Abstract
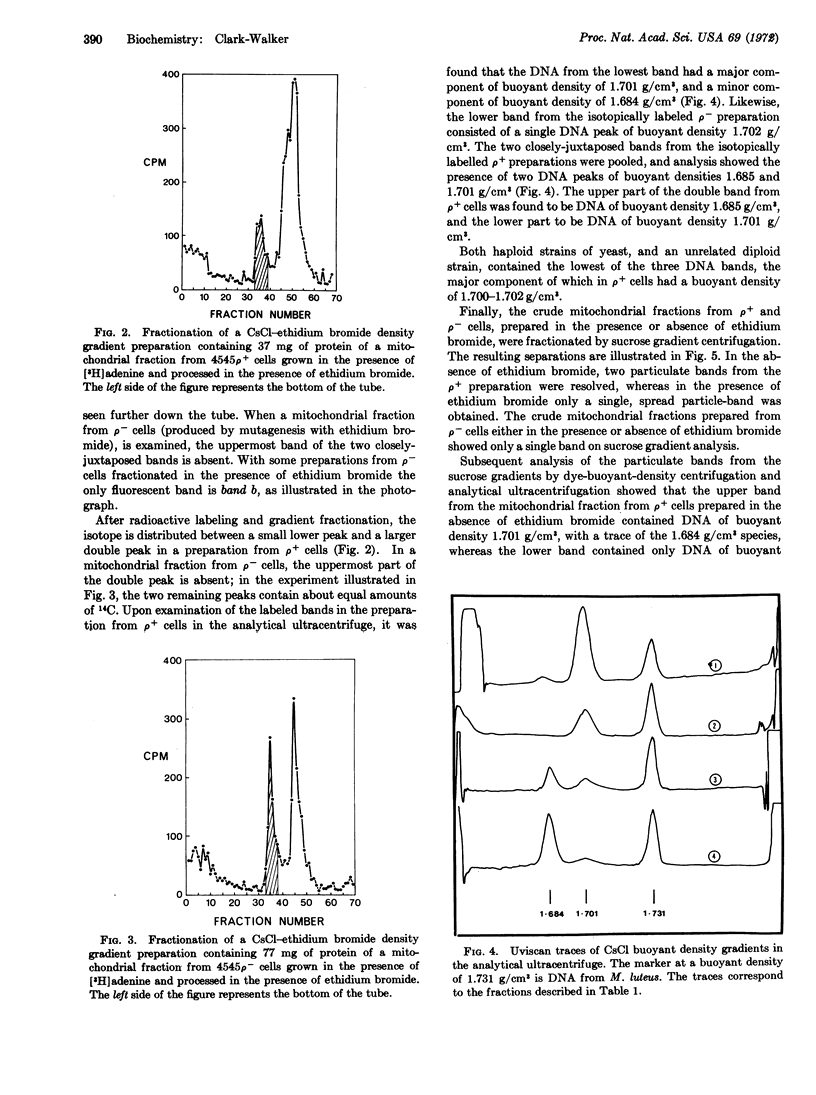
Breakage and fractionation of respiratory competent yeast in the presence of ethidium bromide, and subsequent centrifugation of a detergent lysate of the mitochondrial fraction by the dye-buoyant-density technique, results in the isolation of closed-circular DNA. After removal of bound dye, this DNA has two components when analyzed by equilibrium buoyant density in the analytical ultracentrifuge. A minor component has a buoyant density of 1.684 g/cm3, which is characteristic of mitochondrial DNA, but the major component has a buoyant density of 1.701 g/cm3. This species of DNA is also present in yeast that have been mutagenized to respiratory deficiency in the presence of the highest concentration of ethidium bromide compatible with cell growth. The closed-circular DNA of buoyant density 1.701 g/cm3, and free of linear DNA, is associated with the sole particulate band obtained on sucrose gradient centrifugation of a mitochondrial preparation from respiratory-deficient cells. Two particulate bands are obtained on sucrose gradient centrifugation of a mitochondrial preparation from respiratory-competent cells, the upper band containing DNA of buoyant density 1.701 g/cm3 and the lower band DNA of buoyant density 1.684 g/cm3. The suggestion is advanced, in view of the reputed sedimentation behaviour of yeast peroxisomes, that the closed-circular DNA of buoyant density 1.701 g/cm3 may be located in peroxisomes.
