Abstract
Eleven species of kissing bugs are found in the United States. Their home ranges may be expanding northward, perhaps as a consequence of climate change. At least eight of the species, perhaps all, are reported to harbor Trypanosoma cruzi, the parasite that causes Chagas disease. Because humans are encroaching on kissing bug habitat, there is concern for vector-transmitted Chagas disease in the United States. To date, documented autochthonous cases of Chagas in humans in the United States are rare. Kissing bugs are capable of adapting to new habitats such as human domiciles; however, they do not colonize homes in the United States as in Central and South America. We review the biology, behavior, and medical importance of kissing bugs and the risk they pose for transmission of Chagas disease in the United States. Where possible, descriptions of US species are compared to the epidemiologically important Latin American species.
Keywords: kissing bugs, triatomine, Trypanosoma cruzi, Chagas disease, anaphylaxis, kissing bug bites, pest management
Introduction
Kissing bugs are hematophagous (bloodsucking) insects found almost exclusively in the New World. Aside from being a nuisance following invasion of human habitations, they are important medically because they are vectors of T. cruzi, the protozoan parasite that causes Chagas disease. Kissing bugs (also known as Walapai tigers, vinchucas, or chinches, among many other terms) consist of approximately 140 species. These bugs are classified in the Order, Hemiptera, Family, Reduviidae. There are two tribes, Triatomini and Rhodniini, and two genera are found in the United States, Triatoma and Paratriatoma. (In this report we use the terms, triatomine and kissing bug synonymously.) Kissing bugs likely evolved from a reduviid progenitor that fed on insect prey1 and have specialized in sucking blood from a wide variety of animals, including birds, mammals, reptiles, amphibians, and even invertebrates. Closely related assassin bugs feed on insects and inject toxins and proteases that kill the prey and liquefy its body contents. Assassin bugs, however, are strictly insectivorous, have a painful bite, and are often confused for kissing bugs by the lay public. Kissing bugs are important because they harbor T. cruzi in the terminal portions of the gastrointestinal tract. The kissing bugs acquire this parasite by feeding upon infected vertebrate hosts. The parasite may affect the behavior of the insect host. For example, Mepraia spinolai, a sylvatic diurnal vector in Chile, bites more often and defecates more frequently when harboring T. cruzi.2 In contrast, there did not appear to be measurable changes in the physiology of Triatoma infestans harboring T. cruzi.3,4 Trypanosoma cruzi infection is a fairly common zoonosis in the southern tier of states in the United States (see Wild and domesticated animals infected with Trypanosoma cruzi in the United States section), where kissing bugs are found in 27 states (Fig. 1). The parasite enters the gastrointestinal tract of the kissing bug during the act of sucking blood from an infected vertebrate host. Epimastigotes are then found free in the small intestine of the insect by the thousands (estimated up to 60,000) and pass on to the rectum.5 Infectious metacyclic trypomastigotes line the rectal gland in the terminal part of the abdomen, and their flagella are attached by hemidesmosomes to the gut epithelium.5 Free metacyclic trypomastigotes pass out in the urine/feces. Starvation reduces the parasite count in the abdomen, and if prolonged, parasites in the gastrointestinal tract become non-viable.6 The zoonosis is maintained in wild animals by mammalian wildlife hosts ingesting whole bugs or contaminated feces or contamination of a bite site with infected feces, a characteristic of human acquisition of T. cruzi.
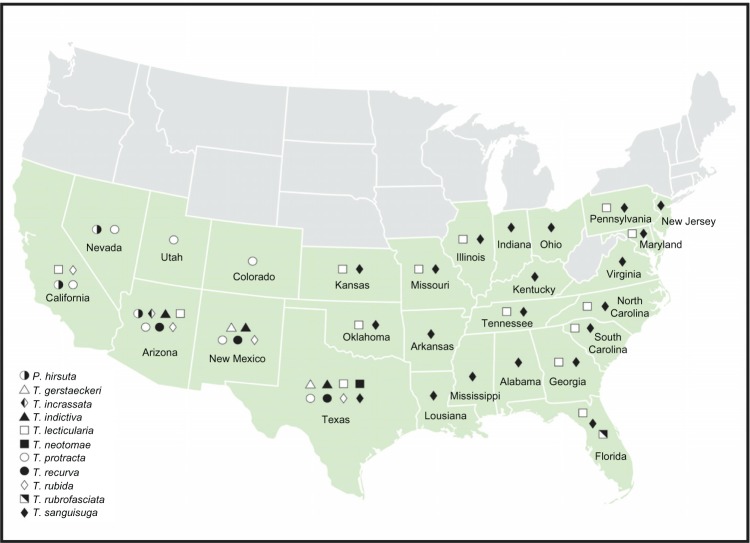
Figure 1.
Map of distribution of kissing bug species in the Continental United States. Information used to make this map represents the latest documentation and taxonomy.
Trypanosoma cruzi is a member of a large group of protozoan parasites of worldwide distribution. Within the genus Trypanosoma are about 10 clades of species, with some specializing on fish, others on amphibians, lizards, crocodiles, or birds, plus those infecting mammals.7 Two clades are of particular interest to humans: the brucei and cruzi clades. Although both clades feed on humans and cause serious disease they independently evolved to use humans as a food source.8 They are also transmitted by different vectors. Within the brucei clade, transmission is mainly via saliva of tsetse flies; in the cruzi clade, transmission is by triatomine bugs through fecal contamination or ingestion. Within the brucei clade are about five important species that are pathogens of domestic livestock, including brucei itself, which is the pathogen responsible for African trypanosomiasis. The cruzi clade includes about 10 species that infect an enormous range of mammals (plus other taxa) including, but not limited to, marsupials, armadillos, bats, civets, monkeys, rodents, raccoons, and carnivores. The species of greatest concern are Trypanosoma rangeli, which infects humans but is not considered pathogenic, and T. cruzi sensu stricto, the pathogen responsible for Chagas disease. Trypanosoma rangeli is transmitted only by bugs in the genus Rhodnius, while T. cruzi is transmitted by a wide variety of bugs within the Triatominae, including Rhodnius, Triatoma, and others.
While feeding on the human host, the epidemiologically important kissing bug species such as T. infestans in South America, defecate on the host’s skin. Trypomastigotes of T. cruzi present in the feces/urine are rubbed into the bite wound or onto mucosal surfaces such as the eyes, nose, and mouth, where they penetrate the epithelium. While vector-borne transmission of T. cruzi is the most common form of disease acquisition, other means of acquiring Chagas disease include congenital, blood transfusion, organ transplantation, and ingestion of contaminated food or drink.
When and how some triatomines became specialist feeders on humans is a mystery. The triatomine subfamily is commonly believed to have originated about 5 million years ago in the New World,9, but fossil evidence from Dominican amber indicates that the bloodsucking origin in triatomines is much more ancient.10 Dates for the arrival of human beings in the New World are debated, but their estimated arrival sometime between 14,000 to 20,000 years ago is relatively recent.
Lent and Wygodzinsky remark that the principal characteristics of domesticated kissing bug species, ie, those colonizing homes and transmitting Chagas disease are (a) adaptation to life in human habitations; (b) high degree of anthropophily; (c) short span of time between blood meal and defecation; and (d), wide geographical distribution.11 These characteristics will be discussed further in later sections of this report.
