Abstract
Ceftolozane/tazobactam: a new option in the treatment of complicated gram-negative infections
INTRODUCTION
One of the major causes of antimicrobial resistance is the overutilization of antimicrobial therapy. Annually, two million people in the United States present with infections resistant to at least one antimicrobial agent of choice typically used to treat that type of infection.1 In treating antimicrobial-resistant infections compared with non–antimicrobial-resistant infections, it is expected that the hospital stay will be extended by 6.4 to 12.7 days,2 mortality will increase twofold,3 and costs will rise by at least $18,588 per occurrence (results reported from a Chicago hospital in 2008).2 This equates to approximately $20 billion per year in direct health care costs alone.3
Gram-negative organisms commonly harboring antimicrobial resistance include the ESKAPE pathogens (Table 1).4 These organisms are responsible for numerous infections, including bacterial meningitis, central venous catheter infections, pneumonia, urinary tract infections (UTIs), and complicated intra-abdominal infections (cIAIs).4,5 The associated mechanisms leading to resistance (Table 1) include production of extended-spectrum β-lactamases (ESBLs) and Klebsiella pneumoniae carbapenamases, decreases in influx porin activity/expression, increases in efflux pumps, and altered penicillin binding proteins (PBPs).6 To combat some of these mechanisms of resistance, Cubist Pharmaceuticals developed a novel investigational antimicrobial agent—an antipseudomonal cephalosporin with a β-lactamase inhibitor, ceftolozane/tazobactam (CXA-201). Due to the increasing number of gram-negative–resistant infections, this entity targets resistant PBP- and ESBL-producing organisms.7,8 One phase 2 and four phase 3 clinical trials of ceftolozane/tazobactam have been completed.9,10
Table 1.
“ESKAPE” Pathogens4
| Enterococcus faecium |
| Staphylococcus aureus |
| Klebsiella pneumoniae |
| Acinetobacter baumannii |
| Pseudomonas aeruginosa |
| Enterobacter species |
| Mechanisms of resistance6 |
| Production of catalytic enzymes (ESBL/carbapenamases) |
| Decrease in the influx porin activity/expression |
| Increase in efflux pumps |
| Altered penicillin binding proteins (PBPs) |
CLINICAL MICROBIOLOGY
Tests of the spectrum of activity against gram-positive aerobic bacteria found that ceftolozane by itself showed activity against Streptococcus species; however, this activity was limited. The addition of tazobactam to ceftolozane led to slight improvements against these gram-positive bacteria. Studies of the ceftolozane/tazobactam combination against gram-positive anaerobic bacteria, specifically Clostridium species, also demonstrated limited activity. On the other hand, ceftolozane’s spectrum of activity against gram-negative aerobic bacteria remained consistent or improved upon the addition of the β-lactamase inhibitor. The spectrum of activity against ceftazidime-resistant and ESBL-harboring Enterobacteriaceae was also significantly improved. In fact, with the addition of tazobactam, lower minimal inhibitory concentrations (MICs) were required to inhibit 90% of isolates (MIC90) in most gram-negative anaerobes. The greatest reductions were observed in Bacteroides and Prevotella species. When tested against ceftazidime-resistant Enterobacteriaceae, ceftolozane/tazobactam was shown to have a twofold more potent effect compared with cefepime and an eightfold more potent effect compared with piperacillin-tazobactam. Moreover, carbapenems such as imipenem/cilastatin and meropenem remained the most active, with 80.4% and 91.6% susceptibility, respectively.8
Ceftolozane/tazobactam also demonstrated superior in vitro activity against ceftazidime-resistant Escherichia coli and K. pneumoniae when compared with ceftriaxone, cefepime, and piperacillin/tazobactam. While the carbapenems retained good activity against this bacterium, the KPC-producing strains of K. pneumoniae remained highly resistant to β-lactam antimicrobials.11 Ceftolozane/tazobactam was shown to be more active than piperacillin/tazobactam, ceftriaxone, and ceftazidime when tested against ceftazidime-resistant strains of Enterobacter and Citrobacter species; however, less activity was noted when compared with cefepime and carbapenems. Activity against ESBL-producing Proteus mirabilis strains was similar to that of piperacillin-tazobactam.
The addition of tazobactam did not alter the activity of ceftolozane appreciably in vitro against P. aeruginosa strains; however, an eightfold increase was observed in the activity of ceftolozane over ceftazidime. In P. aeruginosa strains that were susceptible to ceftazidime and imipenem, ceftolozane/tazobactam was superior to piperacillin/tazobactam and imipenem, and comparable to doripenem. In ceftazidime-resistant P. aeruginosa strains, ceftolozane/tazobactam retained its overall activity. For P. aeruginosa strains with documented resistance to both ceftazidime and imipenem, ceftolozane/tazobactam was shown to have the most activity, followed by doripenem. Based on the available data, the combination of ceftolozane/tazobactam appears to be extremely valuable in the treatment of various resistant bacterial infections, which to date remains a clinical practice dilemma.
