Abstract
Pineal region tumors make up less than 1% of all intracranial neoplasms, with the majority being of germ cell origin. We describe the diagnostic evaluation and treatment of a patient presenting with neurological deficits who was found to have a germinoma of the pineal gland.
CASE REPORT
A 28-year-old woman with an unremarkable past medical history presented to the emergency department with a 6-month history of headaches and the insidious onset of neurologic deficits including hearing loss, slowed speech, fatigue, loss of appetite, hair loss, and presyncopal episodes. She described parietal region headaches that worsened during the day with slight improvement at night. She had previously been seen by her primary physician with complaints of excessive thirst, frequent urination, and abnormal menses and was found to have diminished thyroid function. Neurological examination revealed subtle left face and arm weakness, slight left pronator drift, right-sided tremor, and left-sided dysmetria. Extraocular muscles were abnormal on far leftward gaze, with the left eye demonstrating a mild lateral rectus palsy.
An unenhanced head computed tomography (CT) scan and subsequent gadolinium-enhanced magnetic resonance imaging (MRI) revealed a large midline mass centered in the pineal region with moderate to severe obstructive hydrocephalus (Figure 1). The MRI demonstrated a solid and cystic-enhancing mass with imaging findings suggestive of a pineal germinoma, although there were a number of alternative, albeit less likely, differential pathologic considerations for the appearance of this mass.
Figure 1.
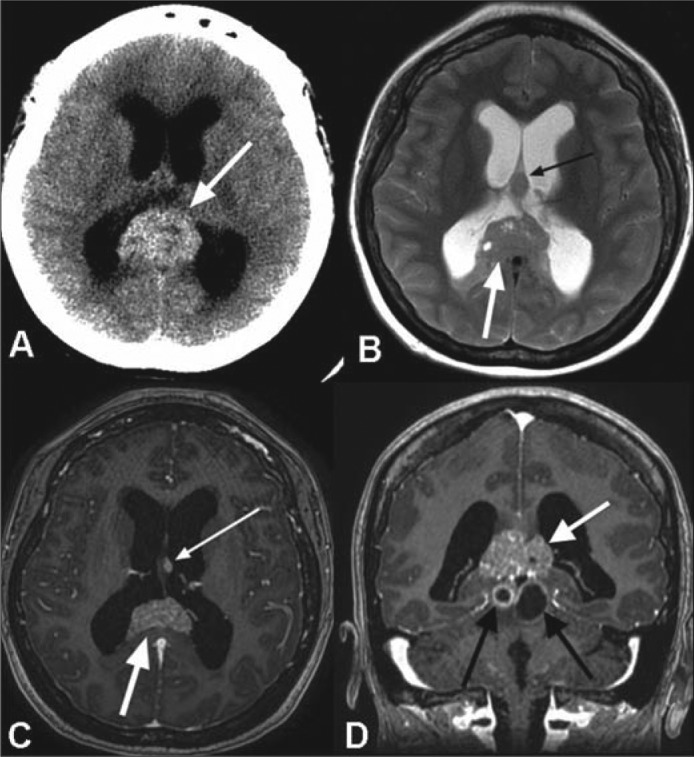
(a) Axial noncontrast CT image demonstrates a pineal region mass (white arrow) with intrinsic hyperdensity and associated obstructive hydrocephalus. (b) Axial T2-weighted, (c) axial, and (d) coronal postcontrast T1-weighted MR images reveal a T2 signal isointense to gray matter with intermixed cystic foci (white arrow in b) and avid enhancement following contrast administration (large white arrows in c and d). A separate T2 hypointense (small black arrow in b) and enhancing (small white arrow in c) nodule is seen at the interface of the septum pellucidum and left forniceal body, which suggests local cerebrospinal fluid dissemination of tumor. Additional, peripherally enhancing cystic foci are present within the midbrain tectum (black arrows in d).
Dexamethasone was initiated for cerebral edema in addition to levetiracetam for seizure prophylaxis. The patient underwent an endoscopic third ventriculostomy with tumor biopsy. Histological analysis was compatible with a germinoma (Figure 2). Initial cerebrospinal fluid (CSF) cytological testing was negative for malignant cells and showed CSF tumor marker levels of 2.5 mIU/mL for beta-human chorionic gonadotropin (beta-HCG) and <0.5 ng/mL for alpha-fetoprotein (AFP). Follow-up serum screening results 1 month later revealed a beta-HCG of 5 mIU/mL and AFP of 1.5 ng/mL. MRI of the spinal axis was negative for drop metastases.
Figure 2.