Biology of Kissing Bugs
Triatomes reside in nests and burrows of a variety of mammals as well as human domiciles, where they feed upon vertebrate and invertebrate blood. Some species are associated with arboreal hosts. Triatoma infestans, historically the most important vector of Chagas disease in South America, has co-evolved with humans to the point that its entire life is spent in human habitations or peridomiciliary structures, such as sheds and corrals.
Kissing bug eggs hatch into wingless larvae, often called nymphs, of which there are five stages, each stage requiring at least one blood meal to molt. The fifth instar or nymph molts to a winged adult that, when given the opportunity, feeds every several weeks, or even more than once a week, depending upon ambient temperature and season of the year.9 When food is unavailable, or conditions unsuitable, T. infestans in South America can survive for 7 months without feeding. The life span of kissing bugs in North America is approximately a year, perhaps longer for the large southern species, Triatoma recurva. In the temperate zone, there is usually one generation per year, whereas in Mesoamerica and parts of tropical South America, several generations of bugs may occur within a year. In captivity, adults of southwestern US species live 4–5 months. Well-fed nymphs give rise to larger adults, but longevity of adults is enhanced by feedings that are more spaced apart.11 Kissing bugs in the Southwest (Figs. 2–4), particularly Triatoma rubida and Triatoma protracta, commonly infest packrat (Neotoma albigula) nests and before or during the summer monsoon rains undergo dispersal flights at dusk. They are attracted to lights in houses and may enter these domiciles where they survive for some time feeding on humans and pets in the household.
Figure 2.

Female Triatoma rubida, a common cause of anaphylaxis in Arizona. (Photograph by Justin Schmidt with permission.)
Figure 4.

Female Triatoma recurva, the largest kissing bug in the United States. (Photograph by Jillian Cowles with permission.)
Kissing bugs, like virtually all other insects communicate with pheromones. Kissing bugs are members of the Order Hemiptera, famous for their stink glands that produce potent allomones used as chemical defensives.12 In kissing bugs, these defensive glands, known as Brindley’s glands13 secrete volatile short-chained fatty acids and alcohols including isobutyric acid, 2-methylbutanol, 3-methylbutanol, and isobutanol.14 Brindley’s secretions serve the dual roles of defense and alarm. Immature bugs lack the glands, but respond with alarm and escape when they detect the odors released by adults that were disturbed.15 Both male and female secretions contain the same chemicals in their Brindley’s glands.14
Sexual communication in triatomines remains poorly understood. Sex pheromones are produced in adult metasternal glands. Unlike sex pheromones that are unique to one sex, they appear to be of identical composition in the glands of both sexes. The situation is further complicated in that only females release the pheromones during early night calling for males. Males appear not to release the glandular secretions at this time.16 This differential attraction of males only by females suggests that females are polyandrous and seek multiple matings with males, whereas males do not act to attract others, something counter to a male’s interest. Then, why do males have metasternal glands and what is their function in males? The answer is unclear – perhaps the glandular secretion acts as a chemical defense to augment that of Brindley’s gland.14
Aggregation of kissing bugs in hiding refuges appears to be mediated in part by pheromones present in feces17 and epicuticular hydrocarbons on the bug body.18 The attraction appears weak and difficult to demonstrate and dependent on the status of individual bugs. Unfed, immature T. infestans were attracted to papers contaminated with feces, whereas recently fed individuals were not attracted.17 The main attractants in epicuticular lipids are the free fatty acids steric (octadecanoic acid) and, at low concentrations, hexacosanoic acid. At high concentrations, hexacosanoic acid is repellent.18
Kissing bugs are also able to sing, although the purpose of stridulating or sound production is unknown.19 The long proboscis of kissing bugs terminates at the rostrum, and when the bug is not feeding, it is folded under the prosternum and lays in a recess or stridulitrum. This elegant structure has chitinous, transverse grooves or sulci that form a sort of washboard. Rubbing the rostrum on the stridulitrum gives rise to sound, a barely audible chirping.
Female kissing bugs readily mate with multiple males and can store sperm for several months. Over her life span, a well-fed female can lay several hundred eggs: T. rubida, 466 and T. protracta, 513 mean eggs/female.20 Dispersal of kissing bugs is accomplished by flying or crawling in adults and crawling in case of nymphs. Passive dispersal can also occur with eggs and/or nymphs attached to hosts. Dispersal flights can be up to a mile21 and may be in response to hunger. Females and males disperse alike, but mate seeking is not the primary reason since fertilized females commonly undergo dispersal flights. Dispersal is also accomplished by pasting eggs to bird feathers as is done by Rhodnius prolixus, a Latin American species that prefers palm tree habitats.20
Distribution of Kissing Bugs in the United States
As mentioned above, kissing bugs are New World bugs with the exception of Triatoma rubrofasciata, which is cosmopolitan and likely distributed worldwide by shipping lines. Species found in the United States are shown in Figure 1. Of the 11 species distributed across the bottom two-thirds of the United States, 10 are Triatoma and 1 is Paratriatoma (a sister species to Triatoma lecticularia22). All species are shared with Mexico except T. rubrofasciata, of which there is only one report in the Continental United States, in Jacksonville, FL.23 Some published distributions may be inaccurate due to merging and splitting of species and name changes over time or reflect vastly different sampling efforts in different states (eg, Texas has been thoroughly sampled and no species are reported in West Virginia likely due to a lack of collections from that state). It is also possible that some specimens were misidentified or distributions have changed over time. These factors may explain the discrepancies in recent reviews.23,24 New citizen science initiatives, where the public is invited to participate by collecting and sending in bugs, will likely result in a wider sampling and may resolve these discrepancies (http://media.wix.com/ugd/a0dc4d_efe96cca543e4f58aec30e352afee657.pdf). Despite these caveats, we do have a good idea of the distribution for those species most frequently reported and/or involved in human bites.
The most broadly distributed is Triatoma sanguisuga, across the entire eastern portion of the United States (Fig. 1 and Table 1) and is a highly infected species25,26 frequently found with human blood meals (Waleckx et al., Emerging Infectious Diseases, in press). We agree with Bern et al.24 that reports of T. sanguisuga west of Texas are likely incorrect, possibly due to an older classification scheme. The geographic range of T. lecticularia overlaps most of the T. sanguisuga range, with the middle missing. There is also a single report of T. lecticularia in Louisiana; however, we have not been able to find any presently.
Table 1.