Keywords: dye-buoyant-density centrifugation, analytical ultracentrifugation, detergent lysate, petite mutant
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazaral M., Helinski D. R. Circular DNA forms of colicinogenic factors E1, E2 and E3 from Escherichia coli. J Mol Biol. 1968 Sep 14;36(2):185–194. doi: 10.1016/0022-2836(68)90374-4. [DOI] [PubMed] [Google Scholar]
- Clark-Walker G. D., Linnane A. W. The biogenesis of mitochondria in Saccharomyces cerevisiae. A comparison between cytoplasmic respiratory-deficient mutant yeast and chlormaphenicol-inhibited wild type cells. J Cell Biol. 1967 Jul;34(1):1–14. doi: 10.1083/jcb.34.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corneo G., Moore C., Sanadi D. R., Grossman L. I., Marmur J. Mitochondrial DNA in yeast and some mammalian species. Science. 1966 Feb 11;151(3711):687–689. doi: 10.1126/science.151.3711.687. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R., Kelly R. B., Kornberg A. A minute circular DNA from Escherichia coli 15. Proc Natl Acad Sci U S A. 1968 Jul;60(3):992–999. doi: 10.1073/pnas.60.3.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring E. S., Grossman L. I., Krupnick D., Cryer D. R., Marmur J. The petite mutation in yeast. Loss of mitochondrial deoxyribonucleic acid during induction of petites with ethidium bromide. J Mol Biol. 1970 Sep 14;52(2):323–335. doi: 10.1016/0022-2836(70)90033-1. [DOI] [PubMed] [Google Scholar]
- Hoffmann H. P., Szabo A., Avers C. J. Cytochemical localization of catalase activity in yeast peroxisomes. J Bacteriol. 1970 Oct;104(1):581–584. doi: 10.1128/jb.104.1.581-584.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg C. P., Borst P., van Bruggen E. F. Mitochondrial DNA. V. A 25 micron closed circular duplex DNA molecule in wild-type yeast mitochondria. Stucture and genetic complexity. Biochim Biophys Acta. 1970 May 21;209(1):1–15. [PubMed] [Google Scholar]
- Hudson B., Upholt W. B., Devinny J., Vinograd J. The use of an ethidium analogue in the dye-buoyant density procedure for the isolation of closed circular DNA: the variation of the superhelix density of mitochondrial DNA. Proc Natl Acad Sci U S A. 1969 Mar;62(3):813–820. doi: 10.1073/pnas.62.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacroute F. Non-Mendelian mutation allowing ureidosuccinic acid uptake in yeast. J Bacteriol. 1971 May;106(2):519–522. doi: 10.1128/jb.106.2.519-522.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent M., Steinert M. Electron microscopy of kinetoplastic DNA from Trypanosoma mega. Proc Natl Acad Sci U S A. 1970 Jun;66(2):419–424. doi: 10.1073/pnas.66.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounolou J. C., Jakob H., Slonimski P. P. Mitochondrial DNA from yeast "petite" mutants: specific changes in buoyant density corresponding to different cytoplasmic mutations. Biochem Biophys Res Commun. 1966 Jul 20;24(2):218–224. doi: 10.1016/0006-291x(66)90723-6. [DOI] [PubMed] [Google Scholar]
- Nagley P., Linnane A. W. Mitochondrial DNA deficient petite mutants of yeast. Biochem Biophys Res Commun. 1970 Jun 5;39(5):989–996. doi: 10.1016/0006-291x(70)90422-5. [DOI] [PubMed] [Google Scholar]
- Pikó L., Blair D. G., Tyler A., Vinograd J. Cytoplasmic DNA in the unfertilized sea urchin egg: physical properties of circular mitochondrial DNA and the occurrence of catenated forms. Proc Natl Acad Sci U S A. 1968 Mar;59(3):838–845. doi: 10.1073/pnas.59.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich E., Luck D. J. Replication and inheritance of mitochondrial DNA. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1600–1608. doi: 10.1073/pnas.55.6.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHERMAN F. Respiration-deficient mutants of yeast. I. Genetics. Genetics. 1963 Mar;48:375–385. doi: 10.1093/genetics/48.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L., Grossman L. I., Marmur J., Kleinschmidt A. K. Physical studies on the structure of yeast mitochondrial DNA. J Mol Biol. 1968 May 14;33(3):907–922. doi: 10.1016/0022-2836(68)90327-6. [DOI] [PubMed] [Google Scholar]
- Sinclair J. H., Stevens B. J., Sanghavi P., Rabinowitz M. Mitochondrial-satellite and circular DNA filaments in yeast. Science. 1967 Jun 2;156(3779):1234–1237. doi: 10.1126/science.156.3779.1234. [DOI] [PubMed] [Google Scholar]
- Szabo A. S., Avers C. J. Some aspects of regulation of peroxisomes and mitochondria in yeast. Ann N Y Acad Sci. 1969 Dec 19;168(2):302–312. doi: 10.1111/j.1749-6632.1969.tb43117.x. [DOI] [PubMed] [Google Scholar]
- Tewari K. K., Vötsch W., Mahler H. R., Mackler B. Biochemical correlates of respiratory deficiency. VI. Mitochondrial DNA. J Mol Biol. 1966 Oct;20(3):453–481. doi: 10.1016/0022-2836(66)90003-9. [DOI] [PubMed] [Google Scholar]