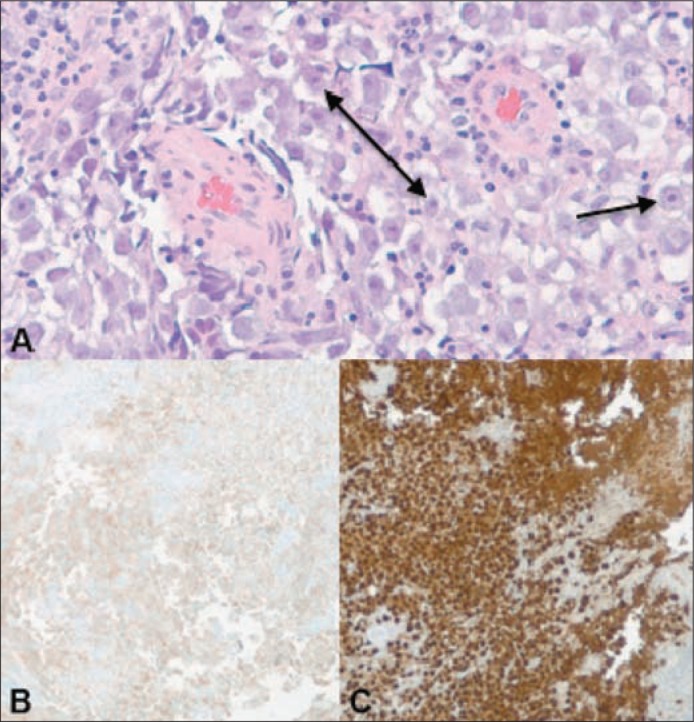
(a) Histologic examination of the biopsy specimen reveals predominantly monotonous polygonal shaped cells with prominent nucleoli and cytoplasm ranging from clear to eosinophilic. Immunohistochemical stains for (b) OCT 3/4 and (c) CD117 reveal strong reactivity. CD117 marker may also be found in other tumors such as gastrointestinal stromal tumors, whereas OCT 4 is relatively specific for germ cells. When CD117 and OCT 4 are used together, they are quite specific for a germinoma.
The patient's postoperative course was complicated by diabetes insipidus treated with intranasal desmopressin. The patient received whole ventricular radiation therapy followed by a boost to the tumor and its margin to a total dose of 4500 cGy using intensity-modulated radiation therapy over the course of 5 weeks. Early posttreatment imaging demonstrated considerable interval improvement in the appearance of the tumor bed (Figure 3); however, surveillance imaging will be needed to confirm complete resolution of the neoplasm.
Figure 3.
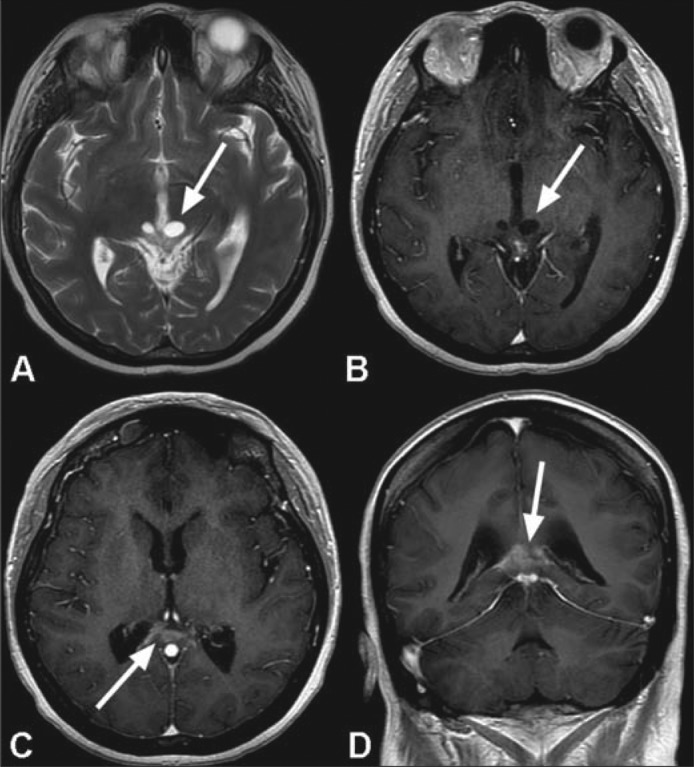
MR images following radiotherapy show a marked improvement in the appearance of the tumor bed. Near complete resolution of the large mass in the pineal fossa is seen with minimal residual enhancement along the margin of the splenium of the corpus callosum (arrows in c and d). There is considerable reduction in the extent of the previously seen peripheral enhancement involving the cystic components within the tectum of the midbrain (arrows in a and b).
DISCUSSION
Pineal region tumors are rare and make up <1% of all intracranial neoplasms (1). Germ cell tumors (GCTs) account for 50% of tumors found in the pineal region, with the majority being pure germinomas (1, 2). Germinomas may be found in the pineal recess (50%–65%), suprasellar region (25%–35%), and basal ganglia/thalamus (5%–10%) (3). The median age of diagnosis for a CNS GCT is 10 to 12 years, with a male predominance of up to 3:1 (1, 4, 5). In individuals with a GCT in the pineal region, male predominance is 12:1 (1, 4, 5). The clinical presentation of a pineal germinoma includes findings of increased intracranial pressure and/or hydrocephalus: headache, nausea, vomiting, papilledema, lethargy, and somnolence. Parinaud syndrome can be found in patients with pineal GCTs and is characterized by paralysis of upward gaze in addition to loss of accommodation, convergence, and pupillary light reflex (3).