US kissing bugs: their habitats and animal hosts.
| SPECIES | GEOGRAPHIC RANGE IN THE UNITED STATES | PRIMARY HOST ASSOCIATIONS | FAVORED HABITAT | LIFE SPAN | ATTRACTED TO HUMAN HABITATIONS |
|---|---|---|---|---|---|
| Triatoma protracta | Southwest | Wood rats | Rodent nests | ~1 year | Yes |
| Triatoma sanguisuga | East | Raccoons, armadillos, opossums, frogs, wood rats, dogs, squirrels, and humans | Rodent and armadillo nests, hollow trees, and woodpiles | ~2 years | Yes |
| Triatoma lecticularia | Southeast | Wood rats and squirrels | Rodent nests | ~1 year | Yes |
| Triatoma rubida | Southwest | Wood rats | Rodent nests and bat refuges | ~1 year | Yes |
| Triatoma gerstaeckeri | Texas, New Mexico | Wood rats, dogs, opossums, and squirrels | Rodent and armadillo nests, corrals, stables, and coops | ~1 year | Yes |
| Paratriatoma hirsuta | Southwest | Wood rats | Rodent nests | ~2 years | No |
| Triatoma indictiva | Arizona, New Mexico, Texas | Wood rats | Rodent nests | Not known | No |
| Triatoma neotomae | Texas | Wood rats | Rodent nests | Not known | No |
| Triatoma rubrofasciata | Florida | Wood rats | Rodent nests | <1 year | Yes |
| Triatoma recurva | Arizona | Squirrels, wood rats, and reptiles | Rodent nests and reptile dens | ~2 years | Yes |
| Triatoma incrassata | Arizona | Squirrels and wood rats | Rodent nests | Not known | Not known |
Triatoma protracta is the most broadly distributed species in the western states: indeed, its distribution is like a mirror image of that of T. sanguisuga, overlapping in Texas. However, it is possibly less infected than T. sanguisuga.23 Triatoma rubida is also an important and widely distributed western species; its range mostly overlaps with T. protracta, covering the southwestern United States. Most reports suggest it is less infected than even T. protracta.23 Triatoma recurva (= Triatoma longipes) is found in several Western states. Although sylvatic, as are all US species, early literature reports indicate it was the species most commonly found in homes in Texas,27 which we are also finding in Arizona. Triatoma gerstaeckeri is only found in two states, Texas and New Mexico; however, it is the most frequently collected species in Texas.28
Species reported in a single state or of which little is known include Triatoma incrassata (only Arizona), Triatoma neotomae (only Texas), and Triatoma indictiva (Arizona, New Mexico, and Texas). In addition to the single report of T. rubrofasciata in the Continental United States, it is also found in Hawaii29 and the Virgin Islands.30
Kissing bugs rely on their hosts for blood meals, so their distribution is associated with their hosts (Table 1): generally, but not exclusively, rodents in the west and armadillos, opossums, and raccoons in the east. However, hosts are not known for all species. Recent molecular work on blood sources should clarify this. The largest factors impacting changes in distribution are likely changes in land use and land cover due to human encroachment and urbanization. This removes natural hosts, and bugs are forced to seek other blood sources, eg, moving into houses and feeding on humans. With climate change, vectors are expected to move northward, although some projections, eg, into the Great Lakes region, are unlikely and the models need to be improved.31 Northward spread of the kissing bugs should not be taken to imply an increased risk of transmission since the bugs already cover the bottom two-thirds of the country and autochthonous transmission is presently rare in the United States.
Kissing Bug Habitats and Host Associations
Triatomine species inhabit a variety of ecological environments. Within these environments, they can range from opportunistic feeders on a variety of host species to specialists feeding on a limited number of host types. Specialist feeders in Latin America include Cavernicola pilosa which feeds on bats, Rhodnius spp. living in palm trees where they feed on birds and arboreal mammals, and Psammolestes spp. which live in bird nests. No specialist feeders are found in the United States. Bird blood meals are suitable for bug survival and reproduction, and chickens are important for maintaining large bug populations, which then feed on humans.32 However, birds are not reservoirs of T. cruzi, possibly due to their higher body temperature and different immune system.
In South and Central America T. infestans, R. prolixus, and Triatoma dimidiata are important vectors of T. cruzi that feed on humans and their animals and are adapted to life with humans.6 These bugs are found in rural, semirural, and urban settings, all of which can be a potential risk for the local inhabitants and travelers alike.33
Kissing bug species found in the United States are sylvatic, only infrequently invading homes. In nature, they live in the burrows and nests of wood rats (Neotoma spp.) and opossums. These are favorite hosts for kissing bug species such as T. sanguisuga, T. lecticularia, and T. protracta. These species of kissing bugs are attracted to light and enter homes, but do not colonize them.11 Triatoma sanguisuga is the most commonly encountered species in the southern United States and has the broadest geographic range. It is associated with nests or burrows of raccoons, armadillos, and opossums, all of which are susceptible to T. cruzi. Triatoma gersteckerii is common in Texas and appears to have adapted to living in dog houses, where dogs subsequently become infected with T. cruzi.28 All Triatoma in the desert Southwest (most common being T. protracta and T. rubida) primarily feed on packrats (N. albigula) and other wood rat species, but also readily feed on a variety of vertebrate hosts, including humans. The Arizona-Sonora Desert Museum in Tucson, Arizona is a living zoo that supports large populations of T. rubida and T. protracta. These bugs plague many museum animals and sometimes feed so extensively that they have caused death by chronic blood loss of animals, including a rare protected mountain rattlesnake.34 We recently showed that these museum-dwelling bugs also commonly feed on human visitors.35
Kissing Bug Feeding Behavior
Kissing bugs rely on multiple sensory systems to locate hosts. These include gradients of carbon dioxide in the air, odors, moisture, hearing, and airflow.12 They are exquisitely sensitive to carbon dioxide exhaled by mammals, being able to detect 75 parts per million above the background air levels of 350 parts per million when a trace of exhaled lactic acid, another attractant, is present. Cold-blooded hosts including reptiles generate very low levels of carbon dioxide; nevertheless, kissing bugs use many other host-generated chemical cues including short-chained aliphatic amines and acids, 7-to 9-carbon aldehydes and alcohols, and lactic acid to locate hosts. The bugs can also detect infrared radiation, enabling them to orient toward warm-blooded hosts from several meters.
Triatomines follow the movement of air currents impregnated with host odors to locate the host.12 Feeding by bugs in human domiciles usually takes place at night, with bugs hiding during the day in cracks and crevices away from the feeding areas. Much less is known about feeding times for sylvatic species. Likely they adjust their activity periods to match the inactive periods of their host, thereby minimizing danger from host defenses. For example, the Southwest species, T. recurva, T. rubida, and T. protracta do not hesitate to approach and feed on rodents in the laboratory during bright midday times,13 an adaptive behavior by bugs that often feed on rodents in their burrows while the rodents are either sleeping or quiescent during the day. This aggressive feeding behavior is not affected by handling of the bugs, and they will often respond by extending their proboscis to feed on the experimenter.
