Abstract
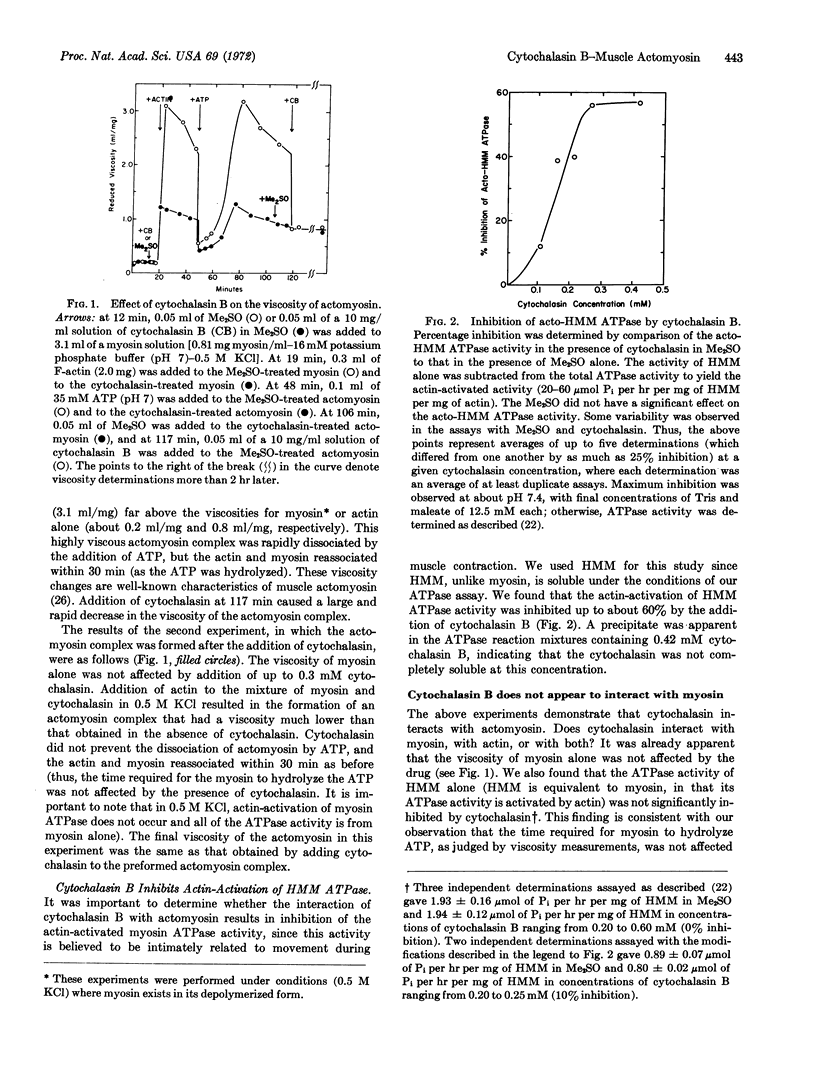
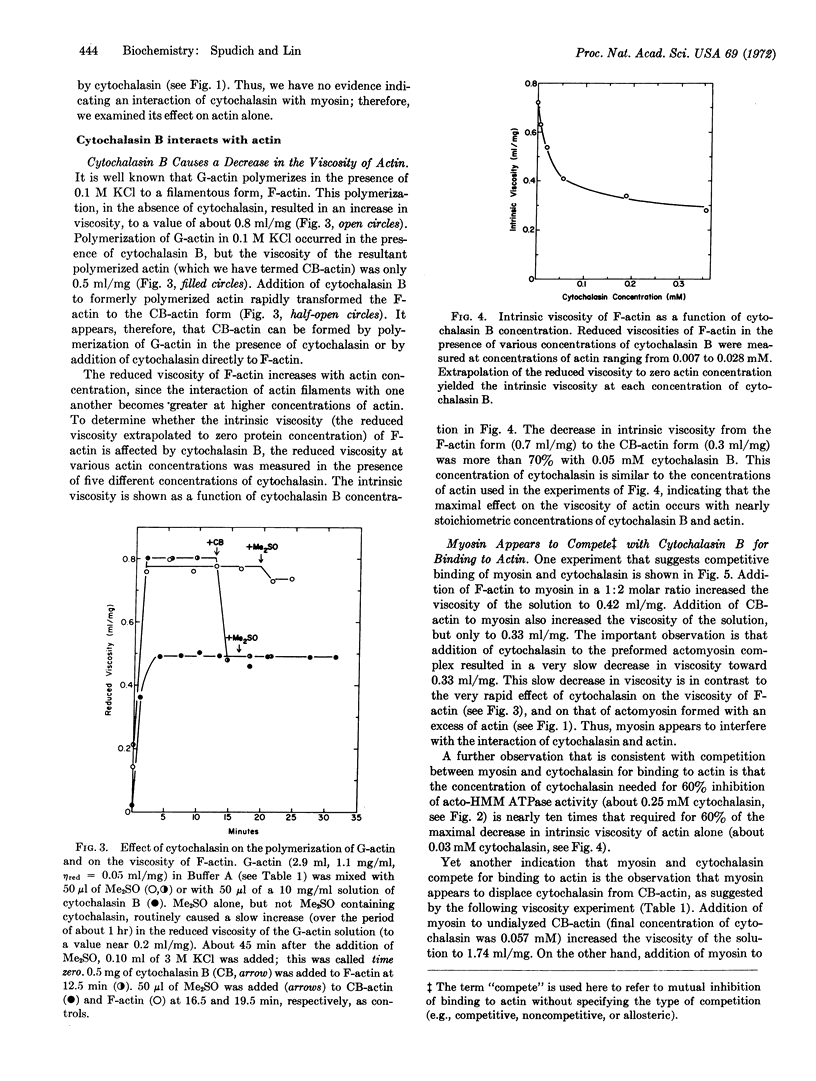
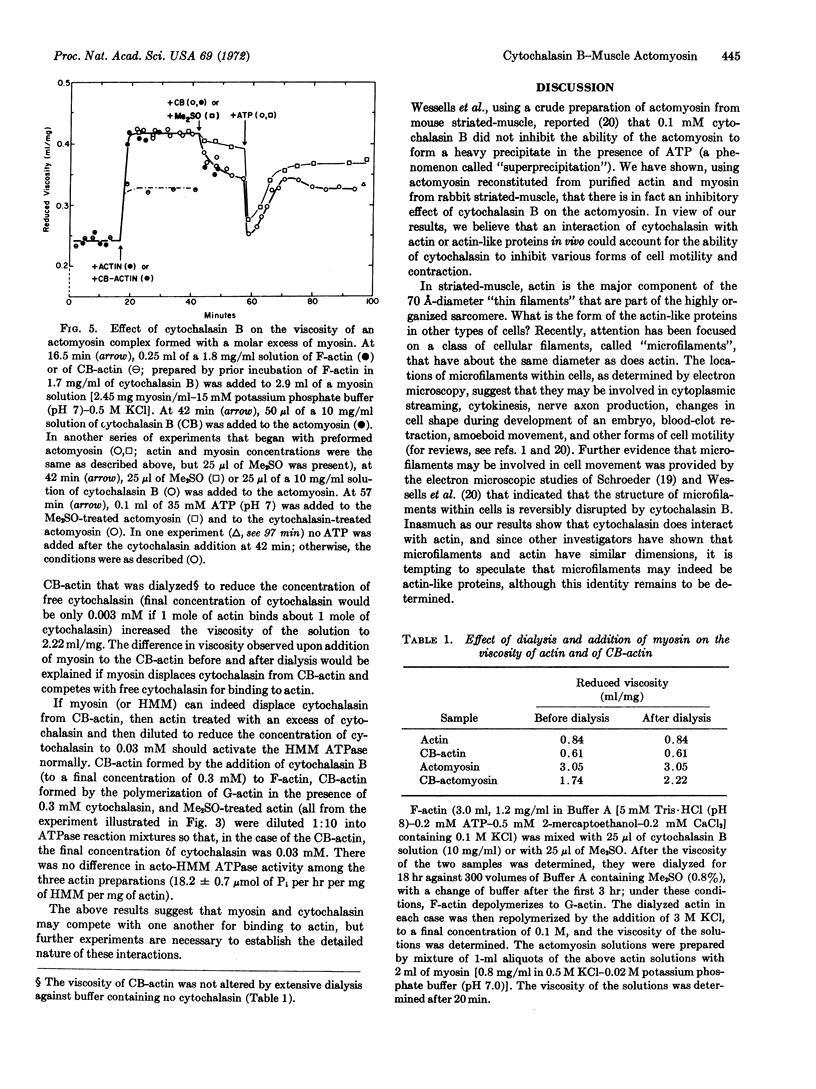
Cytochalasin B, an alkaloid that inhibits a wide variety of cellular movements, interacts with actomyosin, the contractile protein complex of striated muscle. This interaction causes a decrease in viscosity of the actomyosin complex and an inhibition of acto-heavy meromyosin ATPase activity of at least 60%. Cytochalasin B does not affect the viscosity of myosin nor the ATPase activity of heavy meromyosin, suggesting that the drug might interact directly with the actin moiety of the actomyosin complex. Indeed, as judged by viscometry, there is a strong interaction of cytochalasin B with actin, at nearly stoichiometric concentrations. Myosin appears to compete with cytochalasin for binding to actin.
Keywords: cell movement, microfilaments, rabbit striated muscle
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman M. R., Taylor E. W. Further purification and characterization of slime mold myosin and slime mold actin. Biochemistry. 1969 Dec;8(12):4976–4988. doi: 10.1021/bi00840a047. [DOI] [PubMed] [Google Scholar]
- BETTEX-GALLAND M., PORTZEHL H., LUSCHER E. F. Dissociation of thrombosthenin into two components comparable with actin and myosin. Nature. 1962 Feb 24;193:777–778. doi: 10.1038/193777a0. [DOI] [PubMed] [Google Scholar]
- Booyse F. M., Hoveke T. P., Zschocke D., Rafelson M. E., Jr Human platelet myosin. Isolation and properties. J Biol Chem. 1971 Jul 10;246(13):4291–4297. [PubMed] [Google Scholar]
- Carter S. B. Effects of cytochalasins on mammalian cells. Nature. 1967 Jan 21;213(5073):261–264. doi: 10.1038/213261a0. [DOI] [PubMed] [Google Scholar]
- HOFFMANN-BERLING H. Das kontraktile Eiweiss undifferenzierter Zellen. Biochim Biophys Acta. 1956 Mar;19(3):453–463. doi: 10.1016/0006-3002(56)90468-1. [DOI] [PubMed] [Google Scholar]
- Hatano S., Oosawa F. Isolation and characterization of plasmodium actin. Biochim Biophys Acta. 1966 Oct 31;127(2):488–498. doi: 10.1016/0304-4165(66)90402-8. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Bischoff R., Holtzer H. Formation of arrowhead complexes with heavy meromyosin in a variety of cell types. J Cell Biol. 1969 Nov;43(2):312–328. [PMC free article] [PubMed] [Google Scholar]
- Jahn T. L., Bovee E. C. Protoplasmic movements within cells. Physiol Rev. 1969 Oct;49(4):793–862. doi: 10.1152/physrev.1969.49.4.793. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lowey S., Slayter H. S., Weeds A. G., Baker H. Substructure of the myosin molecule. I. Subfragments of myosin by enzymic degradation. J Mol Biol. 1969 May 28;42(1):1–29. doi: 10.1016/0022-2836(69)90483-5. [DOI] [PubMed] [Google Scholar]
- Miki-Noumura T. An actin-like protein of the sea urchin eggs. II. Direct isolation procedure. Dev Growth Differ. 1969 Dec;11(3):219–231. doi: 10.1111/j.1440-169x.1969.00219.x. [DOI] [PubMed] [Google Scholar]
- Nachmias V. T., Huxley H. E. Electron microscope observations on actomyosin and actin preparations from Physarum polycephalum, and on their interaction with heavy meromyosin subfragment I from muscle myosin. J Mol Biol. 1970 May 28;50(1):83–90. doi: 10.1016/0022-2836(70)90105-1. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Korn E. D. Filaments of Amoeba proteus. II. Binding of heavy meromyosin by thin filaments in motile cytoplasmic extracts. J Cell Biol. 1971 Jan;48(1):216–219. doi: 10.1083/jcb.48.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Shelton E., Weihing R. R., Korn E. D. Ultrastructural characterization of F-actin isolated from Acanthamoeba castellanii and identification of cytoplasmic filaments as F-actin by reaction with rabbit heavy meromyosin. J Mol Biol. 1970 May 28;50(1):91–97. doi: 10.1016/0022-2836(70)90106-3. [DOI] [PubMed] [Google Scholar]
- Schroeder T. E. The contractile ring. I. Fine structure of dividing mammalian (HeLa) cells and the effects of cytochalasin B. Z Zellforsch Mikrosk Anat. 1970;109(4):431–449. [PubMed] [Google Scholar]
- Senda N., Shibata N., Tatsumi N., Kondo K., Hamada K. A contractile protein from leucocytes. Its extraction and some of its properties. Biochim Biophys Acta. 1969 May;181(1):191–200. doi: 10.1016/0005-2795(69)90241-4. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Tilney L. G., Mooseker M. Actin in the brush-border of epithelial cells of the chicken intestine. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2611–2615. doi: 10.1073/pnas.68.10.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonomura Y., Appel P., Morales M. On the molecular weight of myosin. II. Biochemistry. 1966 Feb;5(2):515–521. doi: 10.1021/bi00866a017. [DOI] [PubMed] [Google Scholar]
- Weihing R. R., Korn E. D. Acanthamoeba actin. Isolation and properties. Biochemistry. 1971 Feb 16;10(4):590–600. doi: 10.1021/bi00780a008. [DOI] [PubMed] [Google Scholar]
- Wessells N. K., Spooner B. S., Ash J. F., Bradley M. O., Luduena M. A., Taylor E. L., Wrenn J. T., Yamada K. Microfilaments in cellular and developmental processes. Science. 1971 Jan 15;171(3967):135–143. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]



