Abstract
Introduction
We conducted this analysis to determine whether survival of advanced NSCLC patients treated with platin-based chemotherapy doublets involving paclitaxel, docetaxel or gemcitabine was dependent on histological subtypes and treatment regimen.
Methods
We retrospectively analyzed data from E1594, a front-line phase III study in which advanced NSCLC patients were randomized to receive one of four regimens: cisplatin-paclitaxel, cisplatin-gemcitabine, cisplatin-docetaxel, and carboplatin-paclitaxel. Patients were classified into four histology groups: squamous cell (SCC), adeno- (AC), large cell (LCC) and others including not otherwise specified (O/NOS) carcinoma. Logrank test was performed to compare overall survival (OS) and progression free survival (PFS) distributions according to histology as well as treatment.
Results
Of 1,139 patients including 716 men and 423 women, AC was the most common subtype (56.8%), followed by SCC (19.7%), O/NOS (17.0%) and LCC (6.5%). Men were more likely to have SCC and women were more likely to have AC (p=0.002). Among the four histology groups, there was no imbalance in regard to race, performance status, weight loss, brain metastasis or treatment. In each histology group, we found no significant difference in OS and PFS between the four chemotherapy regimens. Conversely, in each treatment arm, the survival outcome was similar between the four histology subtypes.
Conclusions
Our analysis suggests that histology does not predict survival benefit in advanced NSCLC patients treated with first-line platin-based doublets involving paclitaxel, docetaxel or gemcitabine.
Keywords: NSCLC, Histology, Prognostic, Survival, First-line Chemotherapy
INTRODUCTION
Non-small cell lung cancer (NSCLC) accounts for 85% of all lung cancer cases and is the leading cause of cancer death. It includes several histology subtypes, including squamous cell carcinoma (SCC), adenocarcinoma (AC), large cell carcinoma (LCC) and other less common types. Prior to the era of targeted drugs, cytotoxic chemotherapy was the only treatment option for patients with metastatic disease. Until recently, NSCLC patients were treated in the same way with same chemotherapy regimens regardless of histology subtypes. In 2002, the randomized phase III study E1594 demonstrated the equivalence in efficacy of the four platin-based regimens – cisplatin and paclitaxel (CP), cisplatin and gemcitabine (CG), cisplatin and docetaxel (CD), and carboplatin and paclitaxel (CbP).1 Response, one-year, two-year and overall survival was similar between the reference arm CP and each of the three experimental arms. Median survival of the CP arm was 7.8 months in comparison to 8.1 months (CG), 7.4 months (CD), and 8.1 months (CbP), and no arm was statistically significantly different from the others. Results of E1594 and other phase III studies during that period2-4 established platin-doublets involving a third generation cytotoxic drug (taxane, gemcitabine and vinorelbine) as the standard first-line treatment for advanced NSCLC.
In the last decade, the emergence of novel targeted drugs - notably the epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) gefitinib and erlotinib, the vascular endothelial growth factor (VEGF) inhibitor bevacizumab and most recently, the anaplastic lymphoma kinase (ALK) inhibitor crizotinib have changed the landscape of NSCLC therapy. Molecular and clinical investigation has demonstrated an association between tumor genotypes (EGFR mutations and ALK re-arrangement) and certain clinical phenotypes (adenocarcinoma, never smoking) with benefits from EGFR and ALK inhibitors. With the anti-angiogenic drug bevacizumab, due to hemorrhagic risks seen in SCC, it is approved for use with chemotherapy as first-line treatment in patients with non-squamous histology only.