Once a host is located, a hungry bug extends its proboscis and inserts it into the skin of the host. The most terminal part of the proboscis is the rostrum through which sheathed, serrate mandibles are projected to cut the epidermis. A stylet is inserted though the mouthpiece to search for capillaries.11 Sometimes, the host detects the minor sensation caused by a kissing bug’s probing and moves or shifts position. In response, the kissing bug usually retracts somewhat before continuing probing and feeding. Considering that a bug often imbibes more blood than its own weight,3 feeding is relatively rapid, lasting on average 22 minutes for T. protracta, 28 minutes for T. rubida, and between 11 and 28 minutes for the South and Central American T. infestans, T. dimidiata, and R. prolixus.13,14 Blood is mostly composed of water, and the bulkiness of a large blood meal limits a bug’s movement. To eliminate excess water, bugs often defecate/urinate during feeding, at completion of feeding, or shortly after leaving the feeding site. Some species, including those domestic species that frequently transmit Chagas disease, defecate rapidly and frequently, with defecations often on or near the host.14 Frequency and proximity of defecation to the host are considered to be critical factors in transmitting T. cruzi and Chagas disease to humans. Work with T. rubida and T. protracta feeding on immobilized rodents demonstrates that these southwestern species defecate less often and usually at some distance from the host.36 Since T. cruzi is transmitted to humans through the feces of the bugs, the delayed defecation and movement away from the host are likely one reason transmission of Chagas disease from these species is less likely than from South American kissing bug, T. infestans. Trypanosoma cruzi enters the human host by being deposited on the skin and then rubbed into the bite wound or mucous membranes of the eye, nose, and mouth. A particularly pernicious means of contracting Chagas disease is by transmission via ingestion of an infected bug, or the feces of the bug in food-borne Chagas disease.37
When one considers that the infectious form of the parasite, metacyclic trypomastigotes, only appear in the feces of kissing bugs, it is obvious that a critical variable determining parasite transmission is the amount of time that transpires during and after the act of feeding and the first defecation, thus, the emphasis on kissing bug defecation and the spread of Chagas diseaase.11
Kissing Bug Bites
There is considerable misinformation about kissing bug bites. Early accounts of T. sanguisuga stated the bite is “very painful and often even dangerous”38 and “a most painful wound” often confused with a spider bite.39 Shields and Walsh observed T. sanguisuga feeding on humans and concluded that the bite is painless40 as did Darwin on his travels in Argentina.41 Ryckman found that 12 of 481 subjects (2.49%) had a mild reaction (usually pruritus) to the feeding of T. protracta, T. lecticularia, T. dimidiata, or Dipetalogaster maxima.42 From the standpoint of hematophagous insects, it is important the host remain unaware of the presence of the insect; hence, biting is almost always painless. This is supported further by the fact that patients presenting to emergency rooms with anaphylaxis due to kissing bugs bites are rarely aware of a bite preceding anaphylaxis – they awaken at night with intense itching over the body and difficulty breathing.40
The typical kissing bug bite leads to a small raised skin lesion with a central punctum and a inflammatory infiltrate in the subcutaneous tissue.40 Swelling of tissue at the bite site over several days may lead to nodular, urticarial lesions associated with pain and can last up to 7 days.43 Some severe reactions may last for up to a month and include lesions at the bite site. Lymphadenitis and vasculitic rashes such as erythema multiforme may occur as well.40 These delayed allergic responses are uncomfortable and undoubtedly account for the many claims that the bite itself hurts. Romana’s sign (unilateral eyelid edema), found in patients in endemic areas of Chagas, can be a sign of acute disease, but can be caused by other pathologic processes. In the United States, the so-called Romana’s sign is most often a sign of allergy to components of kissing bug saliva or other insects.44
Deterrence of Kissing Bug Bites
Kissing bugs usually feed on humans during nighttime hours, most often in the bedroom. They are usually solitary adults but, on occasion gravid females may enter the home and lay eggs giving rise to nymphs. Bugs are often found between the mattress and box spring or under bedding sheets. In some homes in the Tucson area, three to five adults may be found in the bedroom probably indicating that there are multiple points of easy ingress into the home (see the Pest management of kissing bugs section). We are attempting to develop a deterrent to kissing bug bites because individuals with histories of anaphylaxis or accelerated allergies to kissing bug saliva are at risk for severe allergic responses when bitten. This population will benefit from some barrier, chemical or structural, that protects them from bites – the allergenic substance is in the kissing bug saliva. Most of these individuals live with syringes preloaded with epinephrine in case they awaken with anaphylaxis. Our preliminary work demonstrates that a safe plant product, citronella oil, can deter feeding by T. rubida.45 DEET (N,N-diethyl-meta-toluamide) and picaridin were somewhat effective, but citronella stopped all feeding whatsoever. More studies will determine the effect of the essential oil on other species and in different compositions (liquid, cream, etc.).
Human Chagas Disease in the United States
Due to expansion of home ranges of kissing bugs in the United States, there is a concern that there will be a corresponding increase in the numbers of human cases of Chagas disease. However, several factors need to be operative for acquiring Chagas from kissing bugs in the United States. First, there must be an animal reservoir of T. cruzi present for the bugs to feed upon; this requirement currently exists where kissing bugs live in the United States. Secondly, kissing bugs need frequent access to humans where they can feed upon them. In South America, this is achieved by bugs colonizing homes and peridomestic spaces, whereas in the United States, this does not presently occur due to better housing, frequent use of air conditioning, and pest control. However, an interesting fact is that the very same kissing bug species (T. protracta, T. rubida, and T. recurva) found in the Southwest are also resident in Mexico where they are thought to be vectors of Chagas disease.46
Only seven documented cases of autochthonous human Chagas disease have been reported in the United States. Four of the victims were infants and one was a small child. The infants were probably easy targets for the kissing bugs since in one case bugs were found in the crib of the infant. A case of Chagas in an adult in Louisiana25 was particularly instructive because a wealth of ancillary data bearing on the case was uncovered and reported. The authors searched the home of an older woman who reported being bitten numerous times by kissing bugs. The house was a wooden structure with many access points for bugs and 10 of 18 adult bugs (T. sanguisuga) found in the domicile were positive for T. cruzi by polymerase chain reaction (PCR). However, no nymphs were found in the home indicating that it was unlikely kissing bugs colonized the home. A follow-up study of 298 bugs collected in the vicinity of the home revealed that 180 or ~60% harbored T. cruzi.47 The patient was positive for T. cruzi by serology, and the parasite was cultured from her blood. In this particular case, the required factors were in place including kissing bugs that harbored the parasite as well as the ability of kissing bugs to feed on humans numerous times.
A recent report included 16 more possible autochthonous cases of Chagas disease.48 However, these were discovered by serological testing of asymptomatic blood donors, not ill individuals as were some of the previous seven cases. More than half of the patients in the blood donor group reported spending time (<2 weeks) in endemic areas of Chagas, primarily Mexico. Nevertheless, it is likely some of these individuals, if not all, acquired disease in the United States as all were/are residents of states known to have kissing bugs and animal reservoirs of T. cruzi.
The preceding paragraphs deal with autochthonous cases of human Chagas. However, it is estimated that ~300,000 recent immigrants to the United States have Chagas in its chronic phases. Many of these same individuals live in areas of the United States, where kissing bugs are endemic and hence could transmit T. cruzi to the insect. What effect this would have on the behavior of the kissing bugs is unknown. Coinfection of HIV-1 and T. cruzi as well as immunosuppression pose a further risk of poor outcome. The transmission of T. cruzi from man-to-vector or vector-to-man or another animal host could affect the dynamics of the disease as well as the behavior of the bugs. The potential outcomes of the many possible changes and interactions involving human populations and triatomine populations are unpredictable at this point, but bear watching.
