Abstract
The neurodegenerative effects of Parkinson’s disease (PD) are marked by a selective loss of dopaminergic (DA) neurons. Epidemiological studies suggest that chronic exposure to the pesticide paraquat may increase the risk for PD and DA cell loss. However, combined exposure with additional fungicide(s) including maneb and/or ziram may be required for pathogenesis. To explore potential pathogenic mechanisms, we have developed a Drosophila model of chronic paraquat exposure. We find that while chronic paraquat exposure alone decreased organismal survival and motor function, combined chronic exposure to both paraquat and maneb was required for DA cell death in the fly. To initiate mechanistic studies of this interaction, we used additional genetic reagents to target the ubiquitin proteasome system, implicated in some rare familial forms of PD and the toxic effects of ziram. Genetic inhibition of E1 ubiquitin ligase, but not the proteasome itself, increased DA cell death in combination with maneb but not paraquat. These studies establish a model for long-term exposure to multiple pesticides, and support the idea that pesticide interactions relevant to PD may involve inhibition of protein ubiquitination.
Keywords: Drosophila, Parkinson’s disease, Pesticide, ziram, paraquat, maneb, Ubiquitin proteasome system, E1 ligase
1.0 Introduction
The hallmark of Parkinson’s disease (PD) is the progressive loss of dopaminergic (DA) neurons in the substantia nigra. Rare, familial forms of PD indicate a pathogenic role for disruption of the ubiquitin proteasome system (UPS) and mitochondrion (Lee et al., 2010, Olanow et al., 2006). However, the precise contribution of these pathways to sporadic PD remains unclear. Epidemiological studies demonstrate an increased risk for PD with chronic exposure to specific pesticides, and suggest additive and potentially synergistic effects between the herbicide paraquat and the fungicides maneb and ziram (Costello et al., 2009, Wang et al., 2011). Similarly, rodent models demonstrate that the combined effects of paraquat and maneb exceed that of each toxin alone (Srivastava et al., 2012, Thiruchelvam, 2000), but the mechanisms underlying these interactions are not known. We are using the model organism Drosophila melanogaster to investigate this question, as well as other pharmacologic and genetic interactions potentially relevant to sporadic PD (Lawal et al., 2010, Lawal et al., 2014).
Current Drosophila models of combined pesticide exposure are limited and the combined effects of paraquat plus maneb in the fly are not known. Furthermore, existing models of paraquat administration in Drosophila are acute in nature and cause DA cell death within hours to days (Bonilla et al., 2002, Chaudhuri et al., 2007, Hosamani et al., 2013). Similarly, rodent models of paraquat exposure are relatively acute, and generally conducted over the course of several weeks, a relatively small fraction of the rodent’s lifetime (Liou et al., 1996, McCormack et al., 2002). Thus, neither current fly nor rodent models replicate the chronic time course of exposure seen in many patient populations (Costello et al., 2009). Moreover, most if not all Drosophila studies of acute paraquat exposure have utilized sucrose as a vehicle (Chaudhuri et al., 2007, Humphreys et al., 1993, Legan et al., 2008), which may increase both oxidative stress and mortality in the fly (Rzezniczak et al., 2011).
One potential target of pesticides associated with increased risk for PD is the proteasome and the UPS (Ebrahimi-Fakhari et al., 2012, Wang et al., 2006). However, the effects of proteasome inhibition on DA cell death remain poorly understood (Kordower et al., 2006, Matsui et al., 2010, McNaught et al., 2004, Shin et al., 2011). Furthermore, ubiquitin regulates many cellular processes other than proteasomal degradation, including endocytosis, axon guidance, and synaptic strength (Hamilton et al., 2013, Schmidt et al., 2014). Other pesticide targets potentially relevant to PD include pathways that regulate oxidative stress (e.g. mitochondrial complex III; (Zhang et al., 2003)). Ziram and several other pesticides also inhibit aldehyde dehydrogenase (ALDH), a mitochondrial enzyme that degrades oxidative metabolites of DA and other toxic species (Doorn et al., 2014, Wey et al., 2012).
It is not clear which of these mechanisms may contribute to the toxic effects of specific pesticides in DA neurons and how they may interact (Thrash et al., 2007, Wang et al., 2011). One potential strategy to address these questions is to genetically “mimic” proposed mechanisms of action and determine if this activity is toxic to DA cells. The genetic tools available in Drosophila are particularly useful for this approach. We show here that chronic exposure to maneb plus paraquat, but neither toxin alone, cause DA cell death in the fly and that these effects are only observed in extensively aged flies. Additional experiments using genetic mimics suggest a possible interaction between maneb and inhibition of protein ubiquitination, a mechanism of action previously proposed for ziram (Chou et al., 2008).
2.0 Materials and Methods
2.1 Drosophila strains and maintenance
Drosophila strains were maintained on standard molasses agar media at 25°C with a 12-hour light/dark cycle. Male flies, 2–4 days post-eclosion were used for all experiments. Transgenic UAS lines expressing the “degron” CL1 (Pandey et al., 2007) and the dominant negative proteasomal subunits Pros26 and Prosβ2 (Belote et al., 2002) were gifts of Paul Taylor (U. Penn) and John Belote (Syracuse U.), respectively. F1 progeny derived from genetically crossed Ddc-GAL4 and UAS-Pros26; UAS-Prosβ2 homozygous lines were used for proteasome inhibition experiments. Flies containing a null mutation in the Drosophila Aldh gene generated by imprecise P element excision (Aldh24, (Fry et al., 2006)) were a gift from James Fry (U. Rochester). To ensure that previously reported effects of Aldh24 were not due to spurious mutations, Aldh24 was outcrossed for ten generations into a Canton S. wild type genetic background (Simon, 2003). To follow the recessive null allele, PCR was used to amplify a region of the Aldh gene as defined by the primers aldh-a (also used in (Fry et al., 2006)) gttcttctga cagcacttgt and aldh-31b tcaattaaat cgaacgaacg c.
To generate transgenes expressing RNAi directed against E1 ligase (uba1), a 725 bp fragment was excised from a uba1 cDNA (gh24511, Drosophila Genomics Resource Center, Indiana) via digestion with Sal I, and inserted into the intermediate vector pGEM-11 (Promega, Madison WI). This construct was digested with EcoRI – NotI and the fragment representing uba1 inserted into the symmetrically transcribed RNAi vector sympUAST (Giordano et al., 2002) for injection into Drosophila embryos (Bestgene Chino Hills, CA).
2.2 In vivo assay of proteasome inhibition
Wandering 3rd instar larva of the genotype Ddc-GAL4/UAS-GFP-CL1 and Ddc-GAL4/UAS-GFP-CL1;UAS-E1-RNAi/+ were dissected in PBS and fixed for 40 minutes in a 4% paraformaldehyde solution. The supraesophageal ganglion (the brain) and ventral nerve cord were then mounted on glass slides using Aquamount (PolySciences, Warrington, PA) and visualized using a Zeiss Pascal LSM 5 confocal microscope with a 40x/0.75 EC Plan-Neofluar objective. Z-stack projections of the entire brain and ventral nerve cord were collected and used to count GFP-positive cells.