Recent data with the cytotoxic drug pemetrexed suggested that histology could also influence outcome in NSCLC patients undergoing chemotherapy. Indeed, a subset analysis of a phase III study comparing cisplatin plus pemetrexed (CPem) with cisplatin plus gemcitabine showed that non-SCC patients lived longer with the pemetrexed-containing doublet, while SCC patients did better with the gemcitabine-containing regimen.5 Such clinical data suggested an interaction between histology and chemotherapy in NSCLC. Therefore, we performed this retrospective analysis using data of the study E1594 to determine whether survival of advanced NSCLC patients treated with first-line platin-based chemotherapy doublets involving paclitaxel, docetaxel or gemcitabine depends on histology subtypes.
PATIENTS AND METHODS
Patient Population
We analyzed data of 1,139 patients with advanced NSCLC enrolled in the phase III trial E1594. The inclusion and exclusion criteria and study treatment plan were previously described in the original report by Schiller at al.1 In short, eligible patients had stage IIIB with malignant pleural or pericardial effusion or stage IV or recurrent NSCLC of all histology subtypes; no prior chemotherapy; measurable or non-measurable disease; adequate hematologic, hepatic and renal function; and ECOG performance status 0-2. Patients with stable brain metastases were allowed. After 66 patients with patients with performance status 2 were enrolled, the study was amended to include those with ECOG 0-1 only. Patients were randomly assigned to one of the four platin-based chemotherapy regimens: CP (reference), CG, CD and CbP. Overall survival was the primary endpoint. As previously reported, there was no significant difference in clinical outcome between the reference arm CP and the three experimental arms.
Analytical Method
The primary objective of this retrospective analysis was to determine whether treatment outcome including overall survival (OS) and progression free survival (PFS) depends on histology subtypes. Overall survival was defined as the time from randomization to death from any cause; PFS was defined as the time from randomization to documented disease progression or death from any cause, whichever occurred first. Patients not experiencing an event of interest were censored at the last date of follow-up. Differences between histology and categorical variables were tested for using the chi-square test, and the Kruskal Wallis test was used for continuous variables. The logrank test was used to test for differences in event-time distributions. P-values are two-sided and considered significant at the 0.05 level. No adjustments have been made for multiple comparisons.
RESULTS
Patient Characteristics
Of 1,139 patients, the most common subtype was AC (n = 647; 56.8%), followed by SCC (n = 224; 19.7%), O/NOS (n = 194; 17.0%) and LCC (n = 74; 6.5%). Overall, 80.3% of patients (n = 915) had non-squamous cell carcinoma. Regarding treatment, there were 287 patients in the CP arm, 280 in CG arm, 286 in CD arm, and 286 in CbP arm. Baseline demographics and clinical characteristics of the four histological groups are shown in Table 1. There was a significant difference in gender distribution among the four histology groups, particularly between SCC and AC patients (p = 0.002). Men were accounted for 72.8% of SCC group, but only 59.0% in AC group. Although AC was still the most common histology subtype in both sexes, women overall had higher incidence of AC (62.6% vs. 53.4%) and lower incidence of SCC (14.4% vs. 22.8%) in comparison to men. There was also a significant difference in stage distribution with higher percentage of wet IIIB disease (15.6%) and less stage IV or recurrent disease (84.4%) in patients with AC in comparison with other histology groups (p = 0.03). Otherwise, there was no imbalance in regard of race, performance status, weight loss, brain metastasis or treatment among the four histology subtypes.
Table 1.