Kissing Bug Saliva and its Antigenic Components
Kissing bug bites are the most common cause of insect bites that result in anaphylaxis in the United States. This medical emergency is caused more commonly by stings of bees, wasps, and ants and results from the release of chemical signals in response to proteins in the bug’s saliva. Anaphylaxis usually causes the individual to rush to the emergency room where treatment with epinephrine and other interventions reverse the low blood pressure, swollen airways, and rashes that often accompany anaphylaxis. Victims of kissing bug bite anaphylaxis usually are awakened at night by shortness of breath, difficulty breathing, or generalized itching. Other less ominous allergies include hives, swelling of the eyes, swelling at the site of the bite, and intense, persistent itching. Patients may experience anaphylaxis when rebitten, and at least two adults have died from the condition.49,50
Allergic host responses to kissing bug bites are due to allergens present in the saliva of the bugs. In kissing bug–endemic areas in the United States, allergies are common. About 7% of the inhabitants of one community in California had evidence of IgE allergy to T. protracta.51 Bioassays of salivary gland extracts have demonstrated the presence of allergens capable of eliciting IgE antibody in patients and are highly species specific, ie, there is no cross-reactivity of extracts, eg, between T. rubida and T. protracta.52 The allergens appear to be low–molecular weight proteins.53
In the classical description of anaphylaxis by Poitier and Richet,54 two steps were required for the phenomenon: exposure to an allergen which primed or set the stage followed by repeat exposure to the same allergen after sufficient time (usually weeks) for lymphocytes to produce IgE antibody to the foreign antigen and thus elicit the allergic cascade known as a Type I hypersensitivity reaction.55 This reaction is the most extreme of allergic reactions mediated by IgE antibody. Anaphylaxis has been reported following bites with important Chagas vectors including as R. prolixus and T. infestans, but is apparently not common.56
Currently, there is no effective preventive therapy for anaphylaxis due to kissing bug bites. Immunotherapy can be successful in patients with a history of kissing bug bite anaphylaxis, but is an intensive procedure. Without standardized allergens, it is unlikely to be of much benefit.57
Wild and Domesticated Animals Infected with Trypanosoma Cruzi in the United States
Within the United States, peridomestic transmission of Chagas disease is not yet fully understood with respect to host and vector interactions.49 Although rare in domestic pets other than domestic dogs, infection with T. cruzi is fairly common in several wildlife species with prevalence varying by geographic region as well as host species and characteristics.58 Within domesticated species, reported cases are primarily limited to domestic dogs in the warmer southern states with no cases involving livestock, pigs, or horses.59 Dogs are suspected to be the primary host with respect to T. cruzi transmission between the sylvatic and peridomestic settings.60
The primary wildlife host reservoirs for T. cruzi in the United States are rodent species, especially packrats and wood rats in the genus Neotoma. Raccoons, skunks, opossums, and armadillos also display high rates of infection.24 Worldwide, over 200 species of mammals have been reported as natural hosts for T. cruzi,59 and all mammals may be considered as susceptible to infection.24 In the United States, at least 24 mammal species have been documented as hosts.60 Wood rats, considered to be primary host reservoirs, can be infected with different T. cruzi genotypes59 and also have high rates of coinfection with another novel species of trypanosome. As previously discussed, avian species are resistant to infection.
Infection lasts for the lifetime of the mammalian host in the absence of antitrypanosomal treatment,24 and pathologic cardiac changes have been noted at necropsy in raccoons and opossums. Raccoons appear to be infected with a more virulent genetic strain in which blood parasite levels peak sooner. Additionally, mice injected with raccoon-derived T. cruzi isolates have a 75% mortality rate, whereas mice injected with opossum-derived strains clear the infection and survive.61
Infection rates of T. cruzi in wildlife species are highest in the southern and eastern coastal areas of the United States, where high humidity and lack of a winter freeze result in a higher distribution of insect vectors. In addition to infection of native wildlife species, locally acquired T. cruzi has infected multiple species of nonhuman primates at research facilities in Georgia, Texas, and Louisiana.62 In these cases, affected primates were assumed to have acquired Chagas disease naturally, since most of the individuals were born and raised on site. As in other wildlife species as well as humans, T. cruzi infection of nonhuman primates results in cardiac problems including myocarditis and epicarditis.63
Domestic dogs, the primary domestic species reservoir for T. cruzi, display similar cardiac signs to wildlife species. A dilated form of heart failure develops, typically presenting as exercise intolerance and generalized weakness. Survival time in clinically affected dogs may be up to 60 months, and although treatment is indicated, it rarely results in a cure. Prevention of Chagas disease in dogs involves limiting contact with the insect vector.64
A recent study involving dogs from the Amazon basin demonstrated that a higher seroprevalence of infection in dogs can be considered an indicator of wildlife species loss due to human encroachment into previously undisturbed forests. The reduction of normal sylvatic mammalian fauna in terms of both density and species diversity is associated with higher T. cruzi parasitemia within the remaining wildlife, resulting in subsequent higher perisylvatic infection in domestic dogs locally.65 Ultimately, higher rates of human infection can result. Similar expansion of human settlements into areas supporting active sylvatic T. cruzi disease cycles and subsequent human infection has been documented in the United States as well. Habitat change resulting from urban expansion and development has resulted in displacement of native wildlife hosts in parts of Texas and California, with the low specificity of triatomine host selection resulting in a higher prevalence of domestic dogs serving as host.60
Similar migration of kissing bugs into human habitations as a result of loss of habitat can be expected to continue, a problem further compounded by triatomine vectors in the United States predicted to expand their current ranges in response to climate change.31 Additionally, the high prevalence of T. cruzi infection in rodent and opossum species is problematic since these are opportunistic sylvatic species that acclimate well to living in close proximity to humans in the face of native habitat destruction.24 The maintenance of wildlife biodiversity and habitat serves as dilutional buffer that disperses parasites and reduces contact with humans. This effect is not only important for Chagas disease but with other vector-borne disease such as Lyme and West Nile virus.65 Findings with M. spinolai indicate that host density is directly related to the infection in the insects.66 With human encroachment into previously undisturbed areas, an increased interaction between humans, domestic animals, and wildlife occurs, thereby allowing perisylvatic disease transmission.
Pest Management of Kissing Bugs
Insecticide treatment is the cornerstone of vector control programs for triatomines in South America where domestic and peridomestic species are prevalent and can attain high population densities in and around homes. The sylvatic species found in the United States are only occasional invaders and thus present a different set of problems that rely mainly on nonchemical measures for control, especially habitat modification and exclusion techniques (Table 2).
Table 2.
Nonchemical measures to reduce risk of household infestation by triatomines.
| SANITATION MEASURES: |
|---|
| • Reduce clutter (eg, clothes, piles of paper) inside homes, particularly in the bedroom to reduce potential hiding places for kissing bugs. |
| • Manage vegetation around the home and eliminate clutter (eg, piles of lumber, firewood, and debris) that may provide small animal habitat. |
| Install weather stripping and tight-fitting insect screens on windows and doors, insect-proof dog and cat entrances, and keep fireplace flues shut |
| MANAGE INDOOR AND OUTDOOR LIGHTING AT NIGHT TO MAKE HOMES LESS ATTRACTIVE TO KISSING BUGS: |
| • Move lights away from doors or windows where they may attract insects. |
| • Replace outside white lights with yellow lights. |
| • Keep window curtains and blinds drawn in lighted rooms. |
| Seal potential entryways into homes (eg, foundation cracks and utility line points of entry with caulk, silicone seal, or other appropriate materials |
First and foremost is a thorough inspection of the home both inside and outside. During the active season (around mid-spring to mid-fall), inspections should be carried out on a regular basis to find and destroy stray bugs. During the day they typically hide in dark places in cracks and crevices or under objects. In the bedroom, kissing bugs may hide in bedsheets, blankets, or under the mattress. These potential hiding places should be inspected especially before retiring. Outside the home and during the day, they hide in dark sheltered places such as beneath flowerpots, woodpiles, stone piles, peeling bark, or furniture. In the evening and at night, they emerge from these hiding places and can be seen crawling or resting on surfaces. Special attention should be paid to areas inside and out where pets congregate or sleep.