2.3 Toxin administration
Stock solutions of paraquat (400 mM) (Sigma Aldrich, St. Louis, MO) were made in distilled water. Maneb (100mM) and ziram (200mM) (Chem Services, West Chester, PA) were solubilized in DMSO; all stocks were aliquoted, stored at −20°C and discarded after one freeze-thaw cycle. Stock solutions of toxins were mixed into molten molasses-agar media under a fume hood at the indicated final concentrations, and used within one week. DMSO was added to food containing paraquat so that all treatment groups were exposed to 1% DMSO in the media.
2.4 Survival
Flies were cultured in standard food containing the indicated concentration of toxin(s) and passed onto a fresh food + toxin mixture every 2–3 days for 60 days. Experiments were initiated with 40 flies/vial (n=6 vials/condition) and deaths were recorded at each passage.
2.5 Negative geotaxis
Drosophila motor function was assessed using a climbing (negative geotaxis) assay (Benzer, 1967). Drosophila were passed onto fresh media (with toxin(s)) at 24 hours and 2 hours prior to the assay and climbing was assessed in the dark to eliminate phototactic effects. Six vials with 30–40 flies each were assessed per treatment group. For each assay, flies were loaded into 0.7 x 10 cm vials affixed to a standard countercurrent apparatus (Benzer, 1967). After one-minute acclimation to the dark, the apparatus was tapped on the benchtop and the flies allowed 15 sec to climb from the bottom vial to the top vial. The performance index (PI) for climbing was calculated as the percentage of flies that climbed to the top vial within 15 seconds. Climbing ability was quantified and graphed as a performance index (PI), where PI represents [(Flies at the top vial after 15 sec./total number of flies in bottom+top vials)*100].
2.6 Immunolabeling and DA cell counts
Adult Drosophila brains were fixed in 4% paraformaldehyde then probed with mouse anti-tyrosine hydroxylase primary antibody (Immunostar, Hudson, WI) and a secondary goat anti-mouse Alexa 555 antibody (Molecular Probes, Grand Island, NY) as described previously (Lawal et al., 2010). DA neurons of the PPL1 cluster (Coulom et al., 2004, Lawal et al., 2010) were visualized and on an AxioSkop Zeiss upright microscope with a 40x/0.75 EC Plan-Neofluar objective by manually adjusting the plain of focus through entire brain. For all experiments, DA cells were counted by an observer blind to the genotype of the samples. Images for Figures 2a–c and 3a–d were collected on a Zeiss Pascal LSM 5 confocal microscope with a 40x/0.75 EC Plan-Neofluar objective.
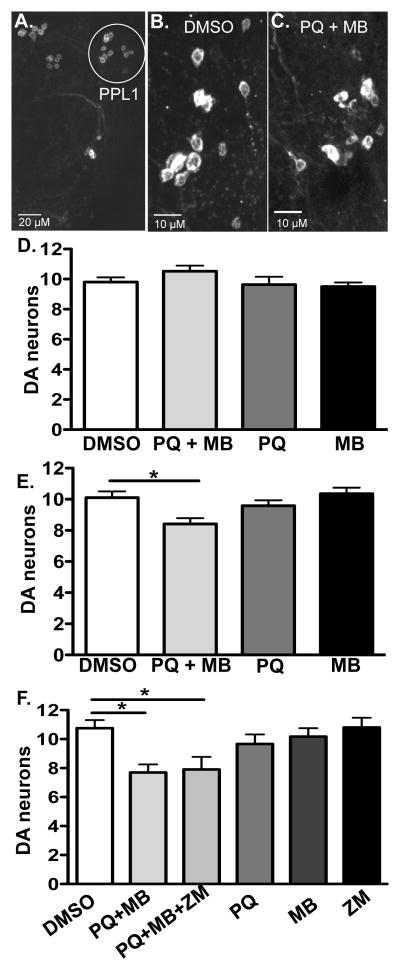
Figure 2. Chronic paraquat and maneb exposure results in dopaminergic neuron loss.
Male flies were incubated on standard media containing DMSO 1%, paraquat 4 mM, maneb 500 μM, ziram 1mM, paraquat 4 mM plus maneb 500 μM, or paraquat 4mM plus maneb 500 μM plus ziram 1mM for four (d) or six weeks (a–c, e, f). a) One half of a bilaterally symmetric wild type, adult fly brain is shown labeled with anti-tyrosine hydroxylase antibody. The PPL1 cluster is highlighted. Dopaminergic neurons of the PPL1 cluster after six weeks of exposure to either DMSO control (b) or 4mM paraquat (PQ) plus 500 μM maneb (MB) (these representative images show 12 and 8 neurons, respectively) (c). Scale bars: a, 20 microns; b, c, 10 microns. d) No significant change in the number of dopaminergic neurons in PPL1 were observed with any pesticide treatment after four weeks of exposure (One-way ANOVA, p > 0.05, n = 10–13 brains and 19–25 PPL1 clusters total per treatment). e) Flies treated with both paraquat and maneb for six weeks show a significant loss of dopaminergic neurons (One-way ANOVA p < 0.005, Bonferroni; post-hoc *p < 0.05; n = 16–22 brains and 28–37 PPL1 clusters total per treatment). f) As in b, exposure to paraquat + maneb resulted in a significant loss of DA cells at 6 weeks. The addition of ziram (ZM) to paraquat + maneb treatment did not result in a further increase in cell loss. Ziram treatment alone did not differ from vehicle controls (DMSO, One-way ANOVA p < 0.01, Bonferroni post-hoc, *p < 0.05; n = 6–14 brains and 10–23 PPL1 clusters total per treatment).
3.0 Results
3.1 Paraquat and paraquat plus maneb exposures result in impaired survival and motor deficits
To assess possible dosage regimens for the chronic application of paraquat, we quantified survival of adult male Drosophila exposed to 0, 4, 6, or 8 mM paraquat in standard molasses cornmeal agar media for four weeks (Figure 1a). Survival was significantly lower in all paraquat treated groups (log rank test, Bonferroni correction for multiple comparisons; p < 0.01) and after four weeks of exposure was 80%, 36%, 0% and 0% at each dose, respectively. Since 4 mM represented the highest tested dose of paraquat that allowed for survival of extensively aged flies, we used this dose for subsequent experiments. The maximum lifespan of a fly under standard culture conditions is 50–80 days with progressive defects in behavioral performance beginning at 10–14 days (Grotewiel et al., 2005, Simon et al., 2006). We estimate that chronic exposure over 4 weeks in the fly is comparable to exposure of rodents for 12 to 18 months or human patients for several decades, through the end of middle age. “Old age” in flies aged may be considered ≥ 5–6 weeks (Grotewiel et al., 2005).
