Abstract
We present a case of hepatic angiosarcoma that presented with disseminated intravascular coagulopathy to highlight the difficulty in diagnosing this disease due its aggressive clinical course, the overlapping features of various coagulopathies, and the nonspecific appearance of angiosarcomas on imaging.
Hepatic angiosarcoma is a rare neoplasm that develops from endothelial cells in the liver. It presents with generalized, nonspecific symptoms such as weight loss, fatigue, and abdominal pain, but on rare occasions coagulopathies may result (1–3). Here we present a case of hepatic angiosarcoma that presented with disseminated intravascular coagulopathy (DIC).
CASE REPORT
An 81-year-old woman was admitted for anemia, thrombocytopenia, and jaundice. Three weeks earlier, she had presented with generalized pruritus and jaundice. She was found to have a hemoglobin level of 4.6 g/dL, hematocrit of 13%, and platelet count of 25,000 μL. She was initially diagnosed with thrombotic thrombocytopenic purpura and was treated with plasma exchange, methylprednisolone, and rituximab without improvement before being transferred to Baylor University Medical Center at Dallas (BUMC). Two years earlier, abdominal computed tomography (CT) disclosed a nodular-appearing liver. A biopsy did not reveal any signs of malignancy or cirrhosis, but noted focal dilatation of the sinusoidal spaces.
On presentation at BUMC, the patient had marked jaundice and diffuse purpura on her extremities. Her abdomen was distended with a positive fluid wave. Hepatosplenomegaly was not appreciated. Laboratory results showed a hemoglobin of 8.9 g/dL; hematocrit of 27.3%; platelet count of 30 μL; schistocytes on the blood smear; total bilirubin of 14.6 mg/dL; direct bilirubin of 8.5 mg/dL; alkaline phosphatase of 109 U/L; fibrinogen of 50 mg/dL (normal 200–400 mg/dL); D-dimer of 34.6 mg/L (normal <.59 mg/L); prothrombin time of 20.7 seconds; and activated thromboplastin time of 30.2 seconds. The patient was given cryoprecipitate and a heparin infusion, and her fibrinogen transiently rose to 153 mg/dL. Magnetic resonance imaging (MRI) of her abdomen showed extensive abnormal patchy enhancement throughout the liver (Figure 1). A transjugular liver biopsy revealed spindle cells suspicious for but not diagnostic of malignancy. The night following the biopsy, swelling and tightness in her right lower extremity was found. CT revealed an expanding hematoma in the right vastus muscle. She was managed with blood products and a reduced concentration of heparin until the family decided to pursue comfort care measures only. She died within 24 hours.
Figure 1.
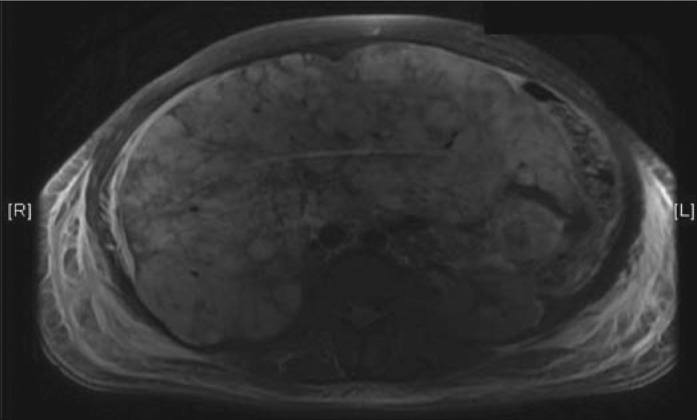
MRI of the liver reveals extensive abnormal patchy enhancement throughout the liver which is favored to relate to intrahepatic shunts/collaterals consistent with possible Budd-Chiari syndrome.
Autopsy revealed a spongy liver with diffuse congestion (Figure 2a). Multiple large cystic spaces contained thrombus. Microscopically, groups of spindle cells with pleomorphic nuclei and eosinophilic cytoplasm formed small vascular channels within the cystic spaces (Figure 2b). Tumor cells also infiltrated the liver parenchyma and the sinusoids. The lining of some cystic spaces suggested venous walls, and portal tract veins also contained infiltrating tumor. No metastatic tumor was present outside of the liver. The tumor cells were immunohistochemically reactive for Factor VIII, FLI-1, and CD34, consistent with hepatic angiosarcoma. Ki-67 measured 50%. There was no granular material to suggest thorotrast.
Figure 2.
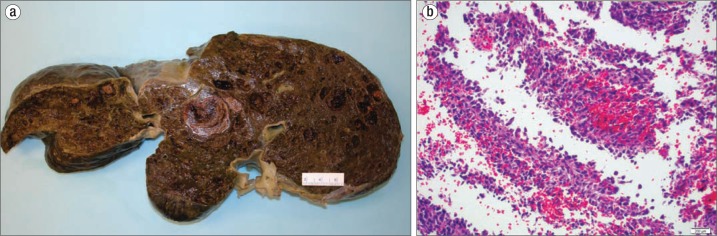
The liver at autopsy. (a) Gross photo. Cut sections of the liver showed diffusely spongy and intensely congested parenchyma with multiple areas of intraparenchymal thrombosis. (b) Spindled cells with moderately pleomorphic nuclei lining vascular channels (hematoxylin and eosin stain, 200×).