RESISTANCE
“The most common and important mechanism through which bacteria can become resistant against β-lactams is by expressing β-lactamases, for example extended-spectrum β-lactamases (ESBLs), plasmid-mediated AmpC enzymes, and carbapenem-hydrolyzing β-lactamases,” van Hoek and colleagues wrote in a review article in Frontiers in Microbiology.12
Takeda et al13 studied the effects of ceftolozane/tazobactam and various comparators exposed to different mechanisms of resistance against P. aeruginosa. E. coli bacteria C600 was used as a host strain to determine the effects of β-lactamases and extended-spectrum β-lactamases. The MICs reported for ceftolozane, ceftazidime, and imipenem were 0.25 mg/L against C600. The β-lactamases (TEM-1, TEM-2, SHV-1, OXA-1) demonstrated minimal effects on the three agents. ESBLs (TEM-3, TEM-4, TEM-5, TEM-6, TEM-7, TEM-8, TEM-9, SHV-2, SHV-3, SHV-4, OXA-2, CTX-M-3, CTX-M-18) reduced ceftolozane’s activity (MICs ranged from 1 mg/L to 32 mg/L) and the activity of ceftazidime (MICs ranged from 4 mg/L to more than 128 mg/L) to an even greater extent. Imipenem was not affected by either agent. None of the agents studied was active against metallo β-lactamase (MBL)–producing P. aeruginosa.
The MIC of ceftolozane/tazobactam against AmpC β-lactamase-producing P. aeruginosa was found to be more potent than that of ceftazidime (MIC, 2 mg/L versus 32 mg/L), suggesting a relatively high stability against this mechanism of resistance. The main mechanisms of resistance against fluoroquinolones (efflux pumps) and carbapenems (OprD) had no effect on the MIC of ceftolozane/tazobactam.
Moya et al14 investigated the resistance mechanisms leading to P. aeruginosa pan-β-lactam-resistance (PBLR) with a focus on the modification of PBP profiles. P. aeruginosa isolates that had developed resistance to antipseudomonal β-lactams (including penicillins, cephalosporins, monobactams, and carbapenems) during treatment of nosocomial infections in intensive care unit patients were used to determine the MIC values for ceftolozane/tazobactam in the presence of various AmpC over-expression levels. The greatest increases in ceftolozane MICs were documented for two strains showing extremely high AmpC expression levels (more than 2,000-fold compared with that of PAO1), while the lowest effect (no modification of MICs) was documented for a strain showing only moderate AmpC overexpression (100-fold higher than that of PAO1). These results suggest that ceftolozane is much less affected by the typical resistance mechanisms associated with P. aeruginosa. In vivo emergence of PBLR strains was shown to be affected by AmpC hyperproduction, in addition to modifications of PBP patterns and other factors. However, ceftolozane maintained some susceptibility.
Cross-resistance associated with ceftolozane/tazobactam, compared with additional antipseudomonal antimicrobials, was minimal. Furthermore, susceptibility has also been maintained in organisms that exhibit resistance to the other commonly used antipseudomonal agents.
PHARMACOKINETICS AND PHARMACODYNAMICS
The pharmacokinetic (PK) properties of ceftolozane have been studied alone and in combination with tazobactam in healthy subjects. Ceftolozane is a parenterally administered cephalosporin that exhibits linear kinetics. Miller et al15 evaluated PK parameters for ceftolozane when administered alone and with tazobactam as a 2:1 ratio in single and multiple ascending doses.
After the administration of single ascending doses, ceftolozane demonstrated linear PK for doses of 500 mg up to 2,000 mg. Doses of tazobactam ranged from 250 mg to 1,000 mg when used in combination with ceftolozane. In the single-dose studies, ceftolozane had a mean plasma half-life (t1/2) of 2.6 hours (range, 2.43–2.64) and a volume of distribution at steady state (Vss) of 5.1 L/h (ceftolozane alone) and 12.3 L/h (ceftolozane/tazobactam). The clearance of ceftolozane, alone and with tazobactam, was shown to occur exclusively via renal elimination. One hundred percent of the drug was recovered in the urine following doses between 500 mg and 2,000 mg. PK parameters including clearance, elimination t1/2, area under the curve (AUC), Vss, and Cmax were similar regardless of whether ceftolozane was administered with or without tazobactam. Mild renal impairment, defined as creatinine clearance (CrCl) of 60–89 mL/min, required no dosage adjustments. However, moderate renal impairment (CrCl of 30–59 mL/min) resulted in a 2.6-fold increase in AUC and a 2.1-fold increase in t1/2 for ceftolozane. The increase in tazobactam was 2.0-fold with respect to the AUC and 1.6-fold for t1/2. A dose reduction of 50% may be required in patients with moderate renal impairment.
