Abstract
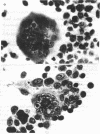
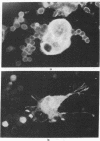
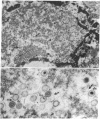
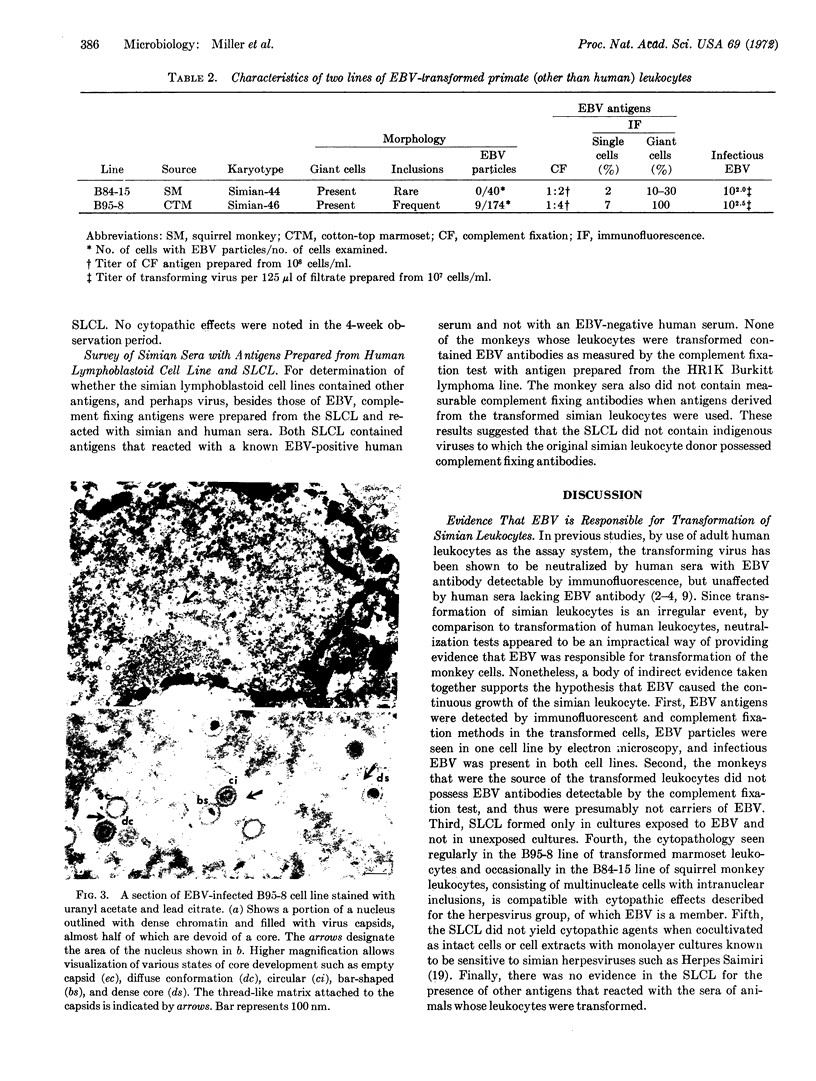
Blood leukocytes of two species of new world primates, other than human, transform following exposure to Epstein-Barr virus. The transformed simian cells produce Epstein-Barr virus antigens and infectious (transforming) virus. The simian lymphoblastoid cells form multinucleate giant cells that appear to be selective sites for the production of Epstein-Barr virus. Multinucleate cells reveal intranuclear inclusions; in both species, a large proportion of giant cells contain Epstein-Barr virus antigen detectable by immunofluorescence.