The differential diagnosis for neoplastic masses in the pineal region includes GCT (pure germinoma, World Health Organization [WHO] grade 2) and nongerminomatous cell tumors, including pineal parenchymal tumors such as pineocytomas (WHO grade 1), pineoblastomas (WHO grade 4), pineal parenchymal tumors of intermediate differentiation (no current WHO grade), and papillary tumors of the pineal region (WHO grade 2–3). Germinomas typically demonstrate increased attenuation relative to gray matter on CT as well as a draped configuration with respect to the posterior third ventricle (6, 7). Lesions are typically isodense to hyperintense to gray matter on T1- and T2-weighted MRI, with cystic and necrotic changes seen in larger masses (6, 7). Avid enhancement is seen following intravenous contrast administration. MRI of the entire neuroaxis and lumbar puncture is recommended to assess for CSF seeding and drop metastases (6). Other lesions encountered in the pineal region include, but are not limited to, exophytic tectal astrocytomas, pineal cysts, epidermoids/dermoids, neurocysticercosis, and tentorial incisure meningiomas.
Establishing a definitive diagnosis of a germinoma is quite important due to the pronounced differences in treatment options compared with other histological subtypes found in this area. Germinomas are particularly sensitive to radiation therapy, and long-term survival rates between 79% and 90% have been routinely achieved (8). External-beam radiation therapy distributes ionizing radiation from a source outside the patient to produce permanent cancer cell DNA strand breaks. The current standard of care predicates the use of whole ventricular radiation therapy with an additional boost to the tumor versus craniospinal irradiation (9). Whole ventricular radiation therapy achieves reduced recurrence rates and decreased spinal failure compared with craniospinal irradiation (9). The addition of neoadjuvant chemotherapy can lead to a reduced radiation dose and volume with localized germinomas, which is of particular importance in pediatric patients (10). Efforts to minimize toxicity may lead to a reduced side effect profile without sacrificing tumor regression (10).
References
- 1.Al-Hussaini M, Sultan I, Abuirmileh N, Jaradat I, Qaddoumi I. Pineal gland tumors: experience from the SEER database. J Neurooncol. 2009;94(3):351–358. doi: 10.1007/s11060-009-9881-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shibui S, Nomura K. Statistical analysis of pineal tumors based on the data of Brain Tumor Registry of Japan. Prog Neurol Surg. 2009;23:1–11. doi: 10.1159/000210049. [DOI] [PubMed] [Google Scholar]
- 3.Ferla S, Spartà S, Giordano R, Zorat PL, Marin G, Meneghetti G. Pineal germinoma: diagnosis, treatment and tumor response. Ital J Neurol Sci. 1987;8(3):267–270. doi: 10.1007/BF02337485. [DOI] [PubMed] [Google Scholar]
- 4.Jennings MT, Gelman R, Hochberg F. Intracranial germ-cell tumors: natural history and pathogenesis. J Neurosurg. 1985;63(2):155–167. doi: 10.3171/jns.1985.63.2.0155. [DOI] [PubMed] [Google Scholar]
- 5.Villano JL, Propp JM, Porter KR, Stewart AK, Valyi-Nagy T, Li X, Engelhard HH, McCarthy BJ. Malignant pineal germ-cell tumors: an analysis of cases from three tumor registries. Neuro Oncol. 2008;10(2):121–130. doi: 10.1215/15228517-2007-054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dumrongpisutikul N, Intrapiromkul J, Yousem DM. Distinguishing between germinomas and pineal cell tumors on MR imaging. AJNR Am J Neuroradiol. 2012;33(3):550–555. doi: 10.3174/ajnr.A2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith AB, Rushing EJ, Smirniotopoulos JG. From the archives of the AFIP: lesions of the pineal region: radiologic-pathologic correlation. Radiographics. 2010;30(7):2001–2020. doi: 10.1148/rg.307105131. [DOI] [PubMed] [Google Scholar]
- 8.Haddock MG, Schild SE, Scheithauer BW, Schomberg PJ. Radiation therapy for histologically confirmed primary central nervous system germinoma. Int J Radiat. Oncol Biol Phys. 1997;38(5):915–923. doi: 10.1016/s0360-3016(97)00135-1. [DOI] [PubMed] [Google Scholar]
- 9.Haas-Kogan DA, Missett BT, Wara WM, Donaldson SS, Lamborn KR, Prados MD, Fisher PG, Huhn SL, Fisch BM, Berger MS, Le QT. Radiation therapy for intracranial germ cell tumors. Int J Radiat Oncol Biol Phys. 2003;56(2):511–518. doi: 10.1016/s0360-3016(02)04611-4. [DOI] [PubMed] [Google Scholar]
- 10.Khatua S, Dhall G, O'Neil S, Jubran R, Villablanca JG, Marachelian A, Nastia A, Lavey R, Olch AJ, Gonzalez I, Gilles F, Nelson M, Panigrahy A, McComb G, Krieger M, Fan J, Sposto R, Finlay JL. Treatment of primary CNS germinomatous germ cell tumors with chemotherapy prior to reduced dose whole ventricular and local boost irradiation. Pediatr Blood Cancer. 2010;55(1):42–46. doi: 10.1002/pbc.22468. [DOI] [PubMed] [Google Scholar]