Tazobactam primarily undergoes renal excretion via active tubular secretion. Coadministration of ceftolozane with tazobactam does not result in an interaction, since ceftolozane is primarily eliminated by glomerular filtration.
Pharmacodynamically, the best correlation to measure the therapeutic efficacy of cephalosporins is the time above MIC of the infecting pathogen (T > MIC). One potential advantage for ceftolozane is its longer half-life, especially when compared with other available cephalosporins. The reported Vss for ceftolozane, 12.9 L, was similar to the average extracellular volume in humans, indicating that ceftolozane may concentrate well at extracellular infection sites. It has also been reported that ceftolozane/tazobactam has excellent lung penetration with low protein binding. The unbound drug is above the MIC of the organism (T > MIC) 40% of the time in plasma and epithelial lining fluid. This was observed in more than 90% of the simulated ventilator-associated pneumonia population for gram-negative organisms such as P. aeruginosa, E. coli, and K. pneumoniae.16
The pharmacodynamics of ceftolozane alone and in combination with tazobactam have been evaluated in various time-kill experiments. Results reveal that ceftolozane/tazobactam exhibited bactericidal activity against various isolates of P. aeruginosa, E. coli, K. pneumoniae, Streptococcus pneumoniae, Burkholderia cepacia, and Moraxella catarrhalis. All isolates demonstrated four to eight times the MIC, with bacterial reductions of 3-log10 within six to eight hours.17 Ceftolozane/tazobactam demonstrated the most in vivo activity against ESBL, producing E. coli with bacterial density reductions ranging from 1.2 to 1.5 log units. While ceftolozane is effective on its own, the addition of tazobactam can extend the activity to include ESBL producers. A greater than 2-log10 bacterial reduction in colony-forming units at 24 hours can be observed when the combination is administered every six or eight hours. Dosing intervals of 12 or 24 hours resulted in as much as a 2-log reduction at 24 hours, exhibiting much less bacterial killing.
Ceftolozane/tazobactam concentrations remain above MIC approximately 40% to 50% of the time between dosage administrations, comparable to other cephalosporins. Monte Carlo modeling revealed that target attainment of 50% T > MIC at 8 mg/L was achieved in 90% of subjects with a 1.5-g dose infused over one hour every eight hours.
Studies compared the efficacy of different ratio combinations of ceftolozane/tazobactam—2:1, 4:1, and 8:1—as well as ceftolozane alone. The most active combination was 2:1. Dosing regimens of 750 mg/375 mg every eight hours and higher prevented the amplification of drug resistance in E. coli and eradicated antimicrobial-resistant subpopulations.17
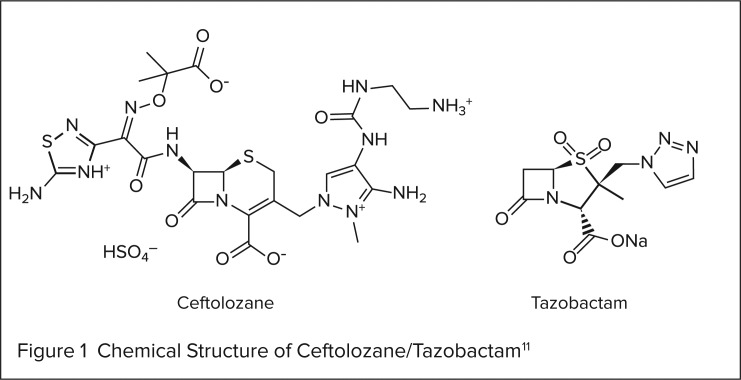
CHEMICAL STRUCTURE11
Ceftolozane/tazobactam is a combination product that contains a cephalosporin (ceftolozane) and a β-lactamase inhibitor (tazobactam). Its chemical structure is illustrated in Figure 1.
Figure 1.
Chemical Structure of Ceftolozane/Tazobactam11
INDICATIONS AND USAGE
Projected clinical indications for ceftolozane/tazobactam include: complicated urinary tract infections (cUTIs), complicated intra-abdominal infections (cIAIs), hospital-acquired bacterial pneumonia, and ventilator-associated bacterial pneumonia, pending the results from ongoing phase 3 clinical trials.