In addition to efforts aimed toward discovery and elimination of kissing bugs, control measures may be needed to remove their wild vertebrate hosts, especially, packrats, a species common around homes in the Southwest. Packrats can have several dozen or more bugs inhabiting each nest. Trapping the packrats and elimination of their nests close to the house can reduce home invasions. However, it is advisable that only the nearest packrat nest and packrats be removed in the hope that the kissing bugs will move to more peripheral nests farther from the house. In the east, opossum nests in trees overhanging the house and armadillo and raccoon nests around and under the house should be removed. After the animals have been removed, a pyrethroid dust or spray should be applied to the old nesting sites to eliminate any remaining kissing bugs. This is crucial because in the absence of their natural host, the bugs will seek out another source of blood, which might end up being the homeowner.17 Removal of prickly pear and cholla cacti eliminates the favorite nesting sites of packrats, and removal of vegetation from near the home deters nesting by armadillos and opossums.
In the case of homeowners who have become allergic to kissing bugs, a directed spray application of pyrethroid insecticides in and around the home may provide some added protection. Most effective indoors is a crack-and-crevice application to potential harborage sites in bedrooms and bathrooms, and outdoors a perimeter treatment along the foundation and eaves, paying close attention to thoroughly treat entryways such as windows and doors to prevent their access into the home. Allergic individuals may elect to sleep under a bed net (mosquito netting) that is tucked in all around the mattress for added protection and use double-sided tape placed on the legs of the bed. Beds should be kept at least one foot away from walls. Sticky trap monitors placed under and around beds may also catch bugs. Because kissing bugs are attracted to white lights, outside porch and yard lighting should be reduced and replaced with yellow lighting, which is not attractive to bugs. During evenings and at night, unnecessary inside lights should be turned off and/or shielded from windows by drawing the curtains.
A major problem in Latin America is reinfestation of housing and peridomestic areas following insecticide spraying, necessitating new approaches like house improvements.67 Many species of bugs like T. dimidiata occupy multiple ecotopes including sylvan, peridomestic, and domestic environments providing reservoirs to readily occupy treated houses.68 For example, Triatoma mexicana of central Mexico and T. dimidiata of the Yucatan, Mexico, and Belize do not colonize homes, but seasonally migrate from sylvan and peridomestic ecotopes and infest homes.69 Thus, the efficacy of insecticide programs is greatly reduced as new populations of triatomines infest homes annually.
Conclusion
Kissing bugs have newfound notoriety in the United States, principally for the severity of allergic reactions to their saliva following a bite and also for the potential to transmit Chagas disease to pets, particularly dogs, and humans. They can be common in many areas of the southern United States, with noteworthy concentrations around San Diego, CA, and Phoenix and Tucson, AZ. With global warming, there may be a further expansion of the geographic distribution of these bugs and along with them, T. cruzi. This fact in itself is not enough to initiate an epidemic of Chagas in the United States, however. In the United States, in contrast to Latin America, many barriers exist to the spread of T. cruzi, including the use of air conditioners, better pest control, homes generally sealed from insect invasion, and lastly, the feeding and defecating behavior of the local kissing bug species appears to be quite different. Latin American species such as T. infestans defecate often during feeding, and their feces containing T. cruzi can be rubbed into the bite site, whereas T. protracta found in the Southwest does not defecate on the host routinely, depositing its feces far from the host. Most species of US kissing bugs enter homes during adult seasonal flight dispersal. These adult dispersals rarely become permanently established colonizations of a home as occurs in Latin America. Although it is now evident that free-roaming kissing bugs feed often on humans, more needs to be learned about these interactions with the bugs in the wild. For example, are nymphal forms feeding on humans since they would be difficult to see. Or, are humans for some reason a very easy target?
Figure 3.

Male Triatoma protracta, another cause of anaphylaxis in California and Arizona. (Photograph by Justin Schmidt with permission.)
Footnotes
ACADEMIC EDITOR: Timothy Kelley, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: SAK, PLD, MM, JOS. Analyzed the data: SAK, PLD, MM, JOS. Wrote the first draft of the manuscript: SAK. Contributed to the writing of the manuscript: SAK, PLD, MM, JOS. Agree with manuscript results and conclusions: SAK, PLD, MM, JOS. Jointly developed the structure and arguments for the paper: SAK, PLD, MM, JOS. Made critical revisions and approved final version: SAK, PLD, MM, JOS. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Schofield CJ, Galvao C. Classification, evolution, and species groups within the Triatominae. Acta Trop. 2009;110:88–100. doi: 10.1016/j.actatropica.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Botto-Mahan C. Trypanosoma cruziinduces life-history trait changes in the wild kissing bug Mepraia spinolai: implications for parasite transmission. Vector Borne Zoonotic Dis. 2009;9:505–10. doi: 10.1089/vbz.2008.0003. [DOI] [PubMed] [Google Scholar]
- 3.Schaub GA. Development of isolated and group-reared 1st instars of Triatoma infestansinfected with. Trypanosoma cruzi Parasitol Res. 1988;74:593–4. doi: 10.1007/BF00531641. [DOI] [PubMed] [Google Scholar]
- 4.Schaub GA. Developmental time and mortality of larvae of Triatoma infestansinfected with Trypanosoma cruzi. Trans R Soc Trop Med Hyg. 1988;82:94–7. [PubMed] [Google Scholar]
- 5.Kollien A, Schmidt J, Schaub GA. Modes of association of Trypanosoma cruziwith the intestinal tract of the vector. Triatoma infestans Acta Trop. 1998;70:127–41. doi: 10.1016/s0001-706x(97)00117-4. [DOI] [PubMed] [Google Scholar]
- 6.Kollien A, Schaub GA. The development of Trypanosoma cruzi (Trypanosomatidae) in the reduviid bug Triatoma infestans (Insecta): influence of starvation. J Eukaryot Microbiol. 1998;45:59–63. doi: 10.1111/j.1550-7408.1998.tb05070.x. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton P. Classification and phylogeny of Trypanosoma cruzi. In: Telleria J, Tibayrenc M, editors. American Trypanosomiasis. Chagas Disease. One Hundred Years of Research. Amsterdam: Elsevier; 2010. pp. 321–38. [Google Scholar]