E1594: Patient Characteristics According to Histology
| Histology |
All Patients n = 1,139 |
||||
|---|---|---|---|---|---|
| Characteristics | Squamous Cell Carcinoma n = 224 |
Adenocarcinoma n = 647 |
Large Cell Carcinoma n = 74 |
Others/Not Otherwise Specified n = 194 |
|
| Age (year) | |||||
| Median | 63 | 61 | 63 | 61 | 61 |
| Range | 39-79 | 25-86 | 44-78 | 32-83 | 25-86 |
|
| |||||
| Sex (%) | |||||
| Male | 72.8 | 59.0 | 68.9 | 61.9 | 62.9 |
| Female | 27.2 | 41.0 | 31.1 | 38.1 | 37.1 |
|
| |||||
| Race (%) | |||||
| White | 86.5 | 85.6 | 89.2 | 84.0 | 85.7 |
| Black | 10.4 | 9.4 | 5.4 | 9.3 | 9.3 |
| Other | 3.1 | 5.0 | 5.4 | 6.7 | 4.9 |
|
| |||||
| Performance Status (%) | |||||
| 0 | 31.7 | 30.9 | 28.4 | 29.4 | 30.6 |
| 1 | 60.7 | 64.0 | 66.2 | 66.0 | 63.8 |
| 2 | 7.6 | 5.1 | 5.4 | 4.6 | 5.5 |
|
| |||||
| Brain metastases (%) | 10.7 | 13.6 | 14.9 | 8.2 | 12.2 |
|
| |||||
| Weight loss >5% (%) | 35.3 | 31.4 | 40.5 | 34.0 | 33.2 |
|
| |||||
| Disease Stage (%) | |||||
| IIIB | 12.1 | 15.6 | 10.8 | 7.7 | 13.3 |
| IV/Recurrent | 87.9 | 84.4 | 89.2 | 92.3 | 86.7 |
|
| |||||
| Treatment Regimens (%) | |||||
| Cisplatin-Paclitaxel | 26.8 | 26.3 | 17.6 | 22.7 | 25.2 |
| Cisplatin-Gemcitabine | 22.3 | 25.5 | 23.0 | 24.7 | 24.6 |
| Cisplatin-Docetaxel | 25.0 | 22.9 | 39.2 | 27.3 | 25.1 |
| Carboplatin-Paclitaxel | 25.9 | 25.3 | 20.3 | 25.3 | 25.1 |
Treatment Outcome
Treatment outcome including median PFS and median OS according to histology is shown in Table 2. Median OS of patients with SCC, AC, LCC, and O/NOS subtypes was 8.1 months, 8.3 months, 7.4 months and 7.2 months, while PFS was 3.6 months, 3.8 months, 4.2 months and 2.9 months, respectively. Overall, there was no significant difference in median OS (p = 0.36) or PFS (p = 0.08) among the four histology groups.
Table 2.
E1594: Treatment Outcome According to Histology
| Survival Outcome | Histology |
|||
|---|---|---|---|---|
| Squamous Cell Carcinoma n = 224 |
Adenocarcinoma n = 647 |
Large Cell Carcinoma n = 74 |
Others/Not Otherwise Specified n = 194 |
|
| Progression Free Survival - mo | ||||
| Median | 3.6 | 3.8 | 4.2 | 2.9 |
| 95% Confidence Interval | 3.0-4.1 | 3.5-4.2 | 2.7-5.6 | 2.6-3.7 |
|
| ||||
| Overall Survival - mo | ||||
| Median | 8.1 | 8.3 | 7.4 | 7.2 |
| 95% Confidence Interval | 7.1-9.5 | 7.5-9.0 | 6.1-9.7 | 6.1-8.6 |
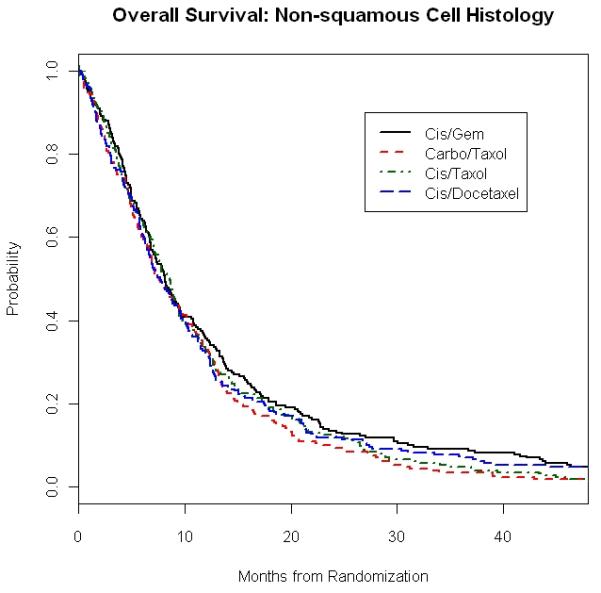
When we looked at outcome according to both histology and chemotherapy regimens, median OS was also similar within each of the four histology groups regardless of treatment given (Table 3). For example, in SCC patients, OS appeared numerically longer with CG (9.4 months) and CbP (9.3 months) than with CP (6.9 months) and CD (8.1 months). However, the difference was not statistically significant with p value of 0.18 (Figure 1A). There was also no significant difference in overall survival in patients with AC between the four chemotherapy regimens, with median OS of 9.1 months (CP), 8.1 months (CG), 7.7 months (CD), and 7.6 months (CbP) with p value of 0.39 (Table 3). Survival outcome to the four doublets was also similar in the combined non-squamous group of AC, LCC and O/NOS (Figure 1B).