CLINICAL PHARMACOLOGY
This combination intravenous (IV) cephalosporin and β-lactamase inhibitor in a fixed 2:1 ratio exerts synergistic effects on susceptible bacterial strains with bactericidal activity.11 Ceftolozane directly inhibits PBPs, resulting in the disruption of cell-wall synthesis and subsequently bacterial cell death. Ceftolozane has been proven to have greater affinity for PBPs when compared with ceftazidime and imipenem.7 The addition of tazobactam to ceftolozane improves the spectrum of activity against resistant strains, including select ESBL-producing organisms.13 This results from tazobactam inhibiting class A β-lactamases in addition to a number of class C β-lactamases, preventing the hydrolysis of ceftolozane and allowing for the enhanced spectrum of activity.19,20
CLINICAL TRIALS
In a phase 2, multicenter, double-blind, randomized clinical trial, the safety and efficacy of ceftolozane/tazobactam were evaluated in 122 patients with cIAI randomized in a 2:1 ratio to either IV ceftolozane/tazobactam 1 g/0.5 g every eight hours and IV metronidazole 0.5 g every eight hours or IV meropenem 1 g every eight hours for four to seven days. The primary endpoint of the study was clinical response at the test of cure (TOC) date, seven to 14 days after treatment. The secondary endpoints were the microbiological response at TOC, safety parameters, and the pharmacokinetics of ceftolozane/tazobactam. Patients were included if they required surgical intervention within 24 hours of antibiotic, were 18 to 90 years of age, and had a diagnosis of cIAI. Patients were excluded if they were pregnant or nursing; had a diagnosis of intra-abdominal wall abscess, noninfectious intra-abdominal process, acute suppurative cholangitis, necrotizing pancreatitis of an infectious source, or pancreatic abscess; had rapidly progressing or life-threatening disease, moderate-to-severe renal dysfunction, an immunocompromising illness, or significant abnormality in a baseline electrocardiogram; or used other IV antibiotics (including carbapenems and cefepime).21 The ceftolozane/tazobactam/metronidazole arm included more high-risk patients (more than 65 years of age, APACHE score higher than 10, and moderate renal impairment). A total of 105 patients were clinically evaluable (CE, per protocol) and 77 were microbiologically evaluable (ME, modified intent to treat [mITT]), with a mean treatment duration of six days. In the ceftolozane/tazobactam/metronidazole arm and the meropenem arm, a clinical response was found in 91.4% and 94.3%, respectively (−2.9%; 95% confidence interval [CI], −13 to −7.2) of the CE subgroup and 88.7% and 95.8% (−7.2%; 95% CI, –18.8 to −4.5) of the ME subgroup. A microbiological response for patients infected with E. coli, the most common pathogen, was 89.5% (34 of 38 patients) and 94.7% (18 of 19 patients) in the ceftolozane/tazobactam/metronidazole and meropenem arms, respectively. Ceftolozane/tazobactam/metronidazole was associated with fewer drug-related adverse effects (8.5%) than meropenem (33.3%).9,17
In two phase 3, multicenter, double-blind, randomized, noninferiority clinical trials, IV ceftolozane/tazobactam 1 g/0.5 g every eight hours was compared with levofloxacin 750 mg IV once every 24 hours in 1,050 patients with cUTIs.17 The primary endpoint was the proportion of patients (in the modified microbiological ITT [mMITT] population) with both a microbiological eradication and clinical cure at the TOC visit (seven days ± two days after last dose). Secondary endpoints included patients (28 to 35 days after the last dose) with clinical cure, patients with microbiological eradication, the rates of eradication for each pathogen, and safety parameters. Patients were included if they were more than 18 years of age presenting with pyuria, clinical signs/symptoms of a cUTI (either pyelonephritis or complicated lower UTI) requiring IV antimicrobial therapy, and provided pretreatment urine cultures. Patients were excluded if they had a moderate-to-severe hypersensitivity to quinolone or β-lactam antimicrobials, had received antimicrobials within 48 hours of the first urine culture, had a UTI judged by investigators to require more than seven days of therapy, or had a permanent urinary stent or obstruction in the urinary tract, prostatitis, renal abscess, ileal loop, vision-ureteral reflux, CrCl less than 30 mL/min, immunocompromising conditions, or presented with more than one of the following: any liver enzyme (AST, ALT, alkaline phosphatase) or total bilirubin more than three times the upper limit of normal, absolute neutrophil count less than 500/μL, platelet count less than 40,000/μL, or hematocrit level less than 20%.22,23 The primary endpoint was achieved in 76.9% and 68.4% of the ceftolozane/tazobactam and levofloxacin mMITT arms, respectively. In the per-protocol group, 83.3% and 75.4% of patients met the primary endpoint in the ceftolozane/tazobactam and levofloxacin arms, respectively. Results were judged to have reached noninferiority. Microbiological eradication rates for ceftolozane/tazobactam and levofloxacin were 80.4% and 72.1% in the mMITT population and 86.2% and 77.6% in the per-protocol population, respectively. Eradication rates of unique pathogens for ceftolozane/tazobactam and levofloxacin are shown in Table 2. Treatment-related adverse effects occurred in 10.3% and 12.0% of the ceftolozane/tazobactam and levofloxacin patients, respectively. Headache was the most common side effect (5.8%) associated with ceftolozane/tazobactam; others included constipation (3.9%), hypertension (3%), nausea (2.8%), and diarrhea (1.9%).10
Table 2.