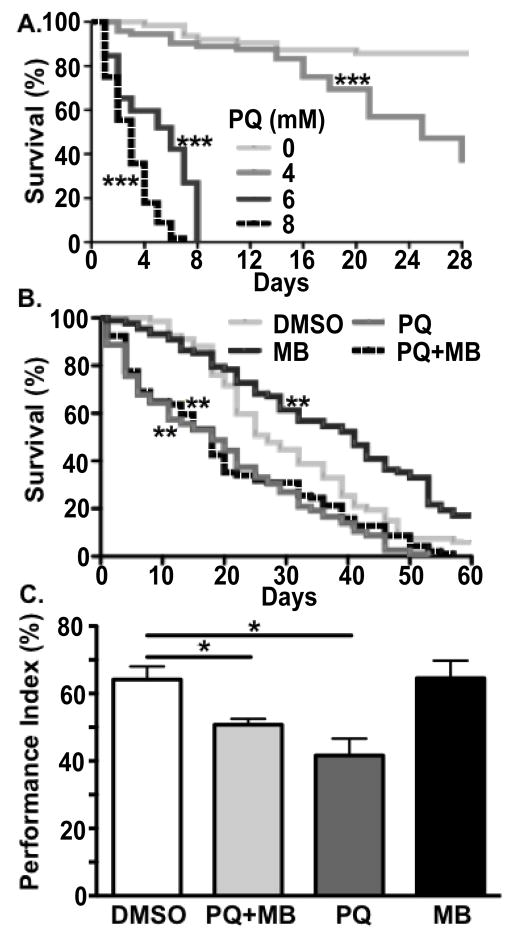
Figure 1. Effects of paraquat and/or maneb exposure on survival and climbing.
a) Survival curves of male wild type (CS) Drosophila exposed to 0, 4, 6, or 8 mM paraquat for 28 days. Only the 0 and 4mM groups showed survivors at 28 days. Survival (b) and climbing ability (c) were assessed for male Drosophila aged on standard media containing either 1% DMSO, 4 mM paraquat (indicated as “PQ”), 500 μM maneb (MB), or 4 mM paraquat plus 500 μM maneb (PQ+MB). All paraquat dose groups showed survival significantly lower than DMSO treatment alone (log-rank test with Bonferroni multiple-comparison correction, ***p < 0.001). b) Flies exposed to paraquat or paraquat plus maneb showed decreased survival versus DMSO control (log-rank test with Bonferroni multiple-comparison correction, **p < 0.005); maneb alone increased survival. c) Flies exposed to paraquat or paraquat + maneb both showed a significantly decreased climbing ability, quantified as performance index (PI, see Methods), versus DMSO control (One-way ANOVA p < 0.005, Bonferroni post-hoc, *p < 0.05).
To initially assess the potential effects of combined chronic exposure to both paraquat and maneb exposure, we first tested survival. Over the course of 8 weeks (60 days), survival of adult male flies was reduced by paraquat or paraquat + maneb compared to maneb alone or vehicle (DMSO) (log-rank test, Bonferroni correction for multiple comparisons; p < 0.001, Figure 1b). Maneb exposure alone showed a modest increase in lifespan compared to vehicle alone (log-rank test, Bonferroni correction for multiple comparisons; p < 0.001).
To test motor function in flies exposed to paraquat and/or maneb we used a well-described negative geotaxis (climbing) assay (Benzer, 1967). No affects on motor function were observed after either 24 or 96 hours of pesticide exposure (data not shown). However, after one-week, both the paraquat and paraquat + maneb treated groups exhibited significantly impaired climbing ability (Figure 1c; one-way ANOVA, post-hoc Bonferroni, p < 0.05).
3.2 Combined chronic exposure to paraquat and maneb results in dopaminergic cell loss
We assessed DA cell loss in Drosophila exposed to pesticides by counting DA neurons in the PPL1 cluster as described (Coulom et al., 2004, Lawal et al., 2010) (Figure 2a–f). DA cell loss was not detected after four weeks exposure to paraquat, maneb, or both, compared to vehicle alone (Figure 2d). By contrast, we observed significant loss of DA neurons at six weeks in flies treated with paraquat + maneb, but neither paraquat nor maneb individually (Figure 2e, one-way ANOVA, post-hoc Bonferroni, p < 0.05). We have previously tested the effects of ziram in Drosophila and failed to detect any significant decrease in survival in flies with chronic exposure (Lawal et al., 2010). Similarly, we report here that ziram did not potentiate the loss of DA cells caused by exposure to paraquat or maneb (Figure 2f).
3.3 Inhibition of E1 ligase alters maneb’s toxicity but not that of paraquat
We hypothesized that ziram’s inability to elicit an effect could be due to toxicokinetic restrictions such as limited adsorption or distribution. Although parenteral administration might be feasible, we speculated that a genetic approach also might be useful to circumvent these issues. Our strategy was to mimic the mechanisms of action that have been suggested to be responsible for ziram’s neurotoxic effects based on of studies in mammalian systems (Chou et al., 2008, Fitzmaurice et al., 2014). In particular, inhibition of ubiquitin E1 ligase by ziram has been proposed to kill mammalian DA cells in vitro (Chou et al., 2008). In addition, inhibition of E1 ligase could be the mechanism underlying ziram’s proposed synergy with paraquat and/or maneb in human populations exposed to all three pesticides (Wang et al., 2011). To genetically inhibit E1 ligase and thereby mimic one of the proposed neurotoxic activities of ziram we developed an RNAi construct to knockdown expression of the Drosophila ortholog of E1 ligase.
To first validate the in vivo biological effects of E1 RNAi expression, we used a previously characterized transgene expressing a GFP labeled proteasome substrate or “degron” (UAS-CL1) (Pandey et al., 2007). The E1 RNAi and degron transgenes were co-expressed in DA and 5HT cells using the Ddc-GAL4 driver (Li et al., 2000). The use of this driver allowed visualization of a discrete number of stereotypically positioned cells (Figure 3a–d). The number of labeled cells in larva co-expressing E1 RNAi was increased two-fold compared to those expressing the degron alone (Figure 3e). These data support the idea that E1 RNAi construct reduced ubiquitination and the subsequent degradation of the GFP marker via proteasomal degradation.
Figure 3. E1 RNAi leads to accumulation of a GFP labeled proteasome substrate.
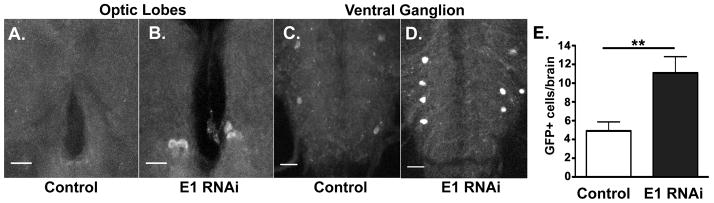
a–d) The optic lobes (OL) (a,b) and ventral ganglion (VG) (c, d) of larva expressing a GFP labeled proteasome substrate (degron) expressed with Ddc-GAL4 in the presence (b,d) or absence (a,c) of UAS-E1 RNAi expression (representative images in a-d show cell counts of 0, 4, 4, and 8, respectively), scale bars 20 microns. c) Quantitation of GFP+ cells in control versus E1 RNAi conditions in whole larval brain. E1 RNAi expressing larva show a significantly higher number of degron GFP-positive cells (Student’s t-test, **p < 0.01).
Flies expressing UAS-E1 RNAi using either Ddc-GAL4 or TH-GAL4 die as pupae (not shown). Therefore, to investigate the potential neurotoxic effects of E1 knock down in adults, we tested additional drivers including elav-GAL4 (Robinow et al., 1991). Pan neuronal expression of E1 RNA using elav-GAL4 allowed survival through adulthood but showed a decreased life span with minimal survival after four weeks (not shown).
