISCUSSION OF THE PH DEPENDENCE OF THE HYDROGEN-DEUTERIUM EXCHANGE OF PROTEINS. C R Trav Lab Carlsberg. 1964;34:299–317. [PubMed] [Google Scholar]
- Laiken S. L., Printz M. P., Craig L. C. Tritium-hydrogen exchange studies of protein models. I. Gramicidin S-A. Biochemistry. 1969 Feb;8(2):519–526. doi: 10.1021/bi00830a010. [DOI] [PubMed] [Google Scholar]
- Laiken S. L., Printz M. P. Kinetic class analysis of hydrogen-exchange data. Biochemistry. 1970 Mar 31;9(7):1547–1553. doi: 10.1021/bi00809a011. [DOI] [PubMed] [Google Scholar]
- PAIVA T. B., PAIVA A. C., SCHERAGA H. A. THE CONFORMATION OF ANGIOTENSIN II IN AQUEOUS SOLUTION. Biochemistry. 1963 Nov-Dec;2:1327–1334. doi: 10.1021/bi00906a026. [DOI] [PubMed] [Google Scholar]
- PALADINI A. C., DELIUS A. E., FRANZE DE FERNANDEZ M. T. Potentiation of the pressor effects of angiotensin in alkaline solution. Biochim Biophys Acta. 1963 Jul 2;74:168–170. doi: 10.1016/0006-3002(63)91353-2. [DOI] [PubMed] [Google Scholar]
- Printz M. P. Tritium-hydrogen exchange studies of polynucleotides. Double-stranded polyriboadenylic acid. Biochemistry. 1970 Jul 21;9(15):3077–3087. doi: 10.1021/bi00817a022. [DOI] [PubMed] [Google Scholar]
- SMEBY R. R., ARAKAWA K., BUMPUS F. M., MARSH M. M. A proposed conformation of isoleucyl-5-angiotensin II. Biochim Biophys Acta. 1962 Apr 23;58:550–557. doi: 10.1016/0006-3002(62)90065-3. [DOI] [PubMed] [Google Scholar]
- Tiffany M. L., Krimm S. New chain conformations of poly(glutamic acid) and polylysine. Biopolymers. 1968;6(9):1379–1382. doi: 10.1002/bip.1968.360060911. [DOI] [PubMed] [Google Scholar]