Table 3.
E1594: Median Overall Survival According to Histology and Chemotherapy Regimens
| Regimens | Histology | |||
|---|---|---|---|---|
| Squamous Cell Carcinoma n = 224 |
Adenocarcinoma n = 647 |
Large Cell Carcinoma n = 74 |
Others/Not Otherwise Specified n = 194 |
|
| Cisplatin-Paclitaxel - mo (95% CI) | 6.9 (5.3-9.4) | 9.1 (7.9-10.9) | 6.1 (2.9-∞) | 6.0 (3.9-9.1) |
| Cisplatin-Gemcitabine - mo (95% CI) | 9.4 (5.7-15.6) | 8.1 (6.8-9.8) | 9.7 (4.5-17.1) | 7.9 (6.3-11.3) |
| Cisplatin-Docetaxel - mo (95% CI) | 8.1 (5.5-11.2) | 7.7 (6.5-9.4) | 6.8 (5.9-11.7) | 8.2 (5.6-12.4) |
| Carboplatin-Paclitaxel - mo (95% CI) | 9.3 (7.3-12.1) | 7.6 (6.6-9.8) | 8.3 (3.6-16.7) | 6.9 (4.9-11.6) |
CI, confidence interval
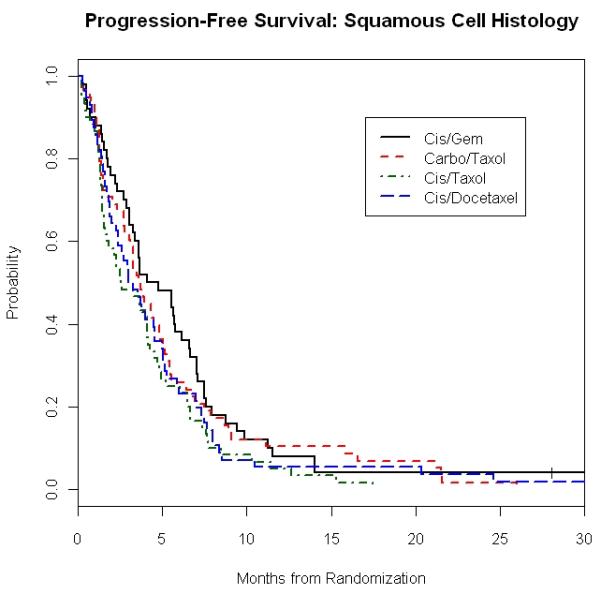
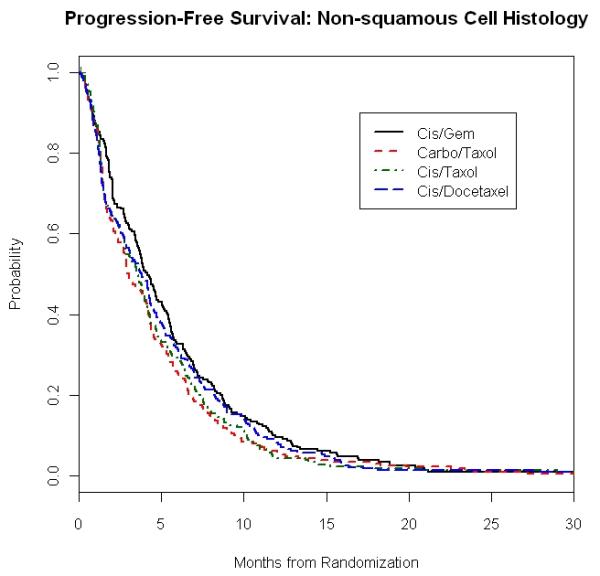
Figure 1. E1594: Overall Survival and Progression Free Survival in Squamous and Non-Squamous Carcinoma Patients According to Treatment Regimens.