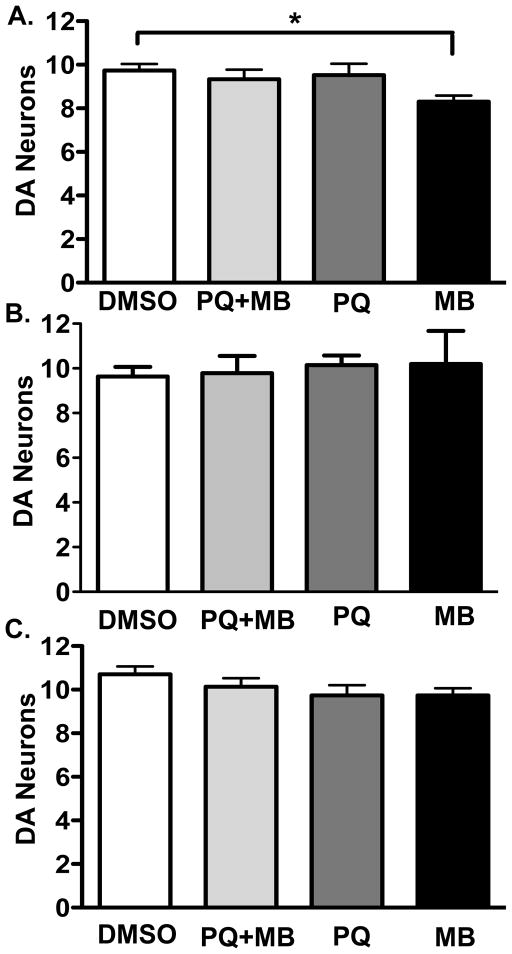
To test the potential interaction of E1 knockdown with additional pesticides, we exposed flies expressing UAS-E1 RNAi with elav-Gal4 to paraquat, maneb or paraquat + maneb for four weeks (Figure 4a). We were unable to perform cell counts at six weeks as in Figure 2d due to the decrease in lifespan. Neither paraquat alone nor paraquat + maneb combined significantly reduced DA cells numbers relative to controls exposed to vehicle alone. By contrast, we observed significant loss of DA neurons in flies expressing E1 RNAi and exposed to maneb for four weeks (Figure 4a, One-way ANOVA, post-hoc Bonferroni, p-value < 0.05). The magnitude of the loss was similar to that observed in wild type flies exposed to paraquat + maneb for six weeks (see Figure 2e). To control for non-specific insertional effects of the transgenes, we similarly tested maneb exposure in flies containing either UAS-E1-RNAi alone or elav-Gal4 alone. We did not detect a loss of DA neurons under these conditions, consistent with the idea that the expression of E1 RNAi was specifically responsible for the observed cell loss (data not shown).
Figure 4. Interaction of paraquat and/or maneb with genetic mimics of ziram.
a) Four week pesticide exposure in E1 RNAi expressing flies results in significantly lower dopaminergic neuron counts for maneb treated groups versus DMSO control (One-way ANOVA p < 0.05, Bonferroni post-hoc; *p < 0.05; n = 11–20 brains and 19–38 PPL1 clusters total per treatment). b) Genetic inhibition of the Pros26 and Pros β2 proteasomal subunits in dopaminergic cells did not show an interaction with any of the indicated pesticide treatments (One-way ANOVA p > 0.05; n = 9–20 brains and 15–38 PPL1 clusters total per treatment). c) Aldehyde dehydrogenase null flies exposed to DMSO 1%, paraquat 4 mM, maneb 500 μM, or paraquat 4 mM plus maneb 500 μM did not show a detectable interaction (One-way ANOVA p > 0.05; n = 6–8 brains and 10–14 PPL1 clusters total, per treatment).
3.4 Inhibition of the proteasome does not appear to synergize with maneb
Conjugation to ubiquitin can have multiple effects including targeting of proteins to the proteasome or autophagosome, regulation of endocytosis, maintenance of synaptic function and alterations in mitochondrial dynamics (Chin et al., 2010, DiAntonio et al., 2004, Schapira, 2011, Schmidt et al., 2014). To test whether proteasome inhibition would mimic the neurotoxic effects of E1 ligase inhibition, we used two previously characterized dominant-negative proteasome subunits, Pros26 and Prosβ2 (Belote et al., 2002). Both constructs are temperature sensitive and show inhibitory effects at the non-permissive temperature (30°C) but not at lower temperatures (e.g. 18°C) (Belote et al., 2002). Flies constitutively expressing one copy of Ddc-Gal4 and each of the Pros subunits consistently died as 3rd instar larva (not shown). To circumvent the lethal effects of proteasome inhibition, cultures were maintained at 18°C throughout development and until 2–4 days post eclosion. The adult flies were then incubated at the non-permissive temperature (30°C) for four weeks in media containing DMSO control, paraquat, maneb, or paraquat + maneb (Figure 4b). In contrast to the results obtained with E1 knockdown, we did not detect a loss of DA cells with proteasomal inhibition in combination with exposure to paraquat, maneb or paraquat + maneb (see Discussion).
3.5 Lack of aldehyde dehydrogenase does not synergize with paraquat and/or maneb
Another proposed mechanism by which pesticides such as ziram may increase neurotoxicity is through inhibition of aldehyde dehydrogenase (ALDH) (Fitzmaurice, 2012, Fitzmaurice et al., 2013, Shen, 2000, Staub et al., 1998, Staub et al., 1995) possibly via metabolism of toxic DA metabolites (Fitzmaurice et al., 2013, Wey et al., 2012). We therefore tested the potential neurotoxic effects of a previously identified mutation in the Drosophila ortholog of mammalian ALDH2 (Fry et al., 2006). Drosophila Aldh null mutants show a reduced lifespan and increased sensitivity to ethanol (Fry et al., 2006). However, we did not detect a DA cell loss at four weeks of age in the absence of additional toxins or in Aldh null flies exposed to paraquat and/or maneb (Figure 4c).
4.0 Discussion
Idiopathic Parkinson’s disease (PD) may require ‘multiple-hits’ and disruption of more than one molecular pathway, by either environmental exposure or genetic variation (Sulzer, 2007). For example, in rodent models, the toxicity of paraquat to DA neurons is dramatically potentiated by maneb (Srivastava et al., 2012, Thiruchelvam, 2000). However, the mechanisms by which these and other agents interact remain obscure (Cicchetti et al., 2005, Li et al., 2005, Thiruchelvam, 2000). To address this question we have developed a chronic exposure model for paraquat and maneb neurotoxicity in the fly. The ease of genetic manipulation in the fly and their short lifespan makes the fly the ideal model organism for studies of chronic neurodegenerative processes. Importantly, flies demonstrate the dopaminergic (DA) cell loss characteristic of PD both by transgenic expression of human PD genes or in the presence of environmental toxins (Lawal et al., 2010, Ming, 2010).
We report that exposure to either paraquat or paraquat and maneb combined results in significantly impaired climbing ability after only one week of exposure and an overall decline in survivorship. More importantly, we demonstrate that six-week exposure to the pesticides paraquat and maneb combined, but neither toxin alone, is associated with DA cell death in the fly. Although a precise comparison to human aging is impossible, the functional decline and proportion of average life span suggests that a 6 week old fly may be comparable to 65–70 year old human (Grotewiel et al., 2005). Interestingly, we did not detect a difference in DA cell death at 4 weeks, which we estimate to be similar to late middle age in humans (Grotewiel et al., 2005, Simon et al., 2006). Prior studies of paraquat exposure in flies show DA cell loss with relatively high doses of paraquat administered in sucrose (Chaudhuri et al., 2007, Lawal et al., 2010); however, in this paradigm flies die within four days of exposure and sucrose itself may be toxic under these conditions (Rzezniczak et al., 2011). Importantly, pathological changes in PD patients usually manifest decades after exposure to high dose pesticide (Goldman, 2014). We suggest that our model of chronic exposure may be useful to test the long-term effects of pesticides, and the mechanisms by which paraquat, maneb and other pesticides and genetic risk alleles may interact.