- 8.Stevens J, Noyes H, Dover G, Gibson W. The ancient and divergent origins of the human pathogenic trypanosomes, Trypanosoma brucei and T. cruzi. Parasitology. 1999;118:107–16. doi: 10.1017/s0031182098003473. [DOI] [PubMed] [Google Scholar]
- 9.Catala S. The biting rate of Triatoma infestans in Argentina. Med Vet Entomol. 1991;5:325–33. doi: 10.1111/j.1365-2915.1991.tb00558.x. [DOI] [PubMed] [Google Scholar]
- 10.Poinar GJ. Triatoma dominicanasp. n. (Hemiptera: Reduviidae: Triatominae), and Trypanosoma antiquussp. n. (Stercoraria: Trypanosomatidae), the first fossil evidence of a triatomine-trypanosomatid vector association. Vector Borne Zoonotic Dis. 2005;5:72–81. doi: 10.1089/vbz.2005.5.72. [DOI] [PubMed] [Google Scholar]
- 11.Lent H, Wygodzinsky P. Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagas’ disease. Bull AMNH. 1979;163:127–520. [Google Scholar]
- 12.Blum M. Chemical Defenses of Arthropods. New York: Academic Press; 1981. [Google Scholar]
- 13.Brindley M. On the metasternal scent glands of certain Heteroptera. Trans R Entomol Soc London. 1930;78:199–208. [Google Scholar]
- 14.Manrique G, Vitta AC, Ferreira RA, et al. Chemical communication in Chagas disease vectors. Source, identity, and potential function of volatiles released by the metasternal and Brindley’s glands of Triatoma infestansadults. J Chem Ecol. 2006;32:2035–52. doi: 10.1007/s10886-006-9127-7. [DOI] [PubMed] [Google Scholar]
- 15.Minoli S, Palottini F, Manrique G. The main components of an alarm pheromone of kissing bugs play multiple roles in the cognitive modulation of the escape response. Front Behav Neurosci. 2013;7:1–10. doi: 10.3389/fnbeh.2013.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pontes G, Bohman J, Rikard Unelius C, Lorenzo M. Metasternal gland volatiles and sexual communication in the triatomine bug. Rhodnius prolixus J Chem Ecol. 2008;34:450–7. doi: 10.1007/s10886-008-9431-5. [DOI] [PubMed] [Google Scholar]
- 17.Schofield C, Patterson J. Assembly pheromone of Triatoma infestansand Rhodnius prolixusnymphs (Hemiptera:Reduviidae) J Med Entomol. 1977 doi: 10.1093/jmedent/13.6.727. [DOI] [PubMed] [Google Scholar]
- 18.Figueiras A, Girotti J, Mijailovsky S, Juarez M. Epicuticular lipids induce aggregation in in Chagas disease vectors. Parasites Vectors. 2009;2:8. doi: 10.1186/1756-3305-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schofield C. Sound production in some triatomine species. Physiol Entomol. 1977;2:43–52. [Google Scholar]
- 20.Zeledon R, Rabinovich JE. Chagas’ disease: an ecological appraisal with special emphasis on its insect vectors. Annu Rev Entomol. 1981;26:101–33. doi: 10.1146/annurev.en.26.010181.000533. [DOI] [PubMed] [Google Scholar]
- 21.Ekkens D. Nocturnal flights of Triatoma (Hemiptera: Reduviidae) in Sabino Canyon, Arizona. J Med Entomol. 1981;18:211–27. [Google Scholar]
- 22.Justi SA, Russo C, Mallet J, Obara M, Galvao C. Molecular phylogeny of Triatomini (Hemiptera: Reduviidae: Triatominae) Parasit Vectors. 2014;7:149. doi: 10.1186/1756-3305-7-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeledon R, Beard CB, Pinto Dias J, Leiby D, Dorn P, Coura JR. An appraisal of the status of Chagas disease in the United States. Amsterdam, Netherlands: Elsevier; 2012. [Google Scholar]
- 24.Bern C, Kjos SA, Yabsley M, Montgomery SP. Trypanosoma cruziand Chagas’ disease in the United States. Clin Microbiol Rev. 2011;24:655–81. doi: 10.1128/CMR.00005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorn P, Perniciaro L, Yabsley M, Roellig D, Balsamo G, Diaz J. Autochthonous transmission of Trypanosoma cruzi, Louisiana. Emerg Infect Dis. 2007;13:605–7. doi: 10.3201/eid1304.061002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cese K, Calliquet K, Dorn P, Wesson D. High Trypanosoma cruzi (Kinetoplastida: Trypanosomadae) prevalence in Triatoma sanguisuga (Hemiptera: Reduviidae) in southeastern Louisiana. J Med Entomol. 2011;48:1091–4. doi: 10.1603/me10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikenga J, Richerson J. Trypanosoma cruzi (Chagas) (protozoa: Kinetoplastida: Trypanosomadae) in invertebrate and vertebrate hosts from Brewster County in Trans-Pecos Texas. J Econ Entomol. 1984;77:126–9. doi: 10.1093/jee/77.1.126. [DOI] [PubMed] [Google Scholar]
- 28.Kjos SA, Snowden KF, Olson JK. Biogeography and Trypanosoma cruziinfection prevalence of Chagas disease vectors in Texas, USA. Vector Borne Zoonotic Dis. 2009;9:41–9. doi: 10.1089/vbz.2008.0026. [DOI] [PubMed] [Google Scholar]
- 29.Usinger RL. The Triatominae of North and Central America and the West Indies and their Public Health Significance. Washington, DC: U.S. Public Health Service; 1944. [Google Scholar]
- 30.Ryckman AE. The Triatominae of North and Central America and the West Indies and their Public Health Significance. Washington, DC: US Public Health Service; 1984. [Google Scholar]
- 31.Garza M, Feria Arroyo T, Casillas E, Sanchez-Cordero V, Rivaldi C. Projected future distributions of vectors of Trypanosoma cruziin North America under climate change scenarios. PLoS Negl Trop Dis. 2014;8:e2818. doi: 10.1371/journal.pntd.0002818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Catalá SS, Crocco LB, Muñoz A, et al. Entomological aspects of Chagas’ disease transmission in the domestic habitat, Argentina. Rev Saude Publica. 2004;38:216–22. doi: 10.1590/s0034-89102004000200010. [DOI] [PubMed] [Google Scholar]
- 33.Diaz J. Recognizing and reducing the risks of Chagas disease (American trypanosomiasis) in travelers. J Travel Med. 2008;15:184–95. doi: 10.1111/j.1708-8305.2008.00205.x. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt J, Stevens L, Dorn P, Mosbacher M, Klotz J, Klotz SA. Kissing bugs in the United States. Kansas School Nat. 2011;57:1–15. [Google Scholar]
- 35.Klotz SA, Schmidt J, Dorn P, Ivanyi C, Sullivan K, Stevens L. Free-roaming kissing bugs, vectors of Chagas disease, feed often on humans in the Southwest. Am J Med. 2014;127:421–6. doi: 10.1016/j.amjmed.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klotz SA, Dorn PL, Klotz JH, et al. Feeding behavior of triatomines from the southwestern United States: an update on potential risk for transmission of Chagas disease. Acta Trop. 2009;111:114–8. doi: 10.1016/j.actatropica.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Nobrega A, Garcia M, Tatto E, et al. Oral transmission of Chagas disease by consumption of acai palm fruit, Brazil. Emerg Infect Dis. 2009;15:653–5. doi: 10.3201/eid1504.081450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kimball B. Conorhinus sanuisugus: Its habits and life history. Trans Annu Meet Kansas Acad Sci. 14:1893–4. 128–31. [Google Scholar]
- 39.Le Conte J. Remarks on two species of American Cimex. Proc Acad Nat Sci Philadelphia. 7:1854–5. 404. [Google Scholar]
- 40.Shields T, Walsh E. Kissing bug bite. Arch Derm. 1956;74:14–21. doi: 10.1001/archderm.1956.01550070016004. [DOI] [PubMed] [Google Scholar]