Keywords: human placental fibroblasts, complement fixation, immunofluorescence, electron microscopy, herpesvirus
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. A., Foley G. E., Frber S., Flowers A., Lazarus H., Hellerstein E. E. Serial transplantation of Burkitt's tumor (EB3) cells in newborn Syrian hamsters and its facilitation by antilymphocyte serum. Cancer Res. 1970 Feb;30(2):338–345. [PubMed] [Google Scholar]
- Adams R. A., Foley G. E., Uzman B. G., Farber S., Lazarus S., Kleinman L. Leukemia: serial transplantation of human leukemic lymphoblasts in the newborn Syrian hamster. Cancer Res. 1967 Apr;27(4):772–783. [PubMed] [Google Scholar]
- Adams R. A., Hellerstein E. E., Pothier L., Foley G. E., Lazarus H., Stuart A. B. Malignant potential of a cell line isolated from the peripheral blood in infectious mononucleosis. Cancer. 1971 Mar;27(3):651–658. doi: 10.1002/1097-0142(197103)27:3<651::aid-cncr2820270321>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Blacklow N. R., Watson B. K., Miller G., Jacobson B. M. Mononucleosis with heterophil antibodies and EB virus infection. Acquisition by an elderly patient in hospital. Am J Med. 1971 Oct;51(4):549–552. doi: 10.1016/0002-9343(71)90260-9. [DOI] [PubMed] [Google Scholar]
- Gerber P., Birch S. M. Complement-fixing antibodies in sera of human and nonhuman primates to viral antigens derived from Burkitt's lymphoma cells. Proc Natl Acad Sci U S A. 1967 Aug;58(2):478–484. doi: 10.1073/pnas.58.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber P. Studies on the transfer of subviral infectivity from SV40-induced hamster tumor cells to indicator cells. Virology. 1966 Apr;28(4):501–509. doi: 10.1016/0042-6822(66)90234-0. [DOI] [PubMed] [Google Scholar]
- Gerper P., Whang-Peng J., Monroe J. H. Transformation and chromosome changes induced by Epstein-Barr virus in normal human leukocyte cultures. Proc Natl Acad Sci U S A. 1969 Jul;63(3):740–747. doi: 10.1073/pnas.63.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogan E. A., Enders J. F., Miller G. Trypsinized placental cell cultures for the propagation of viruses and as "feeder layers". J Virol. 1970 Mar;5(3):406–409. doi: 10.1128/jvi.5.3.406-409.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle G., Henle W. Immunofluorescence in cells derived from Burkitt's lymphoma. J Bacteriol. 1966 Mar;91(3):1248–1256. doi: 10.1128/jb.91.3.1248-1256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Diehl V., Kohn G., Zur Hausen H., Henle G. Herpes-type virus and chromosome marker in normal leukocytes after growth with irradiated Burkitt cells. Science. 1967 Sep 1;157(3792):1064–1065. doi: 10.1126/science.157.3792.1064. [DOI] [PubMed] [Google Scholar]
- Meléndez L. V., Hunt R. D., Daniel M. D., García F. G., Fraser C. E. Herpesvirus saimiri. II. Experimentally induced malignant lymphoma in primates. Lab Anim Care. 1969 Jun;19(3):378–386. [PubMed] [Google Scholar]
- Miller G., Lisco H., Kohn H. I., Stitt D., Enders J. F. Establishment of cell lines from normal adult human blood leukocytes by exposure to Epstein-Barr virus and neutralization by human sera with Epstein-Barr virus antibody. Proc Soc Exp Biol Med. 1971 Sep;137(4):1459–1465. doi: 10.3181/00379727-137-35810. [DOI] [PubMed] [Google Scholar]
- Pope J. H., Horne M. K., Scott W. Identification of the filtrable leukocyte-transforming factor of QIMR-WIL cells as herpes-like virus. Int J Cancer. 1969 May 15;4(3):255–260. doi: 10.1002/ijc.2910040302. [DOI] [PubMed] [Google Scholar]
- Pope J. H., Horne M. K., Scott W. Transformation of foetal human keukocytes in vitro by filtrates of a human leukaemic cell line containing herpes-like virus. Int J Cancer. 1968 Nov 15;3(6):857–866. doi: 10.1002/ijc.2910030619. [DOI] [PubMed] [Google Scholar]
- Speir R. W., Wood O., Liebhaber H., Buckley S. M. Lassa fever, a new virus disease of man from West Africa. IV. Electron microscopy of Vero cell cultures infected with Lassa virus. Am J Trop Med Hyg. 1970 Jul;19(4):692–694. doi: 10.4269/ajtmh.1970.19.692. [DOI] [PubMed] [Google Scholar]
- Steplewski Z., Knowles B. B., Koprowski H. The mechanism of internuclear transmission of SV40-induced complement fixation antigen in heterokaryocytes. Proc Natl Acad Sci U S A. 1968 Mar;59(3):769–776. doi: 10.1073/pnas.59.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Enders J. F. Feline herpesvirus infection in fused cultures of naturally resistant human cells. J Virol. 1969 May;3(5):469–476. doi: 10.1128/jvi.3.5.469-476.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]