Here, we have focused on potential neurotoxic mechanisms previously proposed for ziram, a dithiocarbamate extensively used in the central valley of California and with annual national use approaching 2 million pounds (Shackleford, 2003). In human populations, ziram exposure alone increases PD risk 37%; combined exposure to both paraquat and ziram increases risk further to 82%. Exposure to paraquat, maneb and ziram combined increases PD risk even further to 209% of controls (Wang et al., 2011). The apparent interaction of ziram with paraquat and maneb in epidemiological studies suggests that they might also interact in model systems, but this possibility has not been tested. By extension, we speculate that the combined inhibition of multiple biochemical pathways by these pesticides might have synergistic effects, but this, too has not been tested.
Model systems such as Drosophila are useful for testing the effects of multiple environmental or genetic insults because of their low cost and short life span. In addition, model genetic systems offer the opportunity to explore in vivo the contribution of specific neurotoxic mechanisms of action through the use of genetic tools that mimic the proposed activities of specific pesticides. As shown here and in previous studies (Lawal et al., 2010), ziram itself does not affect the survival, behavior, or DA cell counts in the fly. By contrast, ex vivo exposure of the fly nervous to ziram has robust effects on neuronal function in the fly (manuscript in preparation) that are similar to those seen in cultured mammalian neurons exposed to ziram (Rinetti et al., 2010). We suspect that toxicokinetic issues such as limited absorption in the gut or restricted passage through the glial barrier which envelopes the fly brain may minimize the potential effects of ziram when administered via ingestion. We therefore used a genetic approach to target pathways proposed to be inhibited by ziram, based on studies of cultured mammalian cells (Chou et al., 2008, Fitzmaurice, 2012). This strategy allowed us to specifically test the impact of two proposed mechanisms of action while simultaneously avoiding potential confounds caused by toxicokinetic issues. We propose that this strategy might be generally useful to explore the toxicologic effects of other pesticides in vivo.
Ziram has been shown to inhibit aldehyde dehydrogenase (ALDH) in vitro and ALDH can detoxify DOPAL, a reactive byproduct of dopamine metabolism (Fitzmaurice et al., 2013). We hypothesized that knockout of ALDH might be toxic to DA neurons and potentiate the affects of paraquat and/or maneb exposure. However, we did not detect any loss of DA neurons in flies lacking ALDH alone, nor do we observe any increase in cell loss in ALDH null flies exposed to paraquat and/or maneb for four weeks. Multiple isoforms of ALDH are present in both flies and humans, and their role in DA metabolism is poorly understood (Chakraborty et al., 2011, Marchitti et al., 2007). The mitochondrial ALDH2 and cytosolic ALDH1a1 are thought to be the primary isoforms responsible for DA breakdown in human cells, however, involvement of other ALDH isoforms has not been ruled out (Marchitti et al., 2007). Similarly, the specific roles for particular isoforms of ALDH in the fly have not been fully elucidated (Chakraborty et al., 2011). Therefore, our data must be interpreted with caution and further experiments will be needed to rule out a role for ALDH in neurotoxic processes in Drosophila.
Deregulation of the Ubiquitin Proteasome System (UPS) has been proposed as a possible pathogenic mechanism in both sporadic and genetic forms of PD (Olanow et al., 2006), and multiple pesticides, including ziram, are known to inhibit components of the UPS (Wang et al., 2006). Ziram specifically inhibits the E1 ligase (Chou et al., 2008), the first enzyme of the biochemical cascade required for protein conjugation to ubiquitination (Kleiger et al., 2014). To knockdown E1 expression, we expressed an RNAi transgene directed against the Drosophila E1 ligase. We then exposed flies with pan-neuronal expression of E1 RNAi to either paraquat, maneb, or both pesticides combined and assessed DA cell loss. In conjunction with E1 knock down, we observed significant DA cell loss at 4 weeks in flies exposed to maneb but not in other treatment groups. Decreased survival of flies expressing E1 RNAi prevented similar cell counts at the later 6 week time point used in experiments with wild type flies. It is interesting to note that we could detect DA cell loss by 4 weeks, approximately late middle age, in flies expressing a genetic susceptibility but not the wild type strain. These observations complement previous in vitro studies on the neurotoxic effects of E1 inhibition, and the proposed mechanism of action of ziram (Chou et al., 2008). Our data suggest that E1 inhibition may show synergistic effects with other pesticides in in vivo. We speculate that these results may help to unravel the mechanisms by which these agents interact synergistically to increase the risk for PD in patients.
One downstream effect of E1 inhibition would be decreased targeting of proteins for proteasomal degradation. We therefore postulated that the interaction between E1 inhibition and maneb could result from a decrease in protein degradation via the proteasome. To test this hypothesis, we use temperature sensitive proteasomal subunits to directly inhibit the proteasome and exposed flies to maneb and/or paraquat. In contrast to E1 knockdown, we did not observe an interaction between direct proteasome inhibition and either paraquat or maneb. Our data are consistent with those obtained in vitro in which ziram failed to directly inhibit the activity of the proteasome (Chou et al., 2008). Together, these studies raise the possibility that inhibition of E1 ligase is neurotoxic because of the disruption of ubiquitination-dependent processes other than protein degradation by the proteasome. These might include modifications of the aggresome-autophagy pathway, mitochondria fission and mitophagy, or alpha-synuclein aggregation (Beyer et al., 2013, Chin et al., 2010, Schapira, 2011). Important caveats to this interpretation include the possibility that the inhibitory proteasome subunits were insufficiently active in our studies. Previous studies demonstrate the ability of these constructs have potent effects in a variety of tissues (Neuburger et al., 2006, Schweisguth, 1999). Their activity in our own studies is supported by the observation that expression in cuticle forming tissue was lethal (not shown), and that expression in neurons decreases degradation of a construct targeted to the proteasome. In spite of these observations, it remains possible that higher levels of expression would be required to detect neurotoxic effects in neurons. We also cannot exclude the possibility that other mitigating effects, such as the induction of heat shock (hs) proteins, obscured the effects of proteasome inhibition. Overexpression of hs proteins in adult flies provides neuroprotection against insults relevant to PD (Auluck et al., 2002, Shukla et al., 2014), and exposure to temperatures ≥ 34°C has been shown to significantly increase hs protein expression (Auluck et al., 2005). Temperatures ≤ 30°C do not significantly elevate hs proteins expression in the fly (Auluck et al., 2005) and the maximum temperature used in our experiments was 30°C. Nonetheless, it remains possible that low levels of hs proteins were expressed and that the neuroprotective effects of hs proteins induction masked the neurotoxic effects of proteasome inhibition. Thus, we cannot yet definitively conclude that the neurotoxic effects of E1 inhibition occurred independently of the proteasome.