Eradication Rates of Unique Pathogens for Ceftolozane/Tazobactam and Comparators10
| Complicated Urinary Tract Infection | Complicated Intra-Abdominal Infection | |||||
|---|---|---|---|---|---|---|
| Samples | Ceftolozane/tazobactam | Levofloxacin | Samples | Ceftolozane/tazobactam | Meropenem | |
| Escherichia coli | 546 | 91% | 80% | 426 | 96% | 95% |
| Klebsiella pneumoniae | 48 | 84% | 61% | 53 | 100% | 88% |
| Pseudomonas aeruginosa | 19 | 86% | 58% | 53 | 100% | 100% |
In two phase 3, multicenter, randomized, double-blind trials, the safety and efficacy of ceftolozane/tazobactam for cIAI were studied against meropenem. A total of 993 patients were randomized to receive either IV ceftolozane/tazobactam 1 g/0.5 g every eight hours and IV metronidazole 0.5 g every eight hours or IV meropenem 1 g every eight hours for four to 14 days. The primary endpoint was the clinical cure rate at day 26 to 30 after initiation of treatment; the secondary endpoints were the microbiological cure rates, rates of microbiological cure with a clinical outcome, and safety parameters. Patients were enrolled if they presented with a cIAI requiring surgery within 24 hours of receiving the antimicrobial. Patients were excluded if they were diagnosed with simple appendicitis, acute suppurative cholangitis, infected necrotizing pancreatitis, pancreatic abscess, or pelvic infections, or had additional infections requiring additional gram-negative coverage, rapidly progressing or life-threatening disease, moderate-to-severe renal dysfunction, hepatic disease, or any moderate-to-severe hypersensitivity to β-lactam antimicrobials or metronidazole.24–26 In the mITT population, the primary endpoint was met in 83% of the ceftolozane/tazobactam/metronidazole arm and 87.3% of the meropenem arm, achieving noninferiority. The micro-biological eradication rates for unique pathogens are detailed in Table 2. The most common adverse effects reported were nausea (7.9%), diarrhea (6.2%), fever (5.2%), insomnia (3.5%), and vomiting (3.3%) in the ceftolozane/tazobactam/metronidazole arm, comparable to the meropenem arm. A phase 3, multicenter, randomized, double-blind clinical trial (ASPECT-NP) is being planned to evaluate the safety and efficacy of ceftolozane/tazobactam (3 g IV every eight hours) compared with meropenem (1 g IV every eight hours) in patients with ventilated nosocomial pneumonia. The primary endpoint of 28-day all-cause mortality will be evaluated in the ITT population. Secondary endpoints will include clinical and microbiological response rates, 14-day all-cause mortality, and safety and pharmacokinetic parameters. Patients will be enrolled if they present with ventilator-associated bacterial pneumonia or hospital-acquired bacterial pneumonia; are admitted for more than 48 hours and are intubated or on a ventilator with clinical criteria for ventilated nosocomial pneumonia; and have an APACHE score of 15 to 35. Patients will be excluded if they have moderate-to-severe hypersensitivity to β-lactams, are immunosuppressed, are expected to survive for less than 72 hours, have end-stage renal disease, require dialysis, or have confounding respiratory conditions.27,29 This trial is initiating investigational sites.