DISCUSSION
Liver angiosarcoma is a rare malignancy, accounting for <2% of primary liver malignancies (1). It has a male preponderance and most commonly presents in the fifth or sixth decade of life (4). The most common site for an angiosarcoma is the head and neck, with the liver being the fifth most common site (5). About 30% of liver angiosarcoma cases are associated with environmental exposures to vinyl chloride, arsenic, and thorotrast, a radiocontrast dye used in the 1950s (1). Since exposure to these carcinogens has drastically decreased in recent decades, most cases now are unrelated to these environmental causes. The presentation is nonspecific with fatigue, weight loss, and abdominal discomfort being the most common symptoms, followed by jaundice, ascites, and hepatomegaly.
Hepatic angiosarcomas have an aggressive clinical course with minimal treatment responses and common relapses. Only one-third of cases are diagnosed antemortem and only 25% by closed liver biopsy. Metastases develop in about 50% of patients. Average survival is 6 months, with death resulting from hepatic failure in 50% of patients and from intra-abdominal hemorrhage in 25%. When the tumor is confined to one lobe of the liver, resection is indicated. However, in most cases, the tumor has diffusely infiltrated the tumor at the time of diagnosis. Palliative chemotherapy may be indicated, but a single treatment regimen has not been established.
DIC caused by an angiosarcoma is rare. In one series, DIC occurred in 17% of 42 patients with an angiosarcoma (4). Of the 7 patients with this complication, only one had a hepatic angiosarcoma. On a review of hepatic angiosarcomas, DIC was seen in <5% of cases (1). The prognosis with this complication of the disease is exceedingly poor, with a median survival of <6 months and with <3% of patients surviving beyond 2 years. Even with treatment, the DIC tends to recur (3). Treating DIC with heparin may be poorly tolerated, with additional bleeding complications.
Several theories have been proposed to explain how angiosarcomas may trigger DIC, including the malignancy exposing the basement membrane of the endothelium; a decreased blood supply flow inducing blood cell trapping and lysis, triggering the release of clotting factors; and local inflammation or malignant endothelial cells inducing thromboplastin and tissue factor release. Benign capillary and visceral hemangiomas may also be associated with DIC, as in Kasabach-Merritt syndrome (6).
In this case report, the associated DIC was initially mistaken for thrombotic thrombocytopenic purpura associated with coagulopathy of liver disease. These three hematologic disorders have many overlapping features, and distinguishing between them can be challenging. Thrombotic thrombocytopenic purpura is characterized by a microangiopathic hemolytic anemia (confirmed by the presence of schistocytes on the blood smear) and thrombocytopenia, but typically no other coagulopathy. DIC includes not just anemia and thrombocytopenia, but a coagulopathy associated with thrombin generation, including prolonged prothrombin and activated thromboplastin times, a low fibrinogen, and an increased D-dimer. Hemolysis with schistocytosis is present in 50% of cases. Liver disease can manifest a coagulopathy similar to that of DIC, including anemia, thrombocytopenia, and an abnormal coagulation profile, since most clotting factors are synthesized in the liver. Our patient's blood smear revealed schistocytes most consistent with a microangiopathic hemolytic anemia related to DIC, rather than acanthocytes and target cells more typically noted with liver disease.
Another confounding factor that made the diagnosis elusive was the appearance of the liver on imaging. Classically on T2-weighted MRI, liver angiosarcomas are characterized as multiple lesions of high signal intensity with central regions of low signal intensity. In our case, the MRI showed diffuse abnormal patchy enhancements in the liver, which relates to intrahepatic shunts and collateral vasculature. This finding was more consistent with Budd-Chiari syndrome than malignancy. This imaging appearance, along with the nondiagnostic biopsy sample obtained, prevented the diagnosis of a discrete liver malignancy until autopsy.
References
- 1.Locker GY, Doroshow JH, Zwelling LA, Chabner BA. The clinical features of hepatic angiosarcoma: a report of four cases and a review of the English literature. Medicine (Baltimore) 1979;58(1):48–64. doi: 10.1097/00005792-197901000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Truell JE, Peck SD, Reiquam CW. Hemangiosarcoma of the liver complicated by disseminated intravascular coagulation. A case report. Gastroenterology. 1973;65(6):936–942. [PubMed] [Google Scholar]
- 3.Farid M, Ahn L, Brohl A, Cioffi A, Maki RG. Consumptive coagulopathy in angiosarcoma: a recurrent phenomenon? Sarcoma. 2014;2014:617102. doi: 10.1155/2014/617102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang KF, Leow VM, Hasnan MN, Manisekar KS. Primary hepatic angiosarcoma: difficulty in clinical, radiological, and pathological diagnosis. Med J Malaysia. 2012;67(1):127–128. [PubMed] [Google Scholar]
- 5.Young RJ, Brown NJ, Reed MW, Hughes D, Woll PJ. Angiosarcoma. Lancet Oncol. 2010;11(10):983–991. doi: 10.1016/S1470-2045(10)70023-1. [DOI] [PubMed] [Google Scholar]
- 6.Alliot C, Tribout B, Barrios M, Gontier MF. Angiosarcoma variant of Kasabach-Merritt syndrome. Eur J Gastroenterol Hepatol. 2001;13(6):731–734. doi: 10.1097/00042737-200106000-00020. [DOI] [PubMed] [Google Scholar]