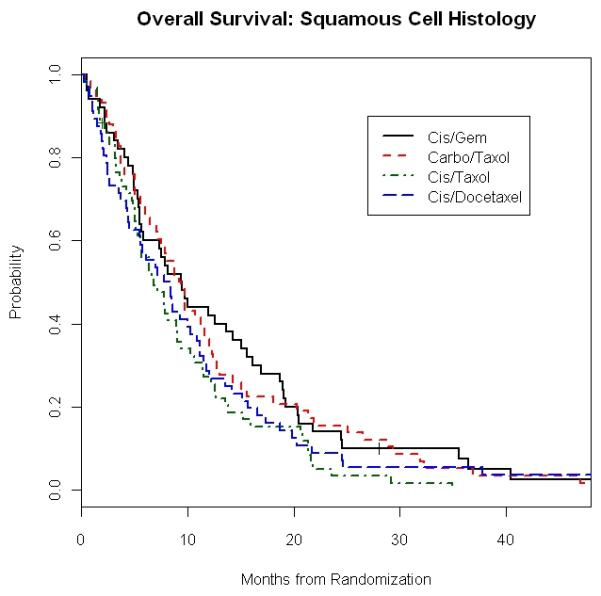
1A. Overall Survival of Squamous Cell Carcinoma Patients
1B. Overall Survival of Non-Squamous Carcinoma Patients
1C. Progression Free Survival of Squamous Carcinoma Patients
1D. Progression Free Survival of Non-Squamous Carcinoma Patients
No significant difference in survival was observed within each of the four chemotherapy arms regardless of histology subtype (Table 3). For instance, in the gemcitabine-containing arm, median OS of the four histology groups was 9.4 months (SCC), 8.1 months (AC), 9.7 months (LCC) and 7.9 months (O/NOS) with a p value of 0.63.
Median PFS according to histology and treatment is presented in Table 4. As with the case of OS, we found that in each histology group, PFS was not affected by the choice of chemotherapy. For example, patients with SCC treated with CP, CG, CD and CbP had a PFS of 2.6 months, 4.3 months, 3.1 months and 3.7 months, respectively (p = 0.20), while results in those with AC were 3.7 months, 4.4 months, 3.7 months and 3.5 months (p=0.19) (Figures 1C and 1D).
Table 4.