ADVERSE DRUG REACTIONS
The adverse-event (AE) profile of ceftolozane/tazobactam from two phase 2 trials (comparing either ceftolozane alone or in combination with tazobactam to ceftazidime or meropenem) suggests that ceftolozane/tazobactam is well tolerated. In the cUTI treatment trial, ceftolozane 1 g every eight hours was compared with ceftazidime 1 g every eight hours for seven to 10 days. The cIAI treatment trial evaluated ceftolozane/tazobactam 1 g/0.5 g with metronidazole 0.5 g every eight hours versus meropenem 1 g every eight hours. Although the reported AEs for ceftolozane and ceftazidime were similar, rates of constipation (9.4% versus 4.8%), nausea (5.9% versus 0%), insomnia (4.7% versus 0%), headache (5.9% versus 0%), hypertension (2.4% versus 0%), phlebitis (2.4% versus 0%), and infusion-site reactions (2.4% versus 0%) were higher with ceftolozane.11 Conversely, ceftazidime demonstrated higher rates of diarrhea (3.5% versus 7.1%). When compared to meropenem, increased rates of ileus (3.6% versus 0%) and anemia (6.1% versus 2.6%) were linked with ceftolozane/tazobactam. Elevations in ALT (7.7%) and AST (5.1%) were identified with meropenem but not with ceftolozane/tazobactam. Based on these phase 2 trials, it appears that ceftolozane/tazobactam is safe and has a tolerable AE profile compared with other β-lactam antimicrobials. In the phase 3 trial comparing ceftolozane/tazobactam with metronidazole to meropenem for cIAI, the AE rates were comparable (44% versus 42.7%). The most common AEs reported with ceftolozane/tazobactam/metronidazole were nausea (7.9%), diarrhea (6.2%), fever (5.2%), insomnia (3.5%), and vomiting (3.3%).25 The rates of AEs in the phase 3 trial comparing ceftolozane/tazobactam to levofloxacin for cUTI treatment were also comparable (34.7% versus 34.4%). Adverse drug events were consistent with those seen in the prior cIAI phase 3 trial and the earlier phase 2 trials; the most common AEs reported with ceftolozane/tazobactam were headache (5.8%), constipation (3.9%), hypertension (3%), nausea (2.8%), and diarrhea (1.9%).28
DRUG INTERACTIONS
Based on previous trial data and ongoing clinical trials, no significant drug–drug or food–drug interactions have been associated with ceftolozane/tazobactam administration. However, drug–drug interactions similar to those observed with the cephalosporin class of antimicrobials and β-lactamase inhibitors (BLIs) should be considered as potential interactions until further drug–drug interactions have been completely elucidated. Moreover, as a result of drug accumulation in renal impairment, caution should be taken when coadministering ceftolozane/tazobactam with other renally eliminated medications due to possible nephrotoxicity.29
CONTRAINDICATIONS
Contraindications for the use of ceftolozane/tazobactam have not been established. However, hypersensitivity reactions are a concern with its use in patients allergic to penicillin-derived products, cephalosporins, or BLI antimicrobials.
PRECAUTIONS AND WARNINGS
Although formal precautions and warnings have not been established, concerns about hypersensitivity reactions, skin rashes, Clostridium difficile (C. difficile)–associated diarrhea, hematological effects, central nervous system effects in patients with renal impairment, and the risk of developing drug-resistant species may be prudent, assuming there are similarities between ceftolozane/tazobactam and other cephalosporins and BLIs.30,31
Hypersensitivity reactions have been reported to be serious and sometimes fatal in patients receiving antimicrobials such as β-lactam/BLI antibiotics. It would be reasonable to screen for individuals at high risk for serious hypersensitivity prior to use of ceftolozane/tazobactam. As with other medications, if a patient experiences an anaphylactic-type reaction while receiving ceftolozane/tazobactam, the drug should be discontinued immediately. In addition, serious skin reactions documented as Stevens-Johnson syndrome and/or toxic epidermal necrolysis have been associated with β-lactams/BLIs; if a patient develops a skin lesion that progresses, the drug should be discontinued immediately. It can be assumed that ceftolozane/tazobactam will pose the same risk of C. difficile as other β-lactams. Antimicrobial agents, in general, alter the normal flora of the colon. In a patient who is taking or has recently discontinued an antimicrobial therapy, a C. difficile diagnosis should be considered if a patient develops watery diarrhea and/or other signs and symptoms suggestive of C. difficile.
As noted in the phase 2 and 3 studies, ceftolozane/tazobactam showed hematological effects such as anemia that were nonsignificant; however, β-lactams have been reported to result in leukopenia/neutropenia. Usually, these effects are the result of prolonged administration and are generally reversible once the drug is discontinued. β-lactams, specifically second-and third-generation cephalosporins, have been shown to increase bleeding risks due to prolonged prothrombin time.30,31
Based on the pharmacokinetic studies conducted, ceftolozane/tazobactam has been shown to accumulate in cases of renal impairment, similar to other β-lactam antimicrobials; therefore, patients with moderate-to-severe renal impairment may require a dose adjustment to prevent accumulation.15,29
With regard to the possible development of antimicrobial-resistant species, appropriate prescribing, adherence, and antimicrobial stewardship should be enforced.