It remains unclear why chronic exposure to maneb alone increased lifespan. It is also unclear why we observed loss of DA neurons with E1 inhibition + maneb but not E1 inhibition + maneb and paraquat. It is possible that we simply failed to detect some toxic effects. However, in some cases, limited exposure to toxins can be protective against other insults (Calabrese, 2008). Exposure to paraquat paradoxically increased the life span in C. elegans on a calorie restrictive diet (Schulz et al., 2007). Paraquat also increased survival in flies expressing decreased levels of the PD-associated protein parkin (Bonilla-Ramirez et al., 2013). A similar phenomenon may have occurred under some of the conditions used in our studies.
4.1 Conclusions
In the ‘multiple-hit’ model of sporadic PD, inhibition of at two least pathways are hypothesized to be necessary for pathogenesis and the apparent lack of individual risk factors to cause disease (Sulzer, 2007). However, multiple genetic and environmental risk factors have been identified and it remains unclear which combinations may be most relevant. We have shown that under the chronic exposure conditions used here, paraquat and maneb combined, but neither paraquat nor maneb alone, cause DA cell loss at six weeks. We have further exploited this chronic exposure paradigm to show synergistic effects of maneb and ubiquitin E1 ligase inhibition. The interactions we observe may be relevant to human populations at risk for PD.
Highlights.
We report a Drosophila model of chronic pesticide exposure relevant to Parkinson’s disease (PD).
We have used this model to demonstrate a neurotoxic interaction between: 1) paraquat and maneb, and 2) between maneb and inhibition of ubiquitin E1 ligase.
These interactions may be relevant to pathophysiologic mechanisms of PD that involve multiple neurotoxic insults.
Acknowledgments
We thank Felix Schweizer for his advice and Marie-Francoise Chesselet for her leadership as principal investigator for the program project grant from the National Institute of Environmental Health and Safety [ES016732].
Funding:
This work was conducted with funding from the National Institute of Environmental Health and Safety (NIEHS), UCLA training grant in Molecular Toxicology, USHHS Ruth L. Kirschstein Institutional National Research Service Award T32 ES015457, (C.A.M. pre-doctoral and H.O.L. post-doctoral) and NIEHS R01ES015747 (D.E.K.), funding from the Parkinson’s Disease Foundation (PDF-SFW-1336 to C.A.M.), from The Brain and Behavior Research Foundation and the Joanne and George Miller and Family Endowed Chair in Depression Research at the UCLA Brain Research Institute (D.E.K.), and an NIEHS program project grant ES016732 (M.F. Chesselet, PI).
Footnotes
Financial interests declaration:
None
Conflict of Interest Form
We declare that there are no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ciara A. Martin, Email: martin.ciaraann@gmail.com.
Angel Barajas, Email: a.barajas40@gmail.com.
George Lawless, Email: glawless@mednet.ucla.edu.
Hakeem O. Lawal, Email: hakeemlawal@gmail.com.
Khadij Assani, Email: khadij.assani@gmail.com.
Yosephine P. Lumintang, Email: y.lumintang@yahoo.com.
Vanessa Nunez, Email: vnunez16@ucla.edu.
David E. Krantz, Email: dkrantz@ucla.edu.
References
- Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson’s disease. Science. 2002;295:865–8. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- Auluck PK1, Meulener MC, Bonini NM. Mechanisms of suppression of alpha-synuclein neurotoxicity by geldanamycin in Drosophila. Journal of Biological Chemistry. 2005;280:2873–8. doi: 10.1074/jbc.M412106200. [DOI] [PubMed] [Google Scholar]
- Belote JM, Fortier E. Targeted expression of dominant negative proteasome mutants in Drosophila melanogaster. Genesis. 2002;34:80–2. doi: 10.1002/gene.10131. [DOI] [PubMed] [Google Scholar]
- Benzer S. Behavioral mutants of Drosophila isolated by countercurrent distribution. Proceedings of the National Academy of Sciences. 1967;58:1112–9. doi: 10.1073/pnas.58.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer K, Ariza A. Alpha-synuclein posttranslational modification and alternative splicing as a trigger for neurodegeneration. Mol Neurobiol. 2013;47:509–24. doi: 10.1007/s12035-012-8330-5. [DOI] [PubMed] [Google Scholar]
- Bonilla E, Medina-Leendertz S, Díaz S. Extension of life span and stress resistance of Drosophila melanogaster by long-term supplementation with melatonin. Experimental Gerontology. 2002;37:629–38. doi: 10.1016/s0531-5565(01)00229-7. [DOI] [PubMed] [Google Scholar]
- Bonilla-Ramirez L, Jimenez-Del-Rio M, Velez-Pardo C. Low doses of paraquat and polyphenols prolong life span and locomotor activity in knock-down parkin Drosophila melanogaster exposed to oxidative stress stimuli: Implication in autosomal recessive juvenile Parkinsonism. Gene. 2013;512:355–63. doi: 10.1016/j.gene.2012.09.120. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Hormesis and medicine. British Journal of Clinical Pharmacology. 2008;66:594–617. doi: 10.1111/j.1365-2125.2008.03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty M, Fry JD. Drosophila lacking a homologue of mammalian aldh2 have multiple fitness defects. Chemico-Biological Interactions. 2011;191:296–302. doi: 10.1016/j.cbi.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri A, Bowling K, Funderburk C, Lawal H, Inamdar A, Wang Z, O’Donnell JM. Interaction of genetic and environmental factors in a Drosophila Parkinsonism model. The Journal of Neuroscience. 2007;27:2457–67. doi: 10.1523/JNEUROSCI.4239-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin LS, Fau OJ, Li L. Parkin-mediated ubiquitin signalling in aggresome formation and autophagy. Biochem Soc Trans. 2010;38:144–9. doi: 10.1042/BST0380144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou AP, Maidment N, Klintenberg R, Casida JE, Li S, Fitzmaurice AG, Fernagut P-O, Mortazavi F, Chesselet M-F, Bronstein JM. Ziram causes dopaminergic cell damage by inhibiting e1 ligase of the proteasome. Journal of Biological Chemistry. 2008;283:34696–703. doi: 10.1074/jbc.M802210200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti F, Lapointe N, Roberge-Tremblay A, Saint-Pierre M, Jimenez L, Ficke BW, Gross RE. Systemic exposure to paraquat and maneb models early Parkinson’s disease in young adult rats. Neurobiology of Disease. 2005;20:360–71. doi: 10.1016/j.nbd.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Costello S, Cockburn M, Bronstein J, Zhang X, Ritz B. Parkinson’s disease and residential exposure to maneb and paraquat from agricultural applications in the Central Valley of California. American Journal of Epidemiology. 2009;169:919–26. doi: 10.1093/aje/kwp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulom H, Birman S. Chronic exposure to rotenone models sporadic Parkinson’s disease in Drosophila melanogaster. The Journal of Neuroscience. 2004;24:10993–8. doi: 10.1523/JNEUROSCI.2993-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiAntonio A, Hicke L. Ubiquitin-dependent regulation of the synapse. Annual Review of Neuroscience. 2004;27:223–46. doi: 10.1146/annurev.neuro.27.070203.144317. [DOI] [PubMed] [Google Scholar]