E1594: Median Progression Free Survival According to Histology and Chemotherapy Regimens
| Regimens | Histology | |||
|---|---|---|---|---|
| Squamous Cell Carcinoma n = 224 |
Adenocarcinoma n = 647 |
Large Cell Carcinoma n = 74 |
Others/Not Otherwise Specified n = 194 |
|
| Cisplatin-Paclitaxel - mo (95% CI) | 2.6 (1.7-4.2) | 3.7 (3.1-4.3) | 3.5 (1.4-∞) | 2.8 (1.8-4.0) |
| Cisplatin-Gemcitabine - mo (95% CI) | 4.3 (3.3-6.6) | 4.4 (3.8-5.4) | 4.5 (2.0-11.5) | 3.4 (2.8-5.1) |
| Cisplatin-Docetaxel - mo (95% CI) | 3.1 (2.4-5.0) | 3.7 (2.6-4.6) | 4.2 (2.9-6.6) | 3.6 (2.7-5.6) |
| Carboplatin-Paclitaxel - mo (95% CI) | 3.7 (3.0-5.0) | 3.5 (2.9-4.2) | 3.9 (1.9-7.8) | 2.2 (1.7-3.9) |
CI, confidence interval
PFS was also independent of histological subtype in each of the four chemotherapy arms. For example, the median PFS in the gemcitabine arm was 4.3 months (SCC), 4.4 months (AC), 4.5 months (LCC) and 3.4 months (O/NOS) with p value of 0.43.
DISCUSSION
Our retrospective analysis based on data of the phase III study E1594 suggested that histology does not influence survival outcome in advanced NSCLC patients treated with first-line platin-based chemotherapy doublets involving paclitaxel, docetaxel or gemcitabine. There was no significant difference in OS or PFS among the four platin-based doublet regimens in patients with squamous as well as non-squamous carcinomas.
The interaction between chemotherapy and NSCLC histology has also been retrospectively examined in other studies. Similar to our findings, analyses from those studies also suggested that histology does not predict survival outcome in NSCLC patients treated with platin-based, non-pemetrexed chemotherapy. In a randomized phase III study comparing three platinum-based doublets in 612 patients, there was no difference in median OS among the three arms of cisplatin and gemcitabine (9.8 months), carboplatin and paclitaxel (9.9 months) and cisplatin and vinorelbine (9.5 months).6 In addition, the study showed no significant treatment-by-histology interaction.7 In the randomized phase III study Global Lung Oncology Branch Trial 3 (GLOB3) comparing cisplatin plus vinorelbine with cisplatin plus docetaxel in 390 patients, the median OS of the vinorelbine arm was similar to that of docetaxel arm (9.9 months vs. 9.8 months, p = 0.58).8 Furthermore, subset analysis showed no difference in survival between the two treatment arms in patients with SCC (8.9 months vs. 9.8 months) and in those with AC (11.7 months vs. 11.6 months) (Table 5). Finally, an analysis based on Surveillance, Epidemiology and End Results (SEER) database of 2,644 patients with stage IIIB/IV NSCLC showed a similar survival, regardless of histology, in patients treated with cisplatin or carboplatin in combination with either gemcitabine or taxane (Table 5).9 Median OS of gemcitabine- and taxane-group was 8.2 months vs. 8.6 months respectively in SCC patients, and 8.1 months vs. 8.2 months in non-SCC patients.
Table 5.
Median Overall Survival According to Histology in Studies Involving Pemetrexed- and Non-Pemetrexed Regimens
| Studies | Setting | Phase | n | Treatment Regimens | Median Overall Survival by Histology (mo) | |
|---|---|---|---|---|---|---|
| SCC | Non-SCC | |||||
| Non-Pemetrexed Regimens | ||||||
| E1594 Schiller 20021 |
First-line | III | 1,139 | Cisplatin-Paclitaxel Cisplatin-Gemcitabine Cisplatin-Docetaxel Carboplatin-Paclitaxel |
6.9 9.4 8.1 9.3 p = NS |
9.1* 8.1* 7.7* 7.6* p = NS |
| GLOB3 Tan 20098 |
First-line | III | 381 | Cisplatin-Vinorelbine Cisplatin-Docetaxel |
8.9 9.8 p = NS |
11.7* 11.6* p = NS |
| Clements 20109 | First-line | SEER Analysis |
2,644 | Cisplatin/Carboplatin-Gemcitabine Cisplatin/Carboplatin -Taxane |
8.2 8.6 p = NS |
8.1 8.2 p = NS |
| Pemetrexed Regimens | ||||||
| Scagliotti 20085 | First-line | III | 1,725 | Cisplatin-Pemetrexed Cisplatin-Gemcitabine |
9.4 10.8 p = 0.05 |
11.8 10.4 p = 0.005 |
| Ciuleanu 200910 | Maintenance | III | 663 | Pemetrexed Placebo |
9.9 10.8 p = NS |
15.5 10.3 p = 0.002 |
| Hanna 200415,16 | Second-line | III | 571 | Pemetrexed Docetaxel |
6.2 7.4 p = 0.018 |
9.3 8.0 p = 0.048 |
Data of patients with adenocarcinoma subtype.