SPECIAL POPULATIONS
No adequate and well-controlled studies have been performed in pregnant women. As a class, cephalosporins are pregnancy category B.32 In the geriatric population, renal function is likely to be decreased, so ceftolozane/tazobactam should be used with caution in patients with moderate-to-severe renal impairment (CrCL less than 30 mL/min).29
CONCLUSION
In 2010, the Infectious Diseases Society of America launched an initiative to stimulate research in novel, safe, and effective antibiotics to combat the growing concern over antimicrobial resistance. This “10 x 20” initiative calls for 10 new antibiotics by the year 2020.33 Cubist Pharmaceuticals has taken advantage of the Generating Antibiotic Incentives Now law, which authorizes fast-track status for qualified infectious disease products such as ceftolozane/tazobactam at the Food and Drug Administration (FDA). According to the abbreviated results from four phase 3 clinical trials, it appears that ceftolozane/tazobactam is a novel, safe, and effective antibiotic for the treatment of cUTIs and cIAIs. In June 2014, the FDA accepted Cubist’s new drug application submission for ceftolozane/tazobactam for the treatment of cUTIs and cIAIs.34 The use of ceftolozane/tazobactam for other clinical indications remains to be seen; however, the current results appear promising.
REFERENCES
- 1.Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. 2013 Apr; Available at: http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf Accessed June 1, 2014. [Google Scholar]
- 2.Roberts RR, Hota B, Ahmad I, et al. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin Infect Dis. 2009;49(8):1175–1184. doi: 10.1086/605630. [DOI] [PubMed] [Google Scholar]
- 3. Alliance for the Prudent Use of Antibiotics. The cost of antibiotic resistance to U.S. families and the health care system. Available at: http://www.tufts.edu/med/apua/consumers/personal_home_5_1451036133.pdf. Accessed June 1, 2014.
- 4.Rice LB. Progress and challenges in implementing the research on ESKAPE pathogens. Infect Control Hosp Epidemiol. 2010;31(suppl 1):S7–S10. doi: 10.1086/655995. [DOI] [PubMed] [Google Scholar]
- 5.Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133–164. doi: 10.1086/649554. [DOI] [PubMed] [Google Scholar]
- 6.Campfield B, Chen K, Kolls JK. Vaccine approaches for multidrug resistant Gram negative infections. Curr Opin Immunol. 2014;28:84–89. doi: 10.1016/j.coi.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moyá B, Zamorano L, Juan C, et al. Affinity of the new cephalosporin CXA-101 to penicillin-binding proteins of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2010;54(9):3933–3937. doi: 10.1128/AAC.00296-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sader HS, Rhomberg PR, Farrell DJ, Jones RN. Antimicrobial activity of CXA-101, a novel cephalosporin tested in combination with tazobactam against Enterobacteriaceae, Pseudomonas aeruginosa, and Bacteroides fragilis strains having various resistance phenotypes. Antimicrob Agents Chemother. 2011;55(5):2390–2394. doi: 10.1128/AAC.01737-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucasti C, Hershberger E, Miller B, et al. A multicenter, double-blind, randomized, phase 2 study to assess the safety and efficacy of ceftolozane/tazobactam (TOL/TAZ) plus metronidazole (MTZ) compared with meropenem (MER) in adult patients with complicated intra-abdominal infections (cIAI). [Abstract K-1709] Poster presentation at the 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy; 2013; Denver, Colorado. September 10–13, 2013. [Google Scholar]
- 10.Cubist Pharmaceuticals. Cubist presents detailed results from positive phase 3 trials of ceftolozane/tazobactam at 2014 European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) Available at: http://www.cubist.com/media/news-releases/cubist-presents-detailed-results-from-positive-pha. Accessed June 1, 2014.
- 11.Hong MC, Hsu DI, Bounthavong M. Ceftolozane/tazobactam: a novel antipseudomonal cephalosporin and β-lactamase-inhibitor combination. Infect Drug Resist. 2013;6:215–223. doi: 10.2147/IDR.S36140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Hoek AH, Mevius D, Guerra B, et al. Acquired antibiotic resistance genes: an overview. Front Microbiol. 2011;2:203. doi: 10.3389/fmicb.2011.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeda S, Nakai T, Wakai Y, et al. In vitro and in vivo activities of a new cephalosporin, FR264205, against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2007;51(3):826–830. doi: 10.1128/AAC.00860-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moyá B, Beceiro A, Cabot g, et al. Pan-β–lactam resistance development in Pseudomonas aeruginosa clinical strains: molecular mechanisms, penicillin-binding protein profiles, and binding affinities. Antimicrob Agents Chemother. 2012;56(9):4771–4778. doi: 10.1128/AAC.00680-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller B, Hershberger E, Benziger D, et al. Pharmacokinetics and safety of intravenous ceftolozane-tazobactam in healthy adult subjects following single and multiple ascending doses. Antimicrob Agents Chemother. 2012;6(6):3086–3091. doi: 10.1128/AAC.06349-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller B, Chandorkar G, Umeh O, et al. Safety and PK of IV ceftolozane/tazobactam 3 g every eight hours and cumulative fraction of response in plasma and epithelial lining fluid in a simulated VAP population. [Abstract A-641] Presentation at the 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy; San Francisco, California. September 9–12, 2012. [Google Scholar]
- 17.Zhanel GG, Chung P, Adam H, et al. Ceftolozane/tazobactam: a novel cephalosporin/β-lactamase inhibitor combination with activity against multidrug-resistant gram-negative bacilli. Drugs. 2014;74(1):31–51. doi: 10.1007/s40265-013-0168-2. [DOI] [PubMed] [Google Scholar]
- 18.Cubist Pharmaceuticals. Ceftolozane/tazobactam—overview.