- 41.Darwin C. Narrative of the surveying voyages of His Majesty’s Ships Adventure and Beagle between the years 1826–36 describing their examination of the southern shores of South America, and the Beagle’s circumnavigation of the globe. Journal and Remarks. 1832–6. III. London: Henry Coburn; 1839. [Google Scholar]
- 42.Ryckman R. Dermatological reactions to the bites of four species of triatominiae (Hemiptera: Reduviidae) and Cimex lectulariousL. (Hemiptera: Cimicidae) Bull Soc Vector Ecol. 1985;10:122–5. [Google Scholar]
- 43.Lynch P, Pinnas J. Kissing bug bites. Cutis. 1978;22:585–9. [PubMed] [Google Scholar]
- 44.Klotz JH, Dorn PL, Logan JL, et al. “Kissing bugs”: potential disease vectors and cause of anaphylaxis. Clin Infect Dis. 2010;50:1629–34. doi: 10.1086/652769. [DOI] [PubMed] [Google Scholar]
- 45.Terriquez J, Klotz SA, Meister E, Klotz J, Schmidt J. Repellency of DEET, picaridin, and three essential oils to Triatoma rubida (Hemiptera: Reduviidae: Triatominae) J Med Entomol. 2013;50:664–7. doi: 10.1603/me12282. [DOI] [PubMed] [Google Scholar]
- 46.Martínez-Ibarra JA, Alejandre-Aguilar R, Paredes-González E, et al. Biology of three species of North American Triatominae (Hemiptera: Reduvidae: Triatominae) fed on rabbits. Mem Inst Oswaldo Cruz. 2007;102:925–30. doi: 10.1590/s0074-02762007000800006. [DOI] [PubMed] [Google Scholar]
- 47.Cesa K, Callouet K, Dorn P, Wesson D. High Trypanosoma cruzi (Kinetoplastida: Trypanosomadae) prevalence in Triatoma sanguisuga (Hemiptera: Reduviidae) in southeastern Louisiana. J Med Entomol. 2011;48:1091–4. doi: 10.1603/me10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cantey PT, Stramer SL, Townsend RL, et al. The United States Trypanosoma cruziInfection Study: evidence for vector-borne transmission of the parasite that causes Chagas disease among United States blood donors. Transfusion. 2012;52:1922–30. doi: 10.1111/j.1537-2995.2012.03581.x. [DOI] [PubMed] [Google Scholar]
- 49.Merced Sun-Star Weekender. Merced, CA: 1975. Bug bite fatal to hornitos man. Anonymous. [Google Scholar]
- 50.Lo Vecchio F, Tran T. Allergic reactions from insect bites. Am J Emerg Med. 2004;22:631. doi: 10.1016/j.ajem.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 51.Marshall N, Liebhaber M, Dyer Z, Saxon A. The prevalence of allergic sensitization to Triatoma protracta (Heteroptera: Reduviidae) in southern California, USA, community. J Med Entomol. 1986;23:117–24. doi: 10.1093/jmedent/23.2.117. [DOI] [PubMed] [Google Scholar]
- 52.Pinnas J, Lindberg R, Chen T, Meinke G. Studies of kissing bug-sensitive patients: evidence for the lack of cross-reactivity between Triatoma protractaand Triatoma rubidasalivary gland extracts. J Allergy Clin Immunol. 1986;77:364–70. doi: 10.1016/s0091-6749(86)80119-1. [DOI] [PubMed] [Google Scholar]
- 53.Marshall N, Chapman M, Saxon A. Species-specific allergens from salivary glands of Triatominae (Heteroptera: Reduviidae) J Allergy Clin Immunol. 1986;78:430–5. doi: 10.1016/0091-6749(86)90029-1. [DOI] [PubMed] [Google Scholar]
- 54.Poitier P, Richet C. De l’action anaphylactique de certains venins. C R Soc Biol (Paris) 1902;54:170–2. [Google Scholar]
- 55.Klotz J, Klotz S, Pinnas J. Animal bites and stings with anaphylactic potential. J Emerg Med. 2009;36:148–56. doi: 10.1016/j.jemermed.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 56.Mott K, Franca J, Barrett T, Hoff R, de Oliveira T, Sherlock I. Cutaneous allergic reactions to Triatoma infestansafter xenodiagnosis. Mem Inst Osaldo Cruz. 1980;75:3–10. doi: 10.1590/s0074-02761980000200001. [DOI] [PubMed] [Google Scholar]
- 57.Moffitt J, Venarske D, Goddard J, Yates A, DeShazo R. Allergic reactions to Triatomabites. Ann Allergy Asthama Immunol. 2003;91:122–8. doi: 10.1016/S1081-1206(10)62165-5. [DOI] [PubMed] [Google Scholar]
- 58.Brown EL, Roellig DM, Gompper ME, et al. Seroprevalence of Trypanosoma cruziamong eleven potential reservoir species from six states across the southern United States. Vector Borne Zoonotic Dis. 2010;10:757–63. doi: 10.1089/vbz.2009.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roellig D, Yabsley M. Infectivity, pathogenicity, and virulence of Trypanosoma cruziisolates from sylvatic animals and vectors, and domestic dogs from the United States in ICR stain mice and SD strain rats. Am J Trop Med Hyg. 2010;83:519–22. doi: 10.4269/ajtmh.2010.09-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kjos SA, Marcet PL, Yabsley MJ, et al. Identification of bloodmeal sources and Trypanosoma cruziinfection in triatomine bugs (Hemiptera: Reduviidae) from residential settings in Texas, the United States. J Med Entomol. 2013;50:1126–39. doi: 10.1603/me12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karsten V, Davis C, Kuhn R. Trypanosoma cruziin wild raccoons and opossums in North Carolina. J Parasitol. 1992;78:547–9. [PubMed] [Google Scholar]
- 62.Dorn P, Daigle M, Combe C, Tate A, Stevens L, Phillippi-Falkenstein K. Low prevalence of Chagas parasite infection in a nonhuman primate colony in Louisiana. J Am Assoc Lab Anim Sci. 2012;51:443–7. [PMC free article] [PubMed] [Google Scholar]
- 63.Mubiru J, Yang A, Dick EJ, Owston M, Sharp R. Correlation between presence of Trypanosoma cruziDNA in heart tissue of baboons and cynomolgus monkeys, and lymphoctyic myocarditis. Am J Trop Med Hyg. 2014;90:627–33. doi: 10.4269/ajtmh.13-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Esch K, Petersen C. Transmission and epidemiology of zoonotic protozoal diseases of companion animals. Clin Microbiol Rev. 2013;26:58–85. doi: 10.1128/CMR.00067-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xavier SC, Roque AL, Lima Vdos S, et al. Lower richness of small wild mammal species and Chagas disease. PLoS Negl Trop Dis. 2012;6:e1647. doi: 10.1371/journal.pntd.0001647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oda E, Solari A, Botto-Mahan C. Effects of mammal host diversity and density in the infection level of a sylvatic kissing bug. Med Vet Entomol. 2014;28:384–90. doi: 10.1111/mve.12064. [DOI] [PubMed] [Google Scholar]
- 67.Monroy C, Bustamante DM, Pineda S, et al. House improvements and community participation in the control of Triatoma dimidiatare-infestation in Jutiapa, Guatemala. Cadernos Saúde Pública. 2009;25:S168–78. doi: 10.1590/s0102-311x2009001300016. [DOI] [PubMed] [Google Scholar]
- 68.Dorn P, Monroy C, Curtis A. Triatoma dimidiata (Latreille, 1811): a review of its diversity across its geographic range and the relationship among populations. Infect Genet Evol. 2007;7:343–52. doi: 10.1016/j.meegid.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 69.Ramirez-Sierra MJ, Herrera-Aguilar M, Gourbiere S, Dumonteil E. Patterns of house infestation dynamics by non-domiciliated Triatoma dimidiatareveal a spatial gradient of infestation in rural villages and potential insect manipulation by. Trypanosoma cruzi Trop Med Int Health. 2009;15:77–86. doi: 10.1111/j.1365-3156.2009.02422.x. [DOI] [PubMed] [Google Scholar]