SEER, Surveillance, Epidemiology and End Results; NS, not significant (p<0.05).
On the other hand, emerging data have suggested that histology could be an important factor in selecting certain chemotherapy drugs such as pemetrexed (Table 5). Subset analysis of a recent randomized phase III study showed that CPem was better than CG in patients with non-squamous carcinoma with a median survival 11.8 months vs. 10.4 months, (p=0.005), while CG appeared superior in those with SCC (10.8 months vs. 9.4 months, p=0.05).5 Based on results of this trial, pemetrexed was approved for use with platinum as first-line therapy in patients with non-squamous NSCLC.
The association between pemetrexed and non-SCC histology in term of clinical benefit was also observed in maintenance setting. A phase III study involving 663 advanced NSCLC patients who had not progressed on first-line chemotherapy demonstrated that maintenance therapy with pemetrexed prolonged median OS (13.4 months vs. 10.6 months, p = 0.012) and PFS (4.3 months vs. 2.6 months, p < 0.0001) in comparison to placebo.10 The preplanned subset analysis showed that survival benefit from pemetrexed was achieved only in non-SCC patients with median OS of 15.5 months vs. 10.3 months (p = 0.002), but not in those with SCC histology. Therefore, maintenance therapy with pemetrexed is approved only in patients with non-SCC. Presumably, the inferior efficacy of pemetrexed in SCC patients is due to a difference in thymidylate synthase (TS) expression, the therapeutic target of this agent, among histology groups. Preclinical studies have shown a higher level of TS activity,11 TS messenger RNA and protein expression12 in SCC in comparison to non-SCC, as well as an inverse correlation between TS expression and sensitivity to pemetrexed in NSCLC models.13,14
Overall, clinical data suggested an inferior clinical outcome to pemetrexed in lung cancer patients with SCC subtype. On the other hand, our analysis based on E1594 as well as results from other studies showed that histology does not predict survival of advanced NSCLC treated with first-line platin-based regimens involving other third generation drugs such as paclitaxel, docetaxel, gemcitabine and vinorelbine. There are several limitations with all histology-and-outcome studies, including ours. First, all were unplanned, retrospective subset analyses. Second, some of histology subsets were small (such as large cell group) or not specified (NOS group).
CONCLUSIONS
Our analysis suggests that histology does not predict survival benefit in advanced NSCLC patients treated with first-line platin-based doublets involving paclitaxel, docetaxel or gemcitabine, and thus should not be used to determine treatment with one of the four regimens used here.
Acknowledgement
This study was conducted by the Eastern Cooperative Oncology Group (Robert L. Comis, M.D., Chair) and supported in part by Public Health Service Grants CA23318, CA66636, CA21115, CA21076, CA49957 and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Footnotes
Conflict of Interest Statement
Tien Hoang - Eastern Cooperative Oncology Group Grant to University of Wisconsin (Institutional Grant).
Suzanne E. Dahlberg - Eastern Cooperative Oncology Group Grant to Dana-Farber Cancer Institute (Institutional Grant).
Joan H. Schiller – Consultant (Bristol-Myers Squibb).