- Doorn JA, Florang VR, Schamp JH, Vanle BC. Aldehyde dehydrogenase inhibition generates a reactive dopamine metabolite autotoxic to dopamine neurons. Parkinsonism & Related Disorders. 2014;20(Supplement 1):S73–S5. doi: 10.1016/S1353-8020(13)70019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi-Fakhari D, Wahlster L, McLean P. Protein degradation pathways in Parkinson’s disease: Curse or blessing. Acta Neuropathologica. 2012;124:153–72. doi: 10.1007/s00401-012-1004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzmaurice AG. Doctral Dissertaion. California Institute of Technology; 2012. The role of pesticide-induced aldehyde dehydrogenase inhibition in the pathogenesis of Parkinson’s disease. [Google Scholar]
- Fitzmaurice AG, Rhodes Sl, Cockburn, Ritz B, Bronstein JM. Aldehyde dehydrogenase variation enhances effect of pesticides associated with Parkinson disease. Neurology. 2014;82:419–26. doi: 10.1212/WNL.0000000000000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzmaurice AG, Rhodes SL, Lulla A, Murphy NP, Lam HA, O’Donnell KC, Barnhill L, Casida JE, Cockburn M, Sagasti A, Stahl MC, Maidment NT, Ritz B, Bronstein JM. Aldehyde dehydrogenase inhibition as a pathogenic mechanism in Parkinson disease. Proceedings of the National Academy of Sciences. 2013;110:636–41. doi: 10.1073/pnas.1220399110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry JD, Saweikis M. Aldehyde dehydrogenase is essential for both adult and larval ethanol resistance in Drosophila melanogaster. Genetics Research. 2006;87:87–92. doi: 10.1017/S0016672306008032. [DOI] [PubMed] [Google Scholar]
- Giordano E, Rendina R, Peluso I, Furia M. RNAi triggered by symmetrically transcribed transgenes in Drosophila melanogaster. Genetics. 2002;160:637–48. doi: 10.1093/genetics/160.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SM. Environmental toxins and Parkinson’s disease. Annual Review of Pharmacology and Toxicology. 2014;54:141–64. doi: 10.1146/annurev-pharmtox-011613-135937. [DOI] [PubMed] [Google Scholar]
- Grotewiel MS, Martin I, Bhandari P, Cook-Wiens E. Functional senescence in Drosophila melanogaster. Ageing Research Reviews. 2005;4:372–97. doi: 10.1016/j.arr.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Hamilton AM, Zito K. Breaking it down: The ubiquitin proteasome system in neuronal morphogenesis. Neural Plasticity. 2013;2013:10. doi: 10.1155/2013/196848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosamani R, Muralidhara Acute exposure of Drosophila melanogaster to paraquat causes oxidative stress and mitochondrial dysfunction. Archives of Insect Biochemistry and Physiology. 2013;83:25–40. doi: 10.1002/arch.21094. [DOI] [PubMed] [Google Scholar]
- Humphreys JM, Hilliker AJ, Phillips JP. Paraquat selection identifies x-linked oxygen defense genes in Drosophila melanogaster. Genome. 1993;36:162–5. doi: 10.1139/g93-021. [DOI] [PubMed] [Google Scholar]
- Kleiger G, Mayor T. Perilous journey: A tour of the ubiquitin–proteasome system. Trends in Cell Biology. 2014;24:352–9. doi: 10.1016/j.tcb.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Kanaan NM, Chu Y, Suresh Babu R, Stansell J, Terpstra BT, Sortwell CE, Steece-Collier K, Collier TJ. Failure of proteasome inhibitor administration to provide a model of Parkinson’s disease in rats and monkeys. Annals of Neurology. 2006;60:264–8. doi: 10.1002/ana.20935. [DOI] [PubMed] [Google Scholar]
- Lawal HO, Chang HY, Terrell AN, Brooks ES, Pulido D, Simon AF, Krantz DE. The Drosophila vesicular monoamine transporter reduces pesticide-induced loss of dopaminergic neurons. Neurobiology of Disease. 2010;40:102–12. doi: 10.1016/j.nbd.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawal HO, Terrell A, Lam HA, Djapri C, Jang J, Hadi R, Roberts L, Shahi V, Chou MT, Biedermann T, Huang B, Lawless GM, Maidment NT, Krantz DE. Drosophila modifier screens to identify novel neuropsychiatric drugs including aminergic agents for the possible treatment of Parkinson’s disease and depression. Molecular Psychiatry. 2014;19:235–42. doi: 10.1038/mp.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-Y, Nagano Y, Taylor JP, Lim KL, Yao T-P. Disease-causing mutations in parkin impair mitochondrial ubiquitination, aggregation, and hdac6-dependent mitophagy. The Journal of Cell Biology. 2010;189:671–9. doi: 10.1083/jcb.201001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legan SK, Rebrin I, Mockett RJ, Radyuk SN, Klichko VI, Sohal RS, Orr WC. Overexpression of glucose-6-phosphate dehydrogenase extends the life span of Drosophila melanogaster. Journal of Biological Chemistry. 2008;283:32492–9. doi: 10.1074/jbc.M805832200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Chaney S, Forte M, Hirsh J. Ectopic g-protein expression in dopamine and serotonin neurons blocks cocaine sensitization in Drosophila melanogaster. Current Biology. 2000;10:211–4. doi: 10.1016/s0960-9822(00)00340-7. [DOI] [PubMed] [Google Scholar]
- Li X, Matsumoto K, Murakami Y, Tezuka Y, Wu Y, Kadota S. Neuroprotective effects of polygonum multiflorum on nigrostriatal dopaminergic degeneration induced by paraquat and maneb in mice. Pharmacology Biochemistry and Behavior. 2005;82:345–52. doi: 10.1016/j.pbb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Liou H-H, Chen R-C, Tsai Y-F, Chen W-P, Chang Y-C, Tsai M-C. Effects of paraquat on the substantia nigra of the wistar rats: Neurochemical, histological, and behavioral studies. Toxicology and Applied Pharmacology. 1996;137:34–41. doi: 10.1006/taap.1996.0054. [DOI] [PubMed] [Google Scholar]
- Marchitti SA, Deitrich RA, Vasiliou V. Neurotoxicity and metabolism of the catecholamine-derived 3,4-dihydroxyphenylacetaldehyde and 3,4-dihydroxyphenylglycolaldehyde: The role of aldehyde dehydrogenase. Pharmacological Reviews. 2007;59:125–50. doi: 10.1124/pr.59.2.1. [DOI] [PubMed] [Google Scholar]
- Matsui H, Ito H, Taniguchi Y, Inoue H, Takeda S, Takahashi R. Proteasome inhibition in medaka brain induces the features of Parkinson’s disease. Journal of Neurochemistry. 2010;115:178–87. doi: 10.1111/j.1471-4159.2010.06918.x. [DOI] [PubMed] [Google Scholar]
- McCormack AL, Thiruchelvam M, Manning-Bog AB, Thiffault C, Langston JW, Cory-Slechta DA, Di Monte DA. Environmental risk factors and Parkinson’s disease: Selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiology of Disease. 2002;10:119–27. doi: 10.1006/nbdi.2002.0507. [DOI] [PubMed] [Google Scholar]