- 19.Drawz SM, Bonomo RA. Three decades of β-lactamase inhibitors. Clin Microbiol Rev. 2010;23(1):160–201. doi: 10.1128/CMR.00037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bush K, Macalintal C, Rasmussen BA, et al. Kinetic interactions of tazobactam with beta-lactamases from all major structural classes. Antimicrob Agents Chemother. 1993;37(4):851–858. doi: 10.1128/aac.37.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Clinicaltrials.gov. Safety and efficacy study to compare IV CXA 101/tazobactam and metronidazole with meropenem in complicated intraabdominal infections. NCT01147640. Available at: http://clinicaltrials.gov/ct2/show/NCT01147640?term=NCT01147640&rank=1. Accessed June 1, 2014.
- 22.Cubist Pharmaceuticals. Cubist announces positive top-line results from phase 3 trial of investigational antibiotic ceftolozane/tazobactam in complicated urinary tract infections. Nov 25, 2013. Available at: http://www.cubist.com/media/news-releases/cubist-announces-positive-top-line-results-fro-(1). Accessed June 1, 2014.
- 23. Clinicaltrials.gov. Study comparing the safety and efficacy of intravenous CXA-201 and intravenous levofloxacin in complicated urinary tract infection, including pyelonephritis. NCT01345929. Available at: http://clinicaltrials.gov/ct2/show/NCT01345929?term=NCT01345929&rank=1. Accessed June 1, 2014.
- 24. Cubist Pharmaceuticals. Cubist announces positive top-line results from phase 3 trial of ceftolozane/tazobactam in intra-abdominal infections. December 16, 2013. Available at: http://investors.cubist.com/Mobile/file.aspx?IID=4093793&FID=21288226. Accessed October 31, 2014.
- 25. Clinicaltrials.gov. Safety and efficacy of intravenous CXA-201 and intravenous meropenem in complicated intraabdominal infections. NCT01445665. Available at: http://clinicaltrials.gov/ct2/show/NCT01445665?term=NCT01445665&rank=1. Accessed June 1, 2014.
- 26. Clinicaltrials.gov. Study comparing the safety and efficacy of intravenous CXA-201 and intravenous meropenem in complicated intraabdominal infections. NCT01445678. Available at: http://clinicaltrials.gov/ct2/show/NCT01445678?term=NCT01445678&rank=1. Accessed June 1, 2014.
- 27. Clinicaltrials.gov. Safety and efficacy study of ceftolozane/tazobactam to treat ventilated nosocomial pneumonia (ASPECT-NP). NCT02070757. Available at: http://clinicaltrials.gov/ct2/show/study/NCT02070757?term=Ceftolozane%2FTazobactam&rank=1 Accessed June 1, 2014.
- 28. Cubist Pharmaceuticals. Cubist presents detailed results from positive phase 3 trials of ceftolozane/tazobactam at 2014 European Congress of Clinical Microbiology and Infectious Diseases (ECCMID). May 9, 2014. Available at: http://investors.cubist.com/Mobile/file.aspx?IID=4093793&FID=23570682. Accessed October 31, 2014.
- 29.Wooley M, Miller B, Krishma G, et al. Impact of renal function on the pharmacokinetics and safety of ceftolozane/tazobactam. Antimicrob Agents Chemother. 2014;58(4):2249–2255. doi: 10.1128/AAC.02151-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rocephin [package insert] South San Francisco, California: Genentech USA, Inc.; 2013. [Google Scholar]
- 31.Zosyn [package insert] Philadelphia, Pennsylvania: Wyeth Pharmaceuticals Inc; 2013. [Google Scholar]
- 32.Merk Manual [website] β-lactams. 2013. Available at: http://www.merck-manuals.com/professional/infectious_diseases/bacteria_and_antibacterial_drugs/%CE%B2-lactams.html. Accessed April 2, 2014.
- 33.Boucher HW, Talbot GH, Benjamin DK, et al. 10 × 20 progress—development of new drugs active against gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(12):1685–1694. doi: 10.1093/cid/cit152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cubist Pharmaceuticals. Cubist announces acceptance of ceftolozane/tazobactam new drug application with priority review. Jun 19, 2014. Available at: http://www.cubist.com/media/news-releases/cubist-announces-acceptance-of-ceftolozanetazobac. Accessed November 4, 2014.