David H. Johnson – Eastern Cooperative Oncology Group Grant to Vanderbilt University (Institutional Grant).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Schiller JH, Harrington D, Belani CP, et al. Comparison of Four Chemotherapy Regimens for Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 2.Bonomi P, Kim K, Fairclough D, et al. Comparison of survival and quality of life in advanced non-small-cell lung cancer patients treated with two dose levels of paclitaxel combined with cisplatin versus etoposide with cisplatin: results of an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2000;18:623–31. doi: 10.1200/JCO.2000.18.3.623. [DOI] [PubMed] [Google Scholar]
- 3.Kelly K, Crowley J, Bunn PA, et al. Randomized Phase III Trial of Paclitaxel Plus Carboplatin Versus Vinorelbine Plus Cisplatin in the Treatment of Patients With Advanced Non-Small-Cell Lung Cancer: A Southwest Oncology Group Trial. J Clin Oncol. 2001;19:3210–3218. doi: 10.1200/JCO.2001.19.13.3210. [DOI] [PubMed] [Google Scholar]
- 4.Fossella F, Pereira JR, von Pawel J, et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX 326 study group. J Clin Oncol. 2003;21:3016–24. doi: 10.1200/JCO.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 5.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–51. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 6.Scagliotti GV, De Marinis F, Rinaldi M, et al. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol. 2002;20:4285–91. doi: 10.1200/JCO.2002.02.068. [DOI] [PubMed] [Google Scholar]
- 7.Scagliotti GV, De Marinis F, Rinaldi M, et al. The role of histology with common first-line regimens for advanced non-small cell lung cancer: a brief report of the retrospective analysis of a three-arm randomized trial. J Thorac Oncol. 2009;4:1568–71. doi: 10.1097/JTO.0b013e3181c06980. [DOI] [PubMed] [Google Scholar]
- 8.Tan EH, Rolski J, Grodzki T, et al. Global Lung Oncology Branch trial 3 (GLOB3): final results of a randomised multinational phase III study alternating oral and i.v. vinorelbine plus cisplatin versus docetaxel plus cisplatin as first-line treatment of advanced non-small-cell lung cance. Ann Oncol. 2009;20:1249–56. doi: 10.1093/annonc/mdn774. [DOI] [PubMed] [Google Scholar]
- 9.Clements KM, Peltz G, Faries DE, et al. Does Type of Tumor Histology Impact Survival among Patients with Stage IIIB/IV Non-Small Cell Lung Cancer Treated with First-Line Doublet Chemotherapy? Chemother Res Pract. 2010;2010:524629. doi: 10.1155/2010/524629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374:1432–40. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 11.Takezawa K, Okamoto I, Tsukioka S, et al. Identification of thymidylate synthase as a potential therapeutic target for lung cancer. Br J Cancer. 2010;103:354–61. doi: 10.1038/sj.bjc.6605793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ceppi P, Volante M, Saviozzi S, et al. Squamous cell carcinoma of the lung compared with other histotypes shows higher messenger RNA and protein levels for thymidylate synthase. Cancer. 2006;107:1589–96. doi: 10.1002/cncr.22208. [DOI] [PubMed] [Google Scholar]
- 13.Ozasa H, Oguri T, Uemura T, et al. Significance of thymidylate synthase for resistance to pemetrexed in lung cancer. Cancer Sci. 2010;101:161–6. doi: 10.1111/j.1349-7006.2009.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takezawa K, Okamoto I, Okamoto W, et al. Thymidylate synthase as a determinant of pemetrexed sensitivity in non-small cell lung cancer. Br J Cancer. 2011;104:1594–601. doi: 10.1038/bjc.2011.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–97. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 16.Peterson P, Park K, Fossella F, et al. Is pemetrexed more effective in adenocarcinoma and large cell lung cancer than in squamous cell carcinoma? A retrospective analysis of a phase III trial of pemetrexed vs docetaxel in previously treated patients with advanced non-small cell lung cancer. J of Thor Oncol. 2007;2:S851. [Google Scholar]


