Abstract
To clarify the geographical distribution of scrub typhus vectors in Korea, a survey of larval trombiculid mites was conducted from 2005 to 2007 by collecting wild small mammals twice a year (spring and autumn) at 24 sites nationwide. A total of 67,325 mites representing 4 genera and 14 species were collected from 783 trapped rodents, corresponding to a chigger index (number of chigger mites per rodent) of 86.0. The predominant mite species were Leptotrombidium pallidum (52.6%), Leptotrombiduim scutellare (27.1%), Leptotrombidium palpale (8.2%), Leptotrombidium orientale (5.6%), and Neotrombicula tamiyai (1.7%). However, the proportions of L. scutellare in southern areas, including endemic provinces such as Jeollabuk-Do (34.3%), Jeollanam-Do (49.0%), and Gyeongsangnam-Do (88%), were relatively higher than in central Korean regions where L. pallidum was predominant. In autumn, the ratio of L. scutellare increased to 42% while the ratio of L. pallidum decreased. The geographical distribution map of the L. scutellare chigger index was identical to the incidence pattern of scrub typhus, whereas those of overall mites and L. pallidum showed no relationship with case incidence patterns. Distribution mapping analysis shows an identical geographical distribution of L. scutellare and epidemic incidence of scrub typhus in South Korea. L. pallidum could be another vector at all other parts of the Korean peninsula, including the eastern and northern regions that have a low level of scrub typhus incidence.
Introduction
Scrub typhus (tsutsugamushi disease) is caused by the rickettsial bacterium, Orientia tsutsugamushi, which are transmitted through bites from infected larval trombiculid mites [1]. Orientia tsutsugamushi is maintained in mite populations by transovarial transmission [2], [3].
In South Korea, scrub typhus is an acute febrile disease most common in the autumn months; the annual case number has fluctuated around 5,000 (approximately 10 per 100,000 persons) since 2004 [4]. As a reportable disease, confirmed cases of scrub typhus have been reported to Korea Centers for Disease Control and Prevention (KCDC) by the National Notifiable Disease Surveillance System since 1994. In 2013, 10,365 cases were reported nationwide; the highest number ever recorded, this total represents a 38.1-fold increase compared to 274 cases reported in 1995 [5]. Over 90% of cases were reported during the epidemic period, from October to November [4], [6].
Known vector species of chigger mites include Leptotrombidium akamushi, L. pallidum, L. scutellare, L. deliense, and L. imphalum in Japan, Taiwan, Thailand, and China, respectively [7], [8], [9], [10]. Ree et al. (1991) first reported L. pallidum as a vector in Korea, with an infection rate of 0.4% in 447 tested mites [11]. L. scutellare is another key vector, with an infection rate of 0.5% [12]. The O. tsutsugamushi bacterium has also been detected in L. palpale, L. orientale, L. zetum, Neotrombicula japonica and Euschoengastia koreaensis chigger mites [13], [14].
The distribution of chigger mites in Korea has been mainly surveyed in epidemic regions, such as Jeollanam-do, Gyeonggi-do, and Chungcheongnam-do [3], [15], [16]. A 2006 survey of 13 localities in Korea from October to November was the first nationwide survey [17].
In this study, we surveyed 24 localities nationwide in spring and autumn from 2005 to 2007 and analyzed the relationship between trombiculid mite geographical distribution and scrub typhus epidemic regions in Korea.
Materials and Methods
Surveillance localities and periods
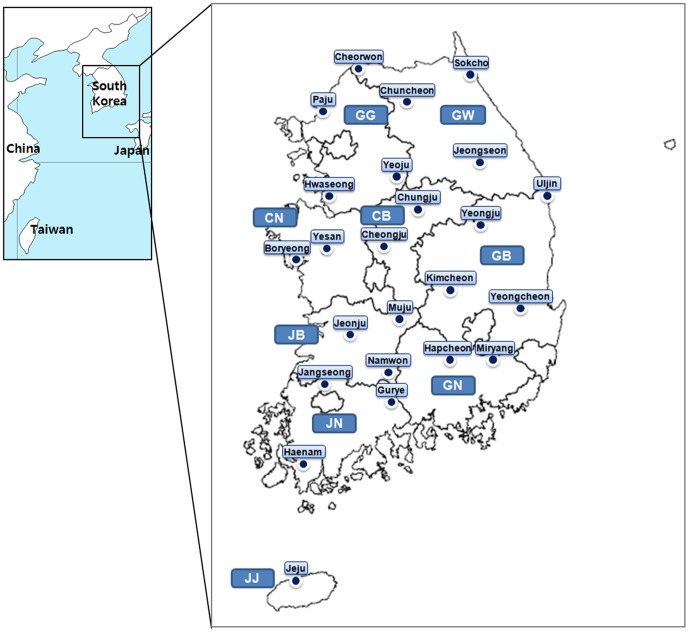
Larval trombiculid mites were collected from wild rodents captured at 24 sites nationwide from 2005 to 2007 (Fig. 1 and S1 Table). Collections were performed at each location in spring (March–May) and autumn (October–November). Collection information, including locality, collection year and month, number of traps installed, and number of rodents captured, is summarized in S1 Table. Rodent trap install sites included rice fields, cropped fields, reservoirs, waterways, hillsides, grass fields and riversides.
Figure 1. Scrub typhus surveillance in the Korean peninsula.
Dots indicate nationwide surveillance locations. Each name indicates a city level locality (-Si, -Gun, and -Gu in the Korean administrative area system). Province abbreviations: GG, Gyeonggi-Do; GW, Gangwon-Do; CB, Chungcheongbuk-Do; CN, Chungcheongnam-Do; GB, Gyeongsangbuk-Do; GN, Gyeongsangnam-Do; JB, Jeollabuk-Do; JN, Jeollanam-Do; JJ, Jeju-Do.
Collection of small mammals and chigger mites
The animal protocol used in this study was reviewed and approved based on ethical procedures and scientific care by the KCDC-Institutional Animal Care and Use Committee (KCDC-IACUC; KCDC-046-13-2A). There was no need for specific permission for each collecting site, because these sites were not located at national parks or protected areas. The selection of collecting sites was supported by each local Public Health Center. The collected rodents were not the endangered or protected species in Korea.
The collection of small mammals was performed at 24 collection sites nationwide. A total of 10 to 15 Sherman live folding traps (3×3×9 inches), baited with a peanut butter spread biscuit, were set up at five to seven points in the collection site at 3–5 m intervals and collected the next morning. Collected wild rodents were euthanized by compressed carbon dioxide (CO2). The wild rodent corpses were hung over glass bowls filled with tap water for 24 h to collect dropped chigger mites. Chigger mites were recovered from the water surface with a fine brush and stored at 4°C for further identification.
Identification of chigger mites
Individual chigger mites were transferred to glass slides and mounted with PVA MTNG medium (Polyvinyl alcohol mounting medium, Bioquip). The specimens were identified to species level under stereo-microscopic examination using morphological keys [18].
ArcGIS geographical analysis
To compare the geographical distribution of vectors, distribution maps were drawn by interpolation using the IDW (Inverse Distance Weighted) technique among Spatial Analyst Tools in ArcGIS 9.0 (2004. Environmental Research Systems Institute, Redlands, CA, USA). Information about patients in South Korea diagnosed with scrub typhus in 2007 was obtained from the National Notifiable Disease Web Statistics System (NNDWSS) of the KCDC (Fig. 2A). Scrub typhus was diagnosed by indirect immune-fluorescent assay and nested polymerase chain reaction (PCR) by regional Institutes of Health and Environment and hospitals and reported to the NNDWSS of the KCDC.
Figure 2. Scrub typhus incidence in 2007 (A), and average monthly number of patients with scrub typhus (B) from 2001 to 2010.
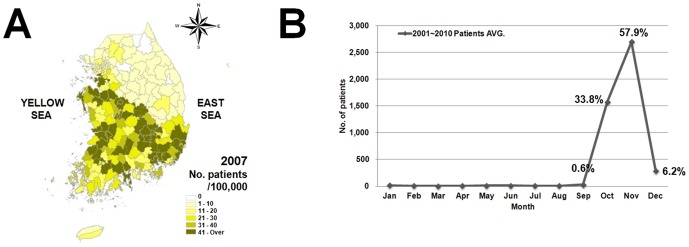
The figure is generated using data from the National Notifiable Disease Web Statistics System (NNDWSS) of the KCDC. Percentage (%) indicates monthly incidence rate.
Results and Discussion
A total of 5,538 traps were installed and 783 wild rodents captured at 24 regions in 9 South Korean provinces (S1 Table). The overall trapping rate was 14.1; the highest trapping rate (30.5%) was recorded in riverside locations. Among trapped rodents, Apodemus agrarius was dominant in all regions, accounting for 87.4% of the collections (data not shown), followed by Crocidura lasiura and Micromys minutus at 8.0% and 4.2%, respectively. From those rodents, 67,325 mites representing 4 genera and 14 species were collected, with a chigger index (C.I., number of chigger mites per rodent) of 86.0 (Table 1). The number and C.I. (43,434 and 108.6) of chigger mites in autumn were 1.8-fold and 1.7-fold higher than in spring (23,891 and 62.4). This result suggests that the high density of chigger mites may affect the high incidence rate (92.3%) of scrub typhus in autumn. In South Korea, the incidence of scrub typhus is generally highest during autumn (over 90%), from September to November, while the incidence in other seasons (spring, summer and winter) ranged from 0.1 to 0.3% (Fig. 2B). However, the difference in chigger indices between spring and autumn does not proportionally explain the difference in scrub typhus incidence in the same seasons.
Table 1. Species of chigger mites from small mammals collected through the nationwide survey in South Korea.
| Locality | S/Aa | No. of captured rodent | No. of chigger mites | Chigger Index (C.I.) | C. ikaoensis | E. korea-ensis | L. gemiti-culum | L. hiranu-mai | L. orientale | L. pallidum | L. palpale | L. scutellare | L. subinter-medium | L. zetum | N. gardellai | N. japonica | N. kwangn-engensis | N. tamiyai | |
| GG | 78 | 7,110 | 91.2 | 10 | 21 | - | - | 427 | 5,237 | 839 | 131 | - | 232 | 2 | 11 | - | 200 | ||
| Hwaseong | S | 8 | 404 | 50.5 | - | - | - | - | 113 | 166 | 68 | - | - | 57 | - | - | - | - | |
| A | 17 | 1,410 | 82.9 | - | 9 | - | - | 47 | 741 | 427 | 131 | - | 41 | - | 11 | - | 2 | ||
| Yeoju | S | 17 | 2,951 | 173.6 | 10 | - | - | - | 166 | 2,640 | 67 | - | - | 38 | - | - | - | 31 | |
| A | 13 | 102 | 7.8 | - | - | - | - | 26 | 70 | 4 | - | - | - | 2 | - | - | - | ||
| Paju | S | 21 | 2,243 | 106.8 | - | 12 | - | - | 75 | 1,620 | 273 | - | - | 96 | - | - | - | 167 | |
| A | 2 | - | 0.0 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| GW | 111 | 9,424 | 84.9 | 4 | 399 | 382 | 22 | 765 | 5,855 | 671 | 14 | - | 26 | 2 | 616 | 476 | 193 | ||
| Chuncheon | S | 13 | 798 | 61.4 | - | - | - | - | 144 | 548 | 84 | - | - | 2 | - | - | - | 21 | |
| A | 15 | 80 | 5.3 | - | - | - | - | 4 | 69 | 2 | - | - | - | - | - | 4 | - | ||
| Sokcho | S | 11 | 271 | 24.6 | - | - | - | - | 123 | 106 | 42 | - | - | - | - | - | - | - | |
| A | 31 | 2,517 | 81.2 | - | - | - | - | 16 | 1,349 | 443 | 14 | - | - | - | 248 | 421 | 26 | ||
| Jeongseon | S | 18 | 242 | 13.4 | - | - | - | - | 2 | 185 | 28 | - | - | - | - | - | - | 28 | |
| A | 3 | 84 | 28.0 | - | 59 | - | - | 10 | - | 10 | - | - | - | - | - | - | 5 | ||
| Cheorwon | S | 11 | 2,691 | 244.6 | - | - | - | 22 | 373 | 2,141 | 36 | - | - | 20 | - | - | 2 | 97 | |
| A | 9 | 2,741 | 304.6 | 4 | 340 | 382 | - | 93 | 1,457 | 26 | - | - | 4 | 2 | 368 | 49 | 16 | ||
| CB | 68 | 6,893 | 101.4 | 10 | 232 | 44 | - | 102 | 5,146 | 284 | 980 | - | 20 | 37 | 12 | 20 | 6 | ||
| Cheongju | S | 18 | 688 | 38.2 | - | 4 | - | - | 6 | 531 | 146 | - | - | - | - | - | - | 2 | |
| A | 26 | 3,056 | 117.5 | 10 | 163 | - | - | 14 | 1,712 | 89 | 980 | - | 18 | 37 | 8 | 20 | 4 | ||
| Chungju | S | 18 | 2,906 | 161.4 | - | - | - | - | - | 2,859 | 47 | - | - | - | - | - | - | - | |
| A | 6 | 243 | 40.5 | - | 66 | 44 | - | 82 | 44 | 2 | - | - | 2 | - | 4 | - | - | ||
| CN | 50 | 4,288 | 85.8 | - | 37 | - | - | 638 | 2,500 | 284 | 792 | - | 34 | - | 2 | - | - | ||
| Boryeong | S | 17 | 781 | 45.9 | - | - | - | - | 184 | 539 | 46 | - | - | 12 | - | - | - | - | |
| A | 16 | 806 | 50.4 | - | - | - | - | 5 | 504 | 188 | 109 | - | - | - | - | - | - | ||
| Yesan | S | 7 | 867 | 123.9 | - | 6 | - | - | 310 | 521 | 30 | - | - | - | - | - | - | - | |
| A | 10 | 1,834 | 183.4 | - | 31 | - | - | 139 | 936 | 20 | 683 | - | 22 | - | 2 | - | - | ||
| JB | 104 | 8,700 | 83.7 | 7 | 67 | - | - | 263 | 4,283 | 770 | 2,986 | 2 | 17 | 2 | 19 | 5 | 279 | ||
| Jeonju | S | 13 | 270 | 20.8 | - | - | - | - | 54 | 214 | 2 | - | - | - | - | - | - | - | |
| A | 6 | 1,207 | 201.2 | - | 49 | - | - | 79 | 382 | 20 | 675 | - | - | 2 | - | - | - | ||
| Namwon | S | 23 | 1,588 | 69.0 | 2 | - | - | - | 51 | 1,106 | 167 | - | - | - | - | - | - | 262 | |
| A | 17 | 2,196 | 129.2 | 3 | 10 | - | - | 22 | 580 | 66 | 1,495 | - | 3 | - | - | 5 | 12 | ||
| Muju | S | 18 | 325 | 18.1 | - | - | - | - | 2 | 286 | 32 | - | 2 | - | - | - | - | 3 | |
| A | 27 | 3,114 | 115.3 | 2 | 8 | - | - | 55 | 1,715 | 483 | 816 | - | 14 | - | 19 | - | 2 | ||
| JN | 126 | 17,251 | 136.9 | 10 | 23 | - | - | 977 | 5,741 | 1,415 | 8,449 | 6 | 90 | 2 | 30 | 13 | 497 | ||
| Gurye | S | 18 | 1,689 | 93.8 | - | - | - | - | 198 | 1,470 | 12 | 1 | 2 | 6 | - | - | - | - | |
| A | 9 | 1,374 | 152.7 | - | 9 | - | - | 70 | 392 | 44 | 837 | 4 | 4 | - | - | 13 | 2 | ||
| Haenam | S | 9 | 144 | 16.0 | - | - | - | - | 120 | - | 14 | 3 | - | 8 | - | - | - | - | |
| A | 32 | 6,370 | 199.1 | 10 | 12 | - | - | 404 | 12 | 372 | 5,519 | - | 10 | 2 | 30 | - | - | ||
| Jangseong | S | 28 | 1,288 | 46.0 | - | 2 | - | - | 95 | 943 | 59 | - | - | 54 | - | - | - | 135 | |
| A | 30 | 6,386 | 212.9 | - | - | - | - | 90 | 2,924 | 915 | 2,090 | - | 8 | - | - | - | 359 | ||
| GB | 167 | 8,112 | 48.6 | 20 | 74 | - | - | 318 | 6,628 | 970 | 28 | - | 43 | 6 | 13 | 11 | 2 | ||
| Kimcheon | S | 23 | 2,410 | 104.8 | - | - | - | - | - | 2,364 | 46 | - | - | - | - | - | - | - | |
| A | 33 | 2,968 | 89.9 | - | - | - | - | - | 2,188 | 739 | 25 | - | 14 | - | 2 | - | - | ||
| Yeongju | S | 25 | 1,014 | 40.6 | 2 | 2 | - | - | 270 | 721 | 16 | - | - | 2 | - | - | - | 2 | |
| A | 5 | 355 | 71.0 | - | - | - | - | 15 | 325 | 2 | - | - | - | - | 2 | 11 | - | ||
| Yeongcheon | S | 28 | 28 | 1.0 | 2 | 1 | - | - | - | - | 20 | - | - | 4 | - | - | - | - | |
| A | 17 | 242 | 14.2 | 14 | 64 | - | - | 6 | 15 | 108 | 3 | - | 23 | - | 9 | - | - | ||
| Uljin | S | 4 | 140 | 35.0 | - | - | - | - | 21 | 119 | - | - | - | - | - | - | - | - | |
| A | 32 | 955 | 29.8 | 2 | 7 | - | - | 6 | 896 | 39 | - | - | - | 6 | - | - | - | ||
| GN | 51 | 4,753 | 93.2 | - | 2 | - | - | 288 | 27 | 251 | 4,183 | - | 1 | - | - | - | 1 | ||
| Miryang | S | 11 | 25 | 2.3 | - | - | - | - | 9 | 11 | 3 | 2 | - | - | - | - | - | - | |
| A | 16 | 4,151 | 259.4 | - | - | - | - | 167 | 16 | 84 | 3,884 | - | - | - | - | - | - | ||
| Hapcheon | S | 11 | 80 | 7.3 | - | - | - | - | 75 | - | 4 | - | - | 1 | - | - | - | - | |
| A | 13 | 497 | 38.2 | - | 2 | - | - | 37 | - | 160 | 297 | - | - | - | - | - | 1 | ||
| JJ | 28 | 794 | 28.4 | - | 24 | - | - | 13 | - | 13 | 667 | - | 78 | - | - | - | - | ||
| Jeju | S | 13 | 48 | 3.7 | - | 2 | - | - | 2 | - | 10 | 1 | - | 34 | - | - | - | - | |
| A | 15 | 746 | 49.7 | - | 22 | - | - | 11 | - | 3 | 666 | - | 44 | - | - | - | - | ||
| Total | S | 383 | 23,891 | 62.4 | 16 | 29 | - | 22 | 2,393 | 19,090 | 1,252 | 7 | 4 | 334 | - | - | 2 | 748 | |
| A | 400 | 43,434 | 108.6 | 45 | 851 | 426 | - | 1,398 | 16,327 | 4,246 | 18,224 | 4 | 207 | 51 | 703 | 523 | 429 | ||
| 783 | 67,325 | 86.0 | 61 | 880 | 426 | 22 | 3,791 | 35,417 | 5,498 | 18,231 | 8 | 541 | 51 | 703 | 525 | 1,177 | |||
S or A indicates spring season or autumn season.
The predominant species identified in this study were Leptotrombidium pallidum (52.6%), L. scutellare (27.1%), L. palpale (8.2%), L. orientale (5.6%), and Neotrombicula tamiyai (1.7%) (Fig. 3A). This finding is similar to a previous report [17]: Lee et al. (2009) found L. pallidum to be the dominant species (74.9%), followed by L. scutellare (18.9%) and L. palpale (2.7%). Lee et al. further showed that L. pallidum and L. scutellare are the predominant scrub typhus vectors, accounting for approximately 90% of the chigger mite population in Korea. In autumn, the ratio of L. scutellare increased to 42.0% while L. pallidum decreased to 37.6% (Fig. 3B). L. palpale density also increased 5.2% to 9.8% in autumn. The increased density of L. scutellare and L. palpale may affect the high autumn incidence of scrub typhus (Fig. 3B). In Korea, spring is the only seeding season, while autumn is the main harvest season. Therefore, the high L. pallidum population without corresponding scrub typhus case increases in spring may be explained by seasonal differences in human behavior patterns that limit human exposure to L. pallidum in spring.
Figure 3. Total percentages (A) and seasonal prevalence (B) of chigger mites collected through Korean national surveys between 2005 and 2007.
“Others” indicates 9 minor species including E. koreaensis.
The highest C.I. was recorded in Jeollanam-Do (JN, 136.9) among 9 provinces, followed by Chungcheongbuk-Do (CB, 101.4), and Gyeongsangnam-Do (GN, 93.2) (Table 1). However, the regions with the highest average incidence over three years (2005 to 2007) were Jeollabuk-Do (JB), Chungcheongnam-Do (CN) and Jeollanam-Do (JN). The prevalence of L. scutellare in southern areas, including endemic provinces such as Jeollabuk-Do (2,986, 34.3%), Jeollanam-Do (8,449, 49.0%), and Gyeongsangnam-Do (4,183, 88%) was relatively higher than in the central areas where L. pallidum was predominant.
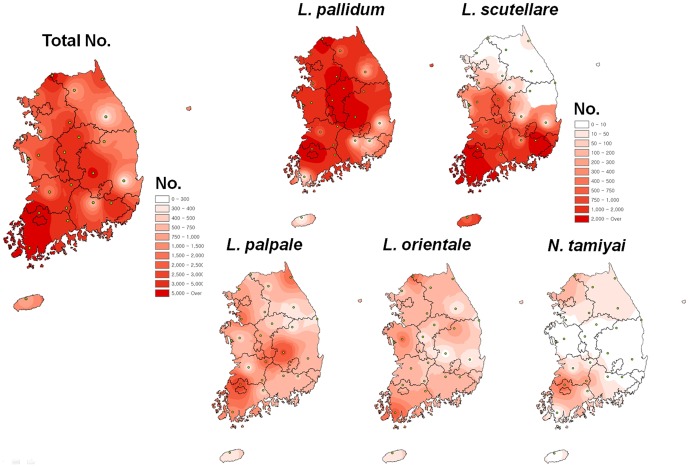
To visualize the geographical distribution of chigger mites in South Korea, we analyzed collection data using the interpolation method of the IDW tool in the ArcGIS program. We first drew distribution maps based on the number of chigger mites for the five predominant species (Fig. 4). L. pallidum, L. palpale and L. orientale were evenly distributed nationwide. However, L. scutellare was found in the western and southern parts of nation and N. tamiyai was not observed in central Korea. Interestingly, of the five species, the distribution pattern of L. scutellare was very similar to regions prevalent for scrub typhus (Fig. 2A and Fig. 4).
Figure 4. Geographical distribution of chigger mites.
The map color indices differ between the total number (0– over 5,000) and species maps (0– over 2,000).
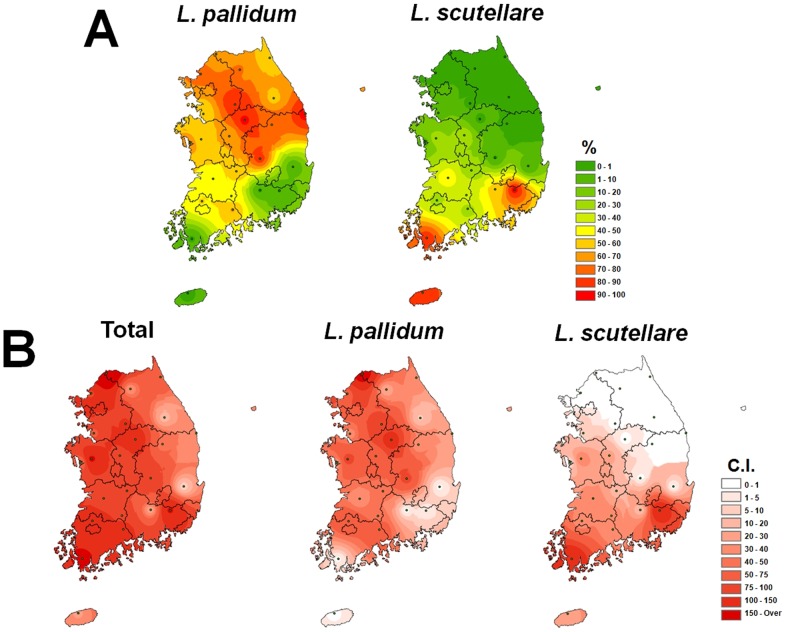
For regional analysis of predominant species, the percentages of L. pallidum and L. scutellare were also mapped (Fig. 5A). L. pallidum accounted for over 50% of mites collected in northern and central regions of Korea, while L. scutellare represented 30% of mites in the western and southern parts (Fig. 5A). In addition, the map of L. scutellare C.I. showed a similar pattern to the prevalence of scrub typhus in South Korea (Fig. 2A and Fig. 5B). Ree et al. (1991) first reported L. pallidum as a vector in Korea, with an infection rate of 0.4% in 447 tested mites [11]. L. scutellare is also known to be another key vector, with an infection rate of 0.5% [12]. O. tsutsugamushi bacteria have also been detected in L. palpale, L. orientale, L. zetum, Neotrombicula japonica and Euschoengastia koreaensis mites [13], [14]. According to Lee et al. (2011), the infection rates of O. tsutsugamushi did not differ significantly among vector mite species (range: 1.5–5.3%); infections rates in L. pallidum and L. scutellare were 1.5% and 3.7%, respectively, and the highest rate (5.3%) was in L. palpale [14]. L. pallidum and L. scutellare are considered major scrub typhus vectors in Korea. Scrub typhus is most prevalent in the western and southern regions of the Korean peninsula (Chungcheongnam-Do (CN), Jeollabuk-Do (JB), Jeollanam-Do (JN), and Gyeongsangnam-Do (GN)). The distribution and C.I. map of L. scutellare was identical to the incidence pattern of scrub typhus, whereas the C.I. maps L. pallidum and chigger mites overall show no relationship with the incidence pattern (Fig.4 and Fig. 5B). However, the L. scutellare C.I. in the Haenam region did not clearly match the scrub typhus incidence pattern; further study of this region is necessary. In conclusion, our distribution mapping analysis suggests that the geographical distribution of L. scutellare is identical to the epidemic incidence of scrub typhus in South Korea. Additionally, L. pallidum could be another vector in the Korean peninsula, including eastern and northern regions.
Figure 5. Geographical distribution based on percentages (A) and C.I. (B) of L. pallidum and L. scutellare.
Chigger index (C.I.) indicates the number of chigger mites on one small mammal.
Supporting Information
Collection sites of small mammals in Korean peninsula.
(DOC)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This work was supported by Korea Centers for Disease Control and Prevention under the budget for health promotion (No. 4800-4851-300). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Seong SY, Choi MS, Kim IS (2001) Orientia tsutsugamushi infection: overview and immune responses. Microbes Infect 3:11–21. [DOI] [PubMed] [Google Scholar]
- 2. Takahashi M, Murata M, Nogami S, Hori E, Kawamura A Jr, et al. (1988) Transovarial transmission of Rickettsia tsutsugamushi in Leptotrombidium pallidum successively reared in the laboratory. Jpn J Exp Med 58:213–218. [PubMed] [Google Scholar]
- 3. Phasomkusolsil S, Tanskul P, Ratanatham S, Watcharapichat P, Phulsuksombati D, et al. (2009) Transstadial and transovarial transmission of Orientia tsutsugamushi in Leptotrombidium imphalum and Leptotrombidium chiangraiensis (Acari: Trombiculidae). J Med Entomol 46:1442–1445. [DOI] [PubMed] [Google Scholar]
- 4. Kweon SS, Choi JS, Lim HS, Kim JR, Kim KY, et al. (2009) Rapid increase of scrub typhus, South Korea, 2001–2006. Emerg Infect Dis 15:1127–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.KCDC (2012) The National Notifiable Disease Surveillance System (NNDSS).
- 6. Lee SH, Lee YS, Lee IY, Lim JW, Shin HK, et al. (2012) Monthly occurrence of vectors and reservoir rodents of scrub typhus in an endemic area of Jeollanam-do, Korea. Korean J Parasitol 50:327–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takahashi M, Misumi H, Urakami H, Nakajima S, Furui S, et al. (2004) Mite vectors (Acari: Trombiculidae) of scrub typhus in a new endemic area in northern Kyoto, Japan. J Med Entomol 41:107–114. [DOI] [PubMed] [Google Scholar]
- 8. Chang WH (1995) Current status of tsutsugamushi disease in Korea. J Korean Med Sci 10:227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuo CC, Huang CL, Wang HC (2011) Identification of potential hosts and vectors of scrub typhus and tick-borne spotted fever group rickettsiae in eastern Taiwan. Med Vet Entomol 25:169–177. [DOI] [PubMed] [Google Scholar]
- 10. Frances SP, Watcharapichat P, Phulsuksombati D, Tanskul P, Linthicum KJ (1999) Seasonal occurrence of Leptotrombidium deliense (Acari: Trombiculidae) attached to sentinel rodents in an orchard near Bangkok, Thailand. J Med Entomol 36:869–874. [DOI] [PubMed] [Google Scholar]
- 11. Ree HI, Lee IY, Cho MK (1991) Determination of the vector species of tsutsugamushi disease in Korea. Kisaengchunghak Chapchi 29:87–92. [DOI] [PubMed] [Google Scholar]
- 12. Ree HI, Lee IY, Cho MK (1992) Study on vector mites of tsutsugamushi disease in Cheju Island, Korea. Kisaengchunghak Chapchi 30:341–348. [DOI] [PubMed] [Google Scholar]
- 13. Ree HI, Lee IY, Jeon SH, Yoshida Y (1997) Geographical distribution of vectors and sero-strains of tsutsugamushi disease at mid-south inland of Korea. Korean J Parasitol 35:171–179. [DOI] [PubMed] [Google Scholar]
- 14. Lee HI, Shim SK, Song BG, Choi EN, Hwang KJ, et al. (2011) Detection of Orientia tsutsugamushi, the causative agent of scrub typhus, in a novel mite species, Eushoengastia koreaensis, in Korea. Vector Borne Zoonotic Dis 11:209–214. [DOI] [PubMed] [Google Scholar]
- 15. Kim HC, Lee IY, Chong ST, Richards AL, Gu SH, et al. (2010) Serosurveillance of scrub typhus in small mammals collected from military training sites near the DMZ, Northern Gyeonggi-do, Korea, and analysis of the relative abundance of chiggers from mammals examined. Korean J Parasitol 48:237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ree HI, Chang WH, Kee S, Lee IY, Jeon SH (1997) Detection of Orientia tsutsugamushi DNA in individual trombiculids using polymerase chain reaction in Korea. Med Entomol Zool 48:197–209. [Google Scholar]
- 17. Lee IY, Kim HC, Lee YS, Seo JH, Lim JW, et al. (2009) Geographical distribution and relative abundance of vectors of scrub typhus in the Republic of Korea. Korean J Parasitol 47:381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ree HI (1990) Fauna and key to the chigger mites of Korea (Acarina: Trombiculidae and Leeuwenhoekiidae). Kor J System Zool 6:57–70. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Collection sites of small mammals in Korean peninsula.
(DOC)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.