- McNaught KSP, Perl DP, Brownell A-L, Olanow CW. Systemic exposure to proteasome inhibitors causes a progressive model of Parkinson’s disease. Annals of Neurology. 2004;56:149–62. doi: 10.1002/ana.20186. [DOI] [PubMed] [Google Scholar]
- Guo M. What have we learned from Drosophila models of Parkinson’s disease? In: Anders B, Cenci MA, editors. Progress in brain research. Elsevier; 2010. pp. 2–16. [DOI] [PubMed] [Google Scholar]
- Neuburger PJ, Saville KJ, Zeng J, Smyth K-A, Belote JM. A genetic suppressor of two dominant temperature-sensitive lethal proteasome mutants of Drosophila melanogaster is itself a mutated proteasome subunit gene. Genetics. 2006;173:1377–87. doi: 10.1534/genetics.106.057976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanow CW, McNaught KSP. Ubiquitin–proteasome system and Parkinson’s disease. Movement Disorders. 2006;21:1806–23. doi: 10.1002/mds.21013. [DOI] [PubMed] [Google Scholar]
- Pandey UB, Nie Z, Batlevi Y, McCray BA, Ritson GP, Nedelsky NB, Schwartz SL, DiProspero NA, Knight MA, Schuldiner O, Padmanabhan R, Hild M, Berry DL, Garza D, Hubbert CC, Yao T-P, Baehrecke EH, Taylor JP. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature. 2007;447:859–63. doi: 10.1038/nature05853. [DOI] [PubMed] [Google Scholar]
- Rinetti GV, Schweizer FE. Ubiquitination acutely regulates presynaptic neurotransmitter release in mammalian neurons. The Journal of Neuroscience. 2010;30:3157–66. doi: 10.1523/JNEUROSCI.3712-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinow S, White K. Characterization and spatial distribution of the elav protein during Drosophila melanogaster development. Journal of Neurobiology. 1991;22:443–61. doi: 10.1002/neu.480220503. [DOI] [PubMed] [Google Scholar]
- Rzezniczak TZ, Douglas LA, Watterson JH, Merritt TJS. Paraquat administration in Drosophila for use in metabolic studies of oxidative stress. Analytical Biochemistry. 2011;419:345–7. doi: 10.1016/j.ab.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Schapira AHV. Mitochondrial pathology in Parkinson’s disease. Mount Sinai Journal of Medicine: A Journal of Translational and Personalized Medicine. 2011;78:872–81. doi: 10.1002/msj.20303. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Finley D. Regulation of proteasome activity in health and disease. Molecular Cell Research. 2014;1843:13–25. doi: 10.1016/j.bbamcr.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metabolism. 2007;6:280–93. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Schweisguth F. Dominant-negative mutation in the β2 and β6 proteasome subunit genes affect alternative cell fate decisions in the Drosophila sense organ lineage. Proceedings of the National Academy of Sciences. 1999;96:11382–6. doi: 10.1073/pnas.96.20.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleford B. Reregistration eligibility decision for ziram pc code: 034805 case. 2003:2180. http://www.epa.gov/oppsrrd1/REDs/ziram_red.pdf.
- Shen M, Lipsky J, Naylor S. Role of disulfiram in the in vitro inhibition of rat liver mitochondrial aldehyde dehydrogenase. Biochem Pharmacol. 2000;60:947–53. doi: 10.1016/s0006-2952(00)00435-4. [DOI] [PubMed] [Google Scholar]
- Shin M, Jan C, Jacquard C, Jarraya B, Callebert J, Launay J-M, Hantraye P, Remy P, Palfi S, Brouillet E. Chronic systemic treatment with a high-dose proteasome inhibitor in mice produces akinesia unrelated to nigrostriatal degeneration. Neurobiology of Aging. 2011;32:2100–2. doi: 10.1016/j.neurobiolaging.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Shukla AK, Pragya P, Chaouhan HS, Tiwari AK, Patel DK, Abdin MZ, Chowdhuri DK. Heat shock protein-70 (hsp-70) suppresses paraquat-induced neurodegeneration by inhibiting jnk and caspase-3 activation in Drosophila model of Parkinson’s disease. PLoS ONE. 2014;9:e98886. doi: 10.1371/journal.pone.0098886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AF, Liang DT, Krantz DE. Differential decline in behavioral performance of Drosophila melanogaster with age. Mechanisms of Ageing and Development. 2006;127:647–51. doi: 10.1016/j.mad.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Simon AFSCAS. Steroid control of longevity in Drosophila melanogaster. Science. 2003;299:1407. doi: 10.1126/science.1080539. [DOI] [PubMed] [Google Scholar]
- Srivastava G, Dixit A, Yadav S, Patel DK, Prakash O, Singh MP. Resveratrol potentiates cytochrome p450 2 d22-mediated neuroprotection in maneb- and paraquat-induced Parkinsonism in the mouse. Free Radical Biology and Medicine. 2012;52:1294–306. doi: 10.1016/j.freeradbiomed.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Staub RE, Quistad GB, Casida JE. Mechanism for benomyl action as a mitochondrial aldehyde dehydrogenase inhibitor in mice. Chemical Research in Toxicology. 1998;11:535–43. doi: 10.1021/tx980002l. [DOI] [PubMed] [Google Scholar]
- Staub RE, Sparks SE, Quistad GB, Casida JE. S-methylation as a bioactivation mechanism for mono- and dithiocarbamate pesticides as aldehyde dehydrogenase inhibitors. Chemical Research in Toxicology. 1995;8:1063–9. doi: 10.1021/tx00050a010. [DOI] [PubMed] [Google Scholar]
- Sulzer D. Multiple hit hypotheses for dopamine neuron loss in Parkinson’s disease. Trends in Neurosciences. 2007;30:244–50. doi: 10.1016/j.tins.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam M, Brockel B, Richfield E, Baggs R, Cory-Slechta D. Potentiated and preferential effects of combined paraquat and maneb on nigrostriatal dopamine systems: Environmental risk factors for Parkinson’s disease? Brain Research. 2000;873:225–34. doi: 10.1016/s0006-8993(00)02496-3. [DOI] [PubMed] [Google Scholar]
- Thrash B, Uthayathas, Karuppagounder SS, Suppiramaniam V, Dhanasekaran M. Paraquat and maneb induced neurotoxicity. Proc West Pharmacol Soc. 2007;50:31–42. [PubMed] [Google Scholar]
- Wang A, Costello S, Cockburn M, Zhang X, Bronstein J, Ritz B. Parkinson’s disease risk from ambient exposure to pesticides. European Journal of Epidemiology. 2011:1–9. doi: 10.1007/s10654-011-9574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-F, Li S, Chou AP, Bronstein JM. Inhibitory effects of pesticides on proteasome activity: Implication in Parkinson’s disease. Neurobiology of Disease. 2006;23:198–205. doi: 10.1016/j.nbd.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Wey MC-Y, Fernandez E, Martinez PA, Sullivan P, Goldstein DS, Strong R. Neurodegeneration and motor dysfunction in mice lacking cytosolic and mitochondrial aldehyde dehydrogenases: Implications for Parkinson’s disease. PLoS ONE. 2012;7:e31522. doi: 10.1371/journal.pone.0031522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Fitsanakis V, Gu G, Jing D, Ao MF, Amarnath V, Montine TJ. Manganese ethylene-bis-dithiocarbamate and selective dopaminergic neurodegeneration in rat: A link through mitochondrial dysfunction. J Neurochem. 2003;84:336–46. doi: 10.1046/j.1471-4159.2003.01525.x. [DOI] [PubMed] [Google